
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Łucja Justyna Walczak-Nowicka | -- | 3929 | 2022-06-28 13:20:12 | | | |
| 2 | Lindsay Dong | Meta information modification | 3929 | 2022-06-29 04:24:22 | | |
Video Upload Options
One of the compounds known as a preservative with a high safety profile is sodium benzoate. While some studies show that it can be used to treat conditions such as depression, pain, schizophrenia, autism spectrum disorders, and neurodegenerative diseases, others report its harmfulness. For example, it was found to cause mutagenic effects, generate oxidative stress, disrupt hormones, and reduce fertility.
1. Introduction
2. The Harmfulness of Sodium Benzoate
It is believed that benzoate can be transformed by decarboxylation into toxic benzene, especially in combination with vitamin C, and then become a compound of high toxicity, mutagenicity, and teratogenicity [17]. There are also reports that sodium benzoate has a weak genotoxic effect. Moreover, it was shown to increase the DNA damage in human lymphocytes in vitro. The compound did not affect the rate of replication, but it did reduce the mitotic rate [18]. Mutagenic and genotoxic effects were also demonstrated in another study on human lymphocytes [19]. This compound caused micronucleus formation and chromosome breakage. In addition, the research shows that sodium benzoate generates oxidative stress and has an adverse effect on the immune system, liver, kidneys, and fertility.
2.1. The Effect of Sodium Benzoate on the Oxidative Stress and Inflammation
The effect of sodium benzoate (6.25, 12.5, 25, 50, and 100 μg/mL) on the increasing oxidative stress was observed in erythrocytes in an in vitro study [20]. After the treatment of cells with benzoate, there was observed increased lipid peroxidation, as well as decreased levels of antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione S-transferase. In another study, its effect on the induction of apoptosis was observed [21]. In addition, inhibition of antioxidant enzymes, decreased levels of glutathione (GSH), increased levels of nitric oxide (NO), and inflammation (increased in IL-6 and TNF α) were noted.
2.2. Effect of Sodium Benzoate on the Embryos
2.3. Effect of Sodium Benzoate on Hormone Levels
2.4. Effect of Sodium Benzoate on Liver and Kidney Function
2.5. Sodium Benzoate and Children’s Hyperactivity
2.6. Sodium Benzoate—Irritating Effect on the Gastric Mucosa
2.7. Sodium Benzoate with Vitamin C
2.8. Effects of Sodium Benzoate on Memory and Anxiety Processes
3. Beneficial Properties of Sodium Benzoate
3.1. Effects of Sodium Benzoate on Oxidative Stress and Inflammation
Inflammatory responses of microglia and astroglia have been observed in various disease entities related to the nervous system. In lipopolysaccharide (LPS)-stimulated microglia cells, benzoate (>100–500 μm) suppresses NO production and decreases inducible nitric oxide synthase (iNOS) expression by inhibiting NFκB activation [45]. Furthermore, it inhibits the production and decreases the expression of TNF- α and IL-1 β. LPS increases the expression of MHC class II and B7-1 and B7-2 stimulatory molecules, and sodium benzoate counteracts these effects by suppressing their expression. In addition, the compound decreases CD11b expression (overexpression is associated with increased microglia activation) in microglia cells. It also inhibits LPS-induced activation of p21ras. It also affects astroglia cells, namely by decreasing the expression of iNOS but also by inhibiting the increased expression of glial fibrillary acidic protein (GFAP). The researchers suggest that the reduction in mevalonate pathway intermediates is likely responsible for the observed anti-inflammatory effects. Moreover, the compound reduces in vivo cholesterol levels in mice by 28% after 7 days of therapy. In another study, similar results were obtained, i.e., reduction in the proinflammatory cytokines TNFα and IL-6, as well as in cholesterol levels (sodium benzoate doses: 250, 500 mg/kg b.w.) [46].
3.2. Sodium Benzoate in Major Depressive Disorder and Anxiety
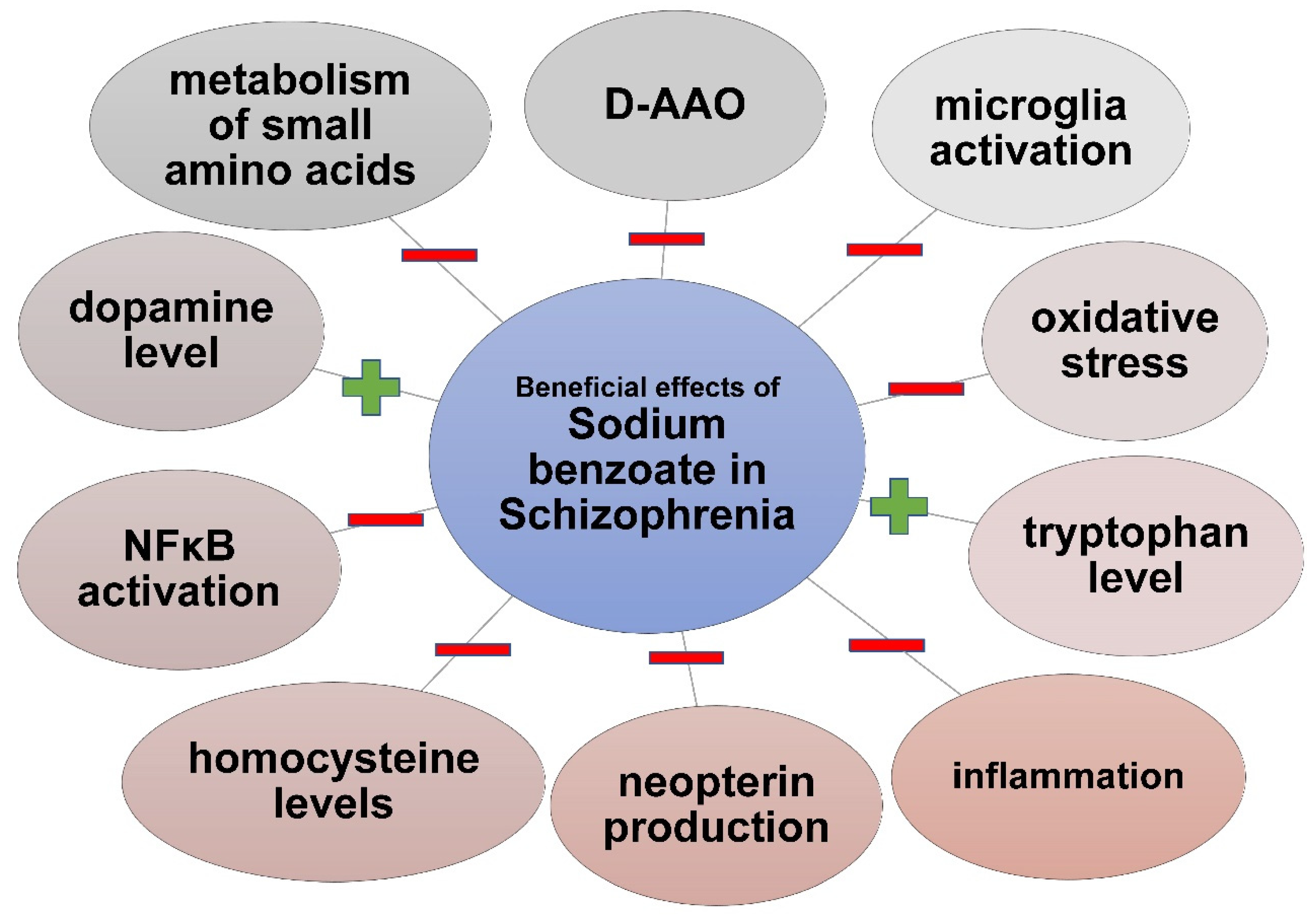
3.3. Sodium Benzoate in Schizophrenia
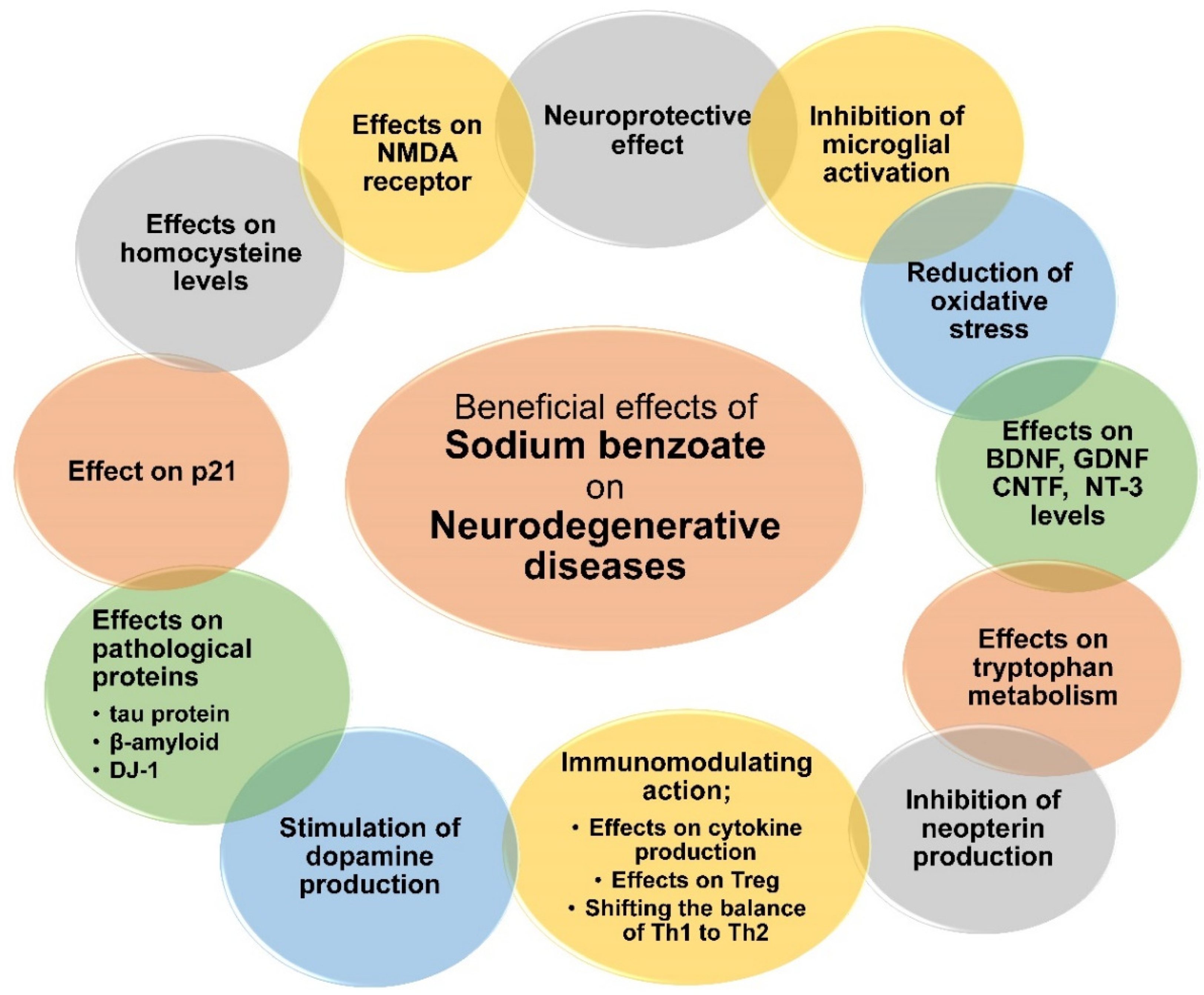
3.4. Sodium Benzoate in Neurodegenerative Diseases
3.5. Sodium Benzoate in Pain Relief
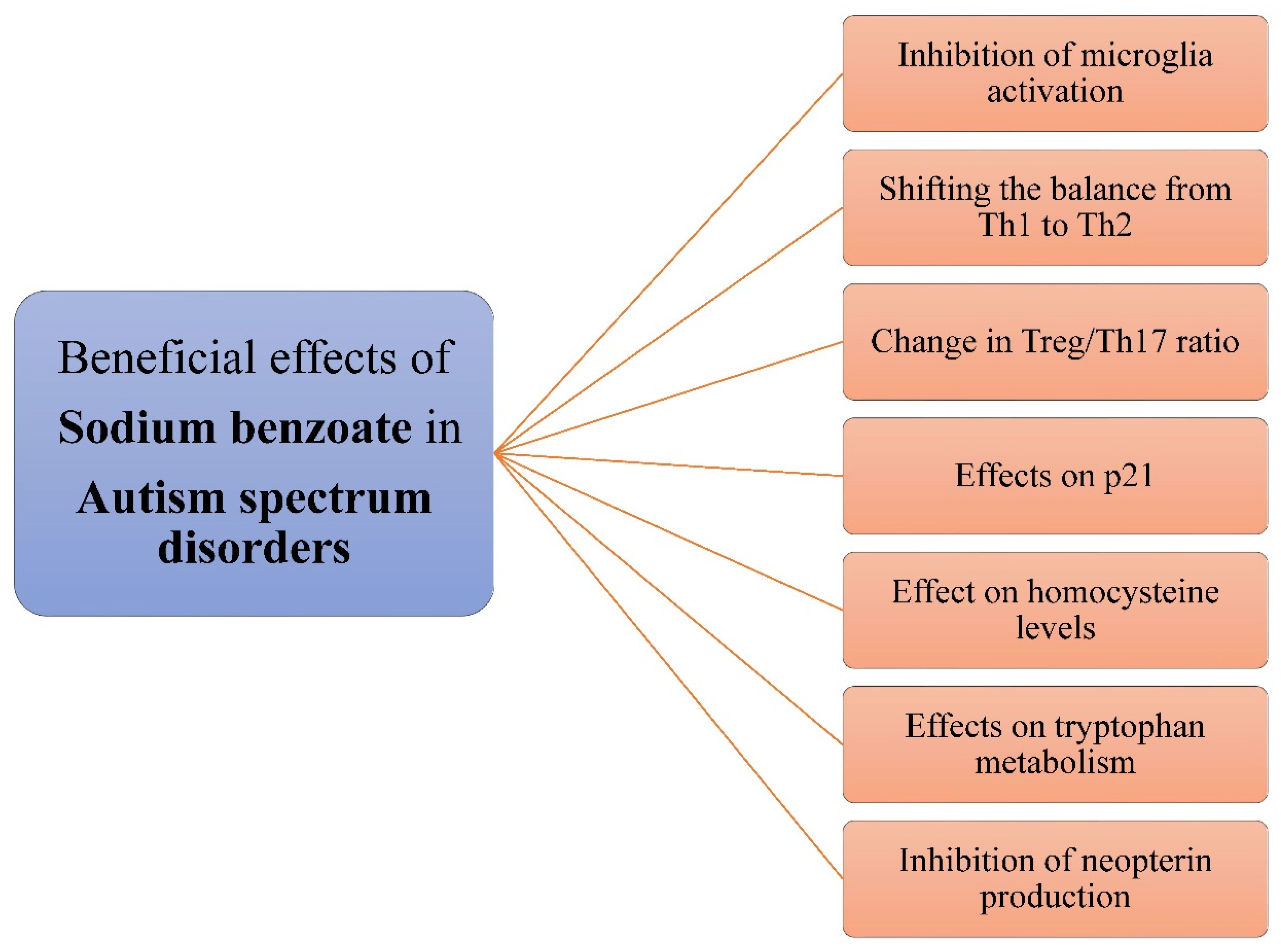
3.6. Sodium Benzoate in Autism Spectrum Disorder
References
- Hartmann, S.; Klaschka, U. Interested Consumers’ Awareness of Harmful Chemicals in Everyday Products. Environ. Sci. Eur. 2017, 29, 29.
- Hartmann, S.; Klaschka, U. Do Consumers Care about Substances of Very High Concern in Articles? Environ. Sci. Eur. 2018, 30, 29.
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Int. 2017, 99, 58–71.
- Cegiełka, A. “Clean Label” as One of the Leading Trends in the Meat Industry in the World and in Poland—A Review. Rocz. Panstw. Zakl. Hig. 2020, 71, 43–55.
- Cheung, T.T.L.; Junghans, A.F.; Dijksterhuis, G.B.; Kroese, F.; Johansson, P.; Hall, L.; De Ridder, D.T.D. Consumers’ Choice-Blindness to Ingredient Information. Appetite 2016, 106, 2–12.
- Davidson, P.M.; Taylor, T.M.; David, J.R.D. Antimicrobials in Food, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-367-17878-9.
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1733 (accessed on 14 November 2021).
- Lennerz, B.; Vafai, S.B.; Delaney, N.F.; Clish, C.B.; Deik, A.A.; Pierce, K.A.; Ludwig, D.S.; Mootha, V.K. Effects of Sodium Benzoate, a Widely Used Food Preservative, on Glucose Homeostasis and Metabolic Profiles in Humans. Mol. Genet. Metab. 2015, 114, 73–79.
- Chen, Y.; Ma, Y.; Ma, W. Pharmacokinetics and Bioavailability of Cinnamic Acid after Oral Administration of Ramulus Cinnamomi in Rats. Eur. J. Drug Metab. Pharmacokinet. 2009, 34, 51–56.
- Zhao, K.; Chen, Y.; Hong, S.; Yang, Y.; Xu, J.; Yang, H.; Zhu, L.; Liu, M.; Xie, Q.; Tang, X.; et al. Characteristics of β-Oxidative and Reductive Metabolism on the Acyl Side Chain of Cinnamic Acid and Its Analogues in Rats. Acta Pharmacol. Sin. 2019, 40, 1106–1118.
- Shahmohammadi, M.; Javadi, M.; Nassiri-Asl, M. An Overview on the Effects of Sodium Benzoate as a Preservative in Food Products. Biotechnol. Health Sci. 2016, 3, 7–11.
- BUPHENYL® (Sodium Phenylbutyrate) Tablets BUPHENYL® (Sodium Phenylbutyrate) Powder. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020572s016,020573s015lbl.pdf (accessed on 10 February 2022).
- AMMONUL. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020645lbl.pdf (accessed on 10 February 2022).
- Weber, R.W.; Hoffman, M.; Raine, D.A.; Nelson, H.S. Incidence of Bronchoconstriction Due to Aspirin, Azo Dyes, Non-Azo Dyes, and Preservatives in a Population of Perennial Asthmatics. J. Allergy Clin. Immunol. 1979, 64, 32–37.
- Settipane, G.A. Aspirin and Allergic Diseases: A Review. Am. J. Med. 1983, 74, 102–109.
- Balatsinou, L.; Di Gioacchino, G.; Sabatino, G.; Cavallucci, E.; Caruso, R.; Gabriele, E.; Ramondo, S.; Di Giampaolo, L.; Verna, N.; Di Gioacchino, M. Asthma Worsened by Benzoate Contained in Some Antiasthmatic Drugs. Int. J. Immunopathol. Pharmacol. 2004, 17, 225–226.
- Piper, J.D.; Piper, P.W. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr. Rev. Food Sci. Food Saf. 2017, 16, 868–880.
- Zengin, N.; Yüzbaşıoğlu, D.; Unal, F.; Yılmaz, S.; Aksoy, H. The Evaluation of the Genotoxicity of Two Food Preservatives: Sodium Benzoate and Potassium Benzoate. Food Chem. Toxicol. 2011, 49, 763–769.
- Pongsavee, M. Effect of Sodium Benzoate Preservative on Micronucleus Induction, Chromosome Break, and Ala40Thr Superoxide Dismutase Gene Mutation in Lymphocytes. BioMed Res. Int. 2015, 2015, 103512.
- Yetuk, G.; Pandir, D.; Bas, H. Protective Role of Catechin and Quercetin in Sodium Benzoate-Induced Lipid Peroxidation and the Antioxidant System in Human Erythrocytes In Vitro. Sci. World J. 2014, 2014, e874824.
- El-Shennawy, L.; Kamel, M.A.E.; Khalaf, A.H.Y.; Yousef, M.I. Dose-Dependent Reproductive Toxicity of Sodium Benzoate in Male Rats: Inflammation, Oxidative Stress and Apoptosis. Reprod. Toxicol. 2020, 98, 92–98.
- Tsay, H.-J.; Wang, Y.-H.; Chen, W.-L.; Huang, M.-Y.; Chen, Y.-H. Treatment with Sodium Benzoate Leads to Malformation of Zebrafish Larvae. Neurotoxicol. Teratol. 2007, 29, 562–569.
- Chen, Q.; Huang, N.-N.; Huang, J.-T.; Chen, S.; Fan, J.; Li, C.; Xie, F.-K. Sodium Benzoate Exposure Downregulates the Expression of Tyrosine Hydroxylase and Dopamine Transporter in Dopaminergic Neurons in Developing Zebrafish. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 85–91.
- Sabour, A.; Ibrahim, I.R. Effect of Sodium Benzoate on Corticosterone Hormone Level, Oxidative Stress Indicators and Electrolytes in Immature Male Rats. Sci. J. Med. Res. 2019, 3, 101–106.
- Saatci, C.; Erdem, Y.; Bayramov, R.; Akalın, H.; Tascioglu, N.; Ozkul, Y. Effect of Sodium Benzoate on DNA Breakage, Micronucleus Formation and Mitotic Index in Peripheral Blood of Pregnant Rats and Their Newborns. Biotechnol. Biotechnol. Equip. 2016, 30, 1179–1183.
- Taheri, S.H.; Sohrabi, D. Teratogenic Effects of Sodium Benzoate on the Rat Fetus. J. Adv. Med. Biomed. Res. 2002, 10, 1–4.
- Jewo, P.I.; Oyeniran, D.A.; Ojekale, A.B.; Oguntola, J.A. Histological and Biochemical Studies of Germ Cell Toxicity in Male Rats Exposed to Sodium Benzoate. J. Adv. Med. Pharm. Sci. 2020, 22, 51–69.
- Kehinde, O.S.; Christianah, O.I.; Oyetunji, O.A. Ascorbic Acid and Sodium Benzoate Synergistically Aggravates Testicular Dysfunction in Adult Wistar Rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 39–46.
- Mahmoud, G.S.; Sayed, S.A.; Abdelmawla, S.N.; Amer, M.A. Positive Effects of Systemic Sodium Benzoate and Olanzapine Treatment on Activities of Daily Life, Spatial Learning and Working Memory in Ketamine-Induced Rat Model of Schizophrenia. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 21–30.
- Khan, I.S.; Dar, K.B.; Ganie, S.A.; Ali, M.N. Toxicological Impact of Sodium Benzoate on Inflammatory Cytokines, Oxidative Stress and Biochemical Markers in Male Wistar Rats. Drug Chem. Toxicol. 2020, 1–10.
- Khodaei, F.; Kholghipour, H.; Hosseinzadeh, M.; Rashedinia, M. Effect of Sodium Benzoate on Liver and Kidney Lipid Peroxidation and Antioxidant Enzymes in Mice. J. Rep. Pharm. Sci. 2019, 8, 217.
- Zeghib, K.; Boutlelis, D.A. Food Additive (Sodium Benzoate)-Induced Damage on Renal Function and Glomerular Cells in Rats; Modulating Effect of Aqueous Extract of Atriplex halimus L. Iran J. Pharm. Res. 2021, 20, 296–306.
- McDougal, E.; Gracie, H.; Oldridge, J.; Stewart, T.M.; Booth, J.N.; Rhodes, S.M. Relationships between Cognition and Literacy in Children with Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Br. J. Dev. Psychol. 2022, 40, 130–150.
- Bateman, B.; Warner, J.; Hutchinson, E.; Dean, T.; Rowlandson, P.; Gant, C.; Grundy, J.; Fitzgerald, C.; Stevenson, J. The Effects of a Double Blind, Placebo Controlled, Artificial Food Colourings and Benzoate Preservative Challenge on Hyperactivity in a General Population Sample of Preschool Children. Arch. Dis. Child. 2004, 89, 506–511.
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567.
- Beezhold, B.L.; Johnston, C.S.; Nochta, K.A. Sodium Benzoate-Rich Beverage Consumption Is Associated with Increased Reporting of ADHD Symptoms in College Students: A Pilot Investigation. J. Atten. Disord. 2014, 18, 236–241.
- Schaubschläger, W.W.; Becker, W.M.; Schade, U.; Zabel, P.; Schlaak, M. Release of Mediators from Human Gastric Mucosa and Blood in Adverse Reactions to Benzoate. Int. Arch. Allergy Appl. Immunol. 1991, 96, 97–101.
- Mcneal, T.P.; Nyman, P.J.; Diachenko, G.W.; Hollifield, H.C. Survey of Benzene in Foods by Using Headspace Concentration Techniques and Capillary Gas Chromatography. J. AOAC Int. 1993, 76, 1213–1219.
- Heshmati, A.; Ghadimi, S.; Mousavi Khaneghah, A.; Barba, F.J.; Lorenzo, J.M.; Nazemi, F.; Fakhri, Y. Risk Assessment of Benzene in Food Samples of Iran’s Market. Food Chem. Toxicol. 2018, 114, 278–284.
- Salviano dos Santos, V.P.; Medeiros Salgado, A.; Guedes Torres, A.; Signori Pereira, K. Benzene as a Chemical Hazard in Processed Foods. Int. J. Food Sci. 2015, 2015, e545640.
- Azuma, S.L.; Quartey, N.K.-A.; Ofosu, I.W. Sodium Benzoate in Non-Alcoholic Carbonated (Soft) Drinks: Exposure and Health Risks. Sci. Afr. 2020, 10, e00611.
- Gardner, L.K.; Lawrence, G.D. Benzene Production from Decarboxylation of Benzoic Acid in the Presence of Ascorbic Acid and a Transition-Metal Catalyst. J. Agric. Food Chem. 1993, 41, 693–695.
- Kamel, M.M.; Razek, A.H. Neurobehavioral Alterations in Male Rats Exposed to Sodium Benzoate. Life Sci. J. 2013, 10, 722–726.
- Noorafshan, A.; Erfanizadeh, M.; Karbalay-doust, S. Sodium Benzoate, a Food Preservative, Induces Anxiety and Motor Impairment in Rats. Neurosciences J. 2014, 19, 24–28.
- Brahmachari, S.; Jana, A.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Reduces Microglial and Astroglial Inflammatory Responses. J. Immunol. 2009, 183, 5917–5927.
- Efekemo, O.; Akaninwor, J.O.; Essien, E.B. Effect of Oral Intake of Sodium Benzoate on Serum Cholesterol and Proinflammatory Cytokine (Tumor Necrosis Factor Alpha and Interleukin-6 ) Levels in the Heart Tissue of Wistar Rats. Asian J. Res. Biochem. 2019, 5, 1–8.
- Modi, K.K.; Roy, A.; Brahmachari, S.; Rangasamy, S.B.; Pahan, K. Cinnamon and Its Metabolite Sodium Benzoate Attenuate the Activation of P21rac and Protect Memory and Learning in an Animal Model of Alzheimer’s Disease. PLoS ONE 2015, 10, e0130398.
- Esnafoglu, E.; Ozturan, D.D. The Relationship of Severity of Depression with Homocysteine, Folate, Vitamin B12, and Vitamin D Levels in Children and Adolescents. Child. Adolesc. Ment. Health 2020, 25, 249–255.
- Chung, K.-H.; Chiou, H.-Y.; Chen, Y.-H. Associations between Serum Homocysteine Levels and Anxiety and Depression among Children and Adolescents in Taiwan. Sci. Rep. 2017, 7, 8330.
- Monje, F.J.; Cabatic, M.; Divisch, I.; Kim, E.-J.; Herkner, K.R.; Binder, B.R.; Pollak, D.D. Constant Darkness Induces IL-6-Dependent Depression-Like Behavior through the NF-ΚB Signaling Pathway. J. Neurosci. 2011, 31, 9075–9083.
- Koo, J.; Marangell, L.b.; Nakamura, M.; Armstrong, A.; Jeon, C.; Bhutani, T.; Wu, J.j. Depression and Suicidality in Psoriasis: Review of the Literature Including the Cytokine Theory of Depression. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1999–2009.
- Makhija, K.; Karunakaran, S. The Role of Inflammatory Cytokines on the Aetiopathogenesis of Depression. Aust. New Zealand J. Psychiatry 2013, 47, 828–839.
- Maier, E.; Kurz, K.; Jenny, M.; Schennach, H.; Ueberall, F.; Fuchs, D. Food Preservatives Sodium Benzoate and Propionic Acid and Colorant Curcumin Suppress Th1-Type Immune Response in Vitro. Food Chem. Toxicol. 2010, 48, 1950–1956.
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56.
- Lindseth, G.; Helland, B.; Caspers, J. The Effects of Dietary Tryptophan on Affective Disorders. Arch. Psychiatr. Nurs. 2015, 29, 102–107.
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Aktualne Tematy w Neuronaukach Behawioralnych; Dantzer, R., Capuron, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 117–138. ISBN 978-3-319-51152-8.
- Muszyńska, B.; Łojewski, M.; Rojowski, J.; Opoka, W.; Sułkowska-Ziaja, K. Surowce naturalne mające znaczenie w profilaktyce i wspomagające leczenie depresji . Psychiatria Polska 2015, 49, 435–453.
- Widner, B.; Laich, A.; Sperner-Unterweger, B.; Ledochowski, M.; Fuchs, D. Neopterin Production, Tryptophan Degradation, and Mental Depression—What Is the Link? Brain Behav. Immun. 2002, 16, 590–595.
- Celik, C.; Erdem, M.; Caycı, T.; Ozdemir, B.; Ozgur Akgul, E.; Kurt, Y.G.; Yaman, H.; Isıntas, M.; Ozgen, F.; Ozsahin, A. The Association between Serum Levels of Neopterin and Number of Depressive Episodes of Major Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 372–375.
- Ciardi, C.; Jenny, M.; Tschoner, A.; Ueberall, F.; Patsch, J.; Pedrini, M.; Ebenbichler, C.; Fuchs, D. Food Additives Such as Sodium Sulphite, Sodium Benzoate and Curcumin Inhibit Leptin Release in Lipopolysaccharide-Treated Murine Adipocytes in Vitro. Br. J. Nutr. 2012, 107, 826–833.
- Ambrus, L.; Westling, S. Leptin, Anxiety Symptoms, and Hypothalamic-Pituitary-Adrenal Axis Activity among Drug-Free, Female Suicide Attempters. Neuropsychobiology 2019, 78, 145–152.
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298.
- Pasquin, S.; Sharma, M.; Gauchat, J.-F. Ciliary Neurotrophic Factor (CNTF): New Facets of an Old Molecule for Treating Neurodegenerative and Metabolic Syndrome Pathologies. Cytokine Growth Factor Rev. 2015, 26, 507–515.
- Modi, K.K.; Jana, M.; Mondal, S.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Upregulates Ciliary Neurotrophic Factor in Astrocytes and Oligodendrocytes. Neurochem. Res. 2015, 40, 2333–2347.
- Peruga, I.; Hartwig, S.; Merkler, D.; Thöne, J.; Hovemann, B.; Juckel, G.; Gold, R.; Linker, R.A. Endogenous Ciliary Neurotrophic Factor Modulates Anxiety and Depressive-like Behavior. Behav. Brain Res. 2012, 229, 325–332.
- Uzbekov, M.; Shikhov, S. Ciliary Neurotrophic Factor Disturbances in Patients with Melancholic Depression. Biomed. J. Sci. Technol. Res. 2019, 13, 10016–10017.
- Jia, C.; Brown, R.W.; Malone, H.M.; Burgess, K.C.; Gill, D.W.; Keasey, M.P.; Hagg, T. Ciliary Neurotrophic Factor Is a Key Sex-Specific Regulator of Depressive-like Behavior in Mice. Psychoneuroendocrinology 2019, 100, 96–105.
- Lin, C.-H.; Chen, P.-K.; Wang, S.-H.; Lane, H.-Y. Effect of Sodium Benzoate on Cognitive Function Among Patients With Behavioral and Psychological Symptoms of Dementia. JAMA Netw. Open 2021, 4, e216156.
- Howley, E.; Bestwick, M.; Fradley, R.; Harrison, H.; Leveridge, M.; Okada, K.; Fieldhouse, C.; Farnaby, W.; Canning, H.; Sykes, A.P.; et al. Assessment of the Target Engagement and D-Serine Biomarker Profiles of the D-Amino Acid Oxidase Inhibitors Sodium Benzoate and PGM030756. Neurochem. Res. 2017, 42, 3279–3288.
- MacKay, M.-A.B.; Kravtsenyuk, M.; Thomas, R.; Mitchell, N.D.; Dursun, S.M.; Baker, G.B. D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression? Front. Psychiatry 2019, 10, 25.
- Saleem, S.; Shaukat, F.; Gul, A.; Arooj, M.; Malik, A. Potential Role of Amino Acids in Pathogenesis of Schizophrenia. Int. J. Health Sci. 2017, 11, 63–68.
- Betts, J.F.; Schweimer, J.V.; Burnham, K.E.; Burnet, P.W.J.; Sharp, T.; Harrison, P.J. D-Amino Acid Oxidase Is Expressed in the Ventral Tegmental Area and Modulates Cortical Dopamine. Front. Synaptic Neurosci. 2014, 6, 11.
- Jana, A.; Modi, K.K.; Roy, A.; Anderson, J.A.; van Breemen, R.B.; Pahan, K. Up-Regulation of Neurotrophic Factors by Cinnamon and Its Metabolite Sodium Benzoate: Therapeutic Implications for Neurodegenerative Disorders. J. Neuroimmune Pharmacol. 2013, 8, 739–755.
- Sharma, M.; Tiwari, M.; Tiwari, R.K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic Clin. Pharmacol. Toxicol. 2015, 117, 287–296.
- Herrmann, W.; Obeid, R. Homocysteine: A Biomarker in Neurodegenerative Diseases. Clin. Chem. Lab. Med. 2011, 49, 435–441.
- Lane, H.-Y.; Tu, C.-H.; Lin, W.-C.; Lin, C.-H. Brain Activity of Benzoate, a D-Amino Acid Oxidase Inhibitor, in Patients With Mild Cognitive Impairment in a Randomized, Double-Blind, Placebo Controlled Clinical Trial. Int. J. Neuropsychopharmacol. 2021, 24, 392–399.
- Repici, M.; Giorgini, F. DJ-1 in Parkinson’s Disease: Clinical Insights and Therapeutic Perspectives. J. Clin. Med. 2019, 8, 1377.
- Xu, W.; Li, T.; Gao, L.; Lenahan, C.; Zheng, J.; Yan, J.; Shao, A.; Zhang, J. Sodium Benzoate Attenuates Secondary Brain Injury by Inhibiting Neuronal Apoptosis and Reducing Mitochondria-Mediated Oxidative Stress in a Rat Model of Intracerebral Hemorrhage: Possible Involvement of DJ-1/Akt/IKK/NFκB Pathway. Front. Mol. Neurosci. 2019, 12, 105.
- Khasnavis, S.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Upregulates Neuroprotective Parkinson Disease Protein DJ-1 in Astrocytes and Neurons. J. Neuroimmune Pharmacol. 2012, 7, 424–435.
- Patel, D.; Jana, A.; Roy, A.; Pahan, K. Cinnamon and Its Metabolite Protect the Nigrostriatum in a Mouse Model of Parkinson’s Disease via Astrocytic GDNF. J. Neuroimmune Pharmacol. 2019, 14, 503–518.
- Chandra, G.; Roy, A.; Rangasamy, S.B.; Pahan, K. Induction of Adaptive Immunity Leads to Nigrostriatal Disease Progression in MPTP Mouse Model of Parkinson’s Disease. J. Immunol. 2017, 198, 4312–4326.
- Rzepiński, Ł.; Maciejek, Z. Heterogenność etiopatogenezy stwardnienia rozsianego w kontekście danych klinicznych, immunohistochemicznych, autopsyjnych oraz aktualnych możliwości terapeutycznych. Pol. Przegląd Neurol. 2018, 14, 1–9.
- Rezaei, N.; Amirghofran, Z.; Nikseresht, A.; Ashjazade, N.; Zoghi, S.; Tahvili, S.; Kamali-Sarvestani, E. In Vitro Effects of Sodium Benzoate on Th1/Th2 Deviation in Patients with Multiple Sclerosis. Immunol. Investig. 2016, 45, 679–691.
- Brahmachari, S.; Pahan, K. Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Modifies T Cells at Multiple Steps and Inhibits Adoptive Transfer of Experimental Allergic Encephalomyelitis. J. Immunol. 2007, 179, 275–283.
- Zhao, W.; Konno, R.; Zhou, X.-J.; Yin, M.; Wang, Y.-X. Inhibition of D-Amino-Acid Oxidase Activity Induces Pain Relief in Mice. Cell. Mol. Neurobiol. 2008, 28, 581–591.
- Zhao, W.-J.; Gao, Z.-Y.; Wei, H.; Nie, H.-Z.; Zhao, Q.; Zhou, X.-J.; Wang, Y.-X. Spinal D-Amino Acid Oxidase Contributes to Neuropathic Pain in Rats. J. Pharmacol. Exp. Ther. 2010, 332, 248–254.
- Gong, N.; Gao, Z.-Y.; Wang, Y.-C.; Li, X.-Y.; Huang, J.-L.; Hashimoto, K.; Wang, Y.-X. A Series of D-Amino Acid Oxidase Inhibitors Specifically Prevents and Reverses Formalin-Induced Tonic Pain in Rats. J. Pharmacol. Exp. Ther. 2011, 336, 282–293.
- Wei, H.; Gong, N.; Huang, J.-L.; Fan, H.; Ma, A.-N.; Li, X.-Y.; Wang, Y.-X.; Pertovaara, A. Spinal D-Amino Acid Oxidase Contributes to Mechanical Pain Hypersensitivity Induced by Sleep Deprivation in the Rat. Pharmacol. Biochem. Behav. 2013, 111, 30–36.
- Blouin, M.; Han, Y.; Burch, J.; Farand, J.; Mellon, C.; Gaudreault, M.; Wrona, M.; Lévesque, J.-F.; Denis, D.; Mathieu, M.-C.; et al. The Discovery of 4-carbonyl)Amino]Cyclopropyl}benzoic Acid (MK-2894), A Potent and Selective Prostaglandin E2 Subtype 4 Receptor Antagonist. J. Med. Chem. 2010, 53, 2227–2238.
- Kramar, H.; Stepaniuk, H.; Voloshchuk, N.; Taran, I.; Kovalenko, S. Experimental study of pain-relieving mechanisms of 4--benzoic acid (PK-66 COMPOUND). Georgian Med. News 2018, 283, 148–154.
- Mondal, S.; Pahan, K. Cinnamon Ameliorates Experimental Allergic Encephalomyelitis in Mice via Regulatory T Cells: Implications for Multiple Sclerosis Therapy. PLoS ONE 2015, 10, e0116566.
- Kundu, M.; Mondal, S.; Roy, A.; Martinson, J.L.; Pahan, K. Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Enriches Regulatory T Cells via STAT6-Mediated Upregulation of TGF-β. J. Immunol. 2016, 197, 3099–3110.
- Yang, P. A Pilot Trial of Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added on Augmentative and Alternative Communication Intervention for Non-Communicative Children with Autism Spectrum Disorders. Transl. Med. 2017, 7, 1000192.
- Görker, I.; Tüzün, Ü. Autistic-like Findings Associated with a Urea Cycle Disorder in a 4-Year-Old Girl. J. Psychiatry Neurosci. 2005, 30, 133–135.
- Kernohan, K.D.; McBride, A.; Hartley, T.; Rojas, S.K.; Care4Rare Canada Consortium; Dyment, D.A.; Boycott, K.M.; Dyack, S. P21 Protein-Activated Kinase 1 Is Associated with Severe Regressive Autism, and Epilepsy. Clin. Genet. 2019, 96, 449–455.
- Fuentes-Albero, M.; Cauli, O. Homocysteine Levels in Autism Spectrum Disorder: A Clinical Update. Endocr Metab Immune Disord. Drug Targets 2018, 18, 289–296.
- Guo, B.-Q.; Li, H.-B.; Ding, S.-B. Blood Homocysteine Levels in Children with Autism Spectrum Disorder: An Updated Systematic Review and Meta-Analysis. Psychiatry Res. 2020, 291, 113283.
- Sweeten, T.L.; Posey, D.J.; McDougle, C.J. High Blood Monocyte Counts and Neopterin Levels in Children With Autistic Disorder. Am. J. Psychiatry 2003, 160, 1691–1693.
- Zhao, H.; Yin, S.; Fan, J. High Plasma Neopterin Levels in Chinese Children with Autism Spectrum Disorders. Int. J. Dev. Neurosci. 2015, 41, 92–97.
- Boccuto, L.; Chen, C.-F.; Pittman, A.R.; Skinner, C.D.; McCartney, H.J.; Jones, K.; Bochner, B.R.; Stevenson, R.E.; Schwartz, C.E. Decreased Tryptophan Metabolism in Patients with Autism Spectrum Disorders. Mol. Autism 2013, 4, 16.
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J.; Chirumbolo, S.; Bjørklund, G. Tryptophan Status in Autism Spectrum Disorder and the Influence of Supplementation on Its Level. Metab. Brain Dis. 2017, 32, 1585–1593.




