
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Caruso Scortichini | -- | 4372 | 2022-06-28 08:20:28 | | | |
| 2 | Vivi Li | Meta information modification | 4372 | 2022-06-28 08:39:42 | | |
Video Upload Options
To reduce the impact of chemical pesticides on the environment, there are relevant efforts to enhance the possibility of controlling plant diseases using environmentally friendly biocontrol agents or natural products that show pathogen control capacity. The European Union, FAO, and the United Nations largely promote and finance projects and programs in order to introduce crop protection principles that can attain sustainable agriculture. Preventive measures related to the choice of cultivars, soil fertility, integrated pest management (IPM), and organic farming strategies are still the basis for obtaining satisfactory crop yields and reducing classical pesticide utilisation through the application of commercially available and ecofriendly control agents. Effective pathogen detection at borders to avoid quarantine pathogens is mandatory to reduce the risk of future epidemics.
1. Introduction
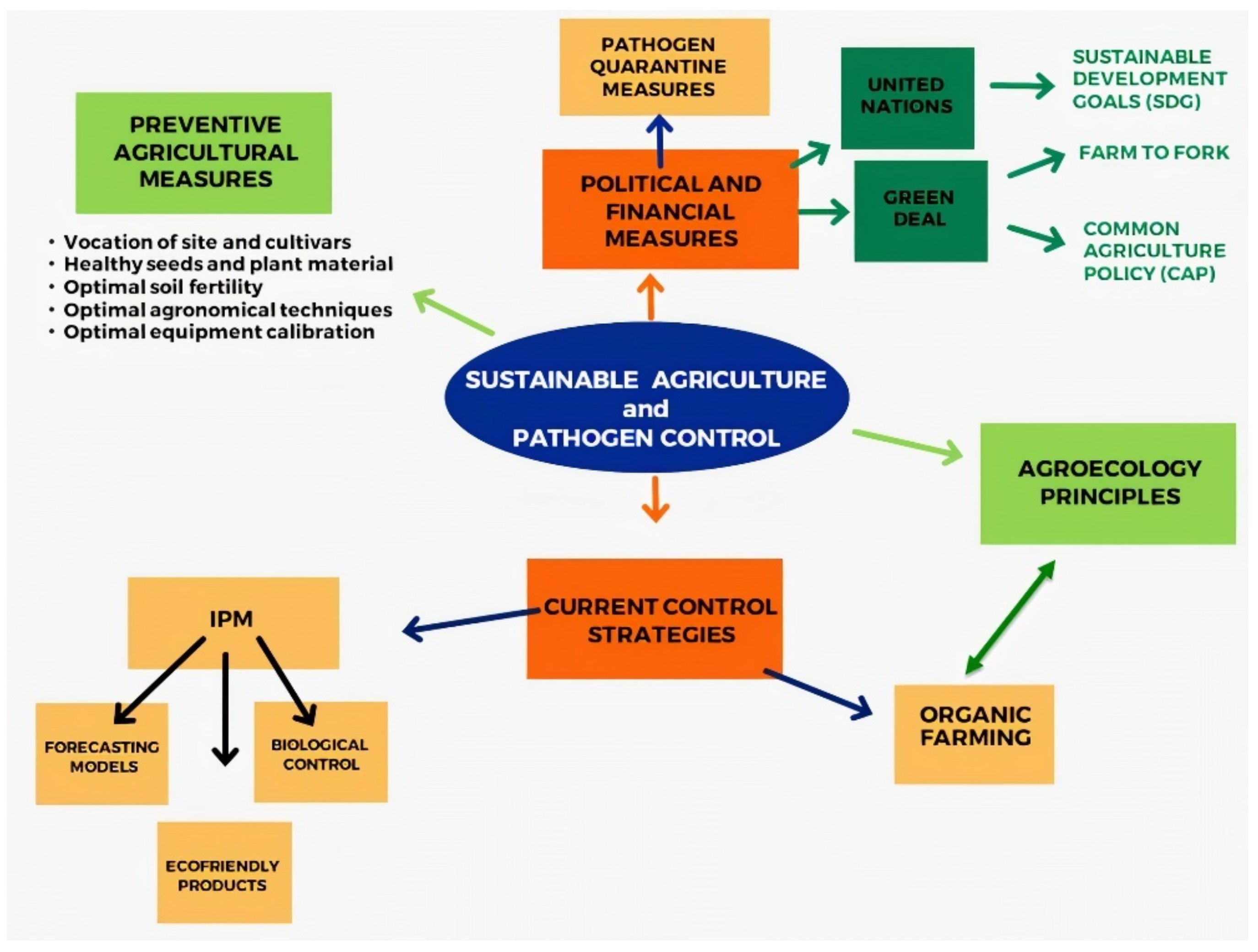
2. The Basis for a Sustainable Disease Control: The Preventive Measures
2.1. Suitability and Selection of the Site and Cultivars
2.2. Healthy Seeds and Plant Material
2.3. Optimal Soil Fertility and Agronomical Techniques
3. Sustainable Agriculture and Pathogen Control
3.1. The Basis for an Effective Sustainable Pathogen Control
3.2. Current Control Strategies
3.2.1. Disease-Forecasting Models
3.2.2. Biological Control
References
- Siebrecht, N. Sustainable agriculture and its implementation gap-overcoming obstacles to implementation. Sustainability 2021, 12, 3853.
- Carlisle, L.; Montenegro de Wit, M.; Delonge, M.S.; Iles, A.; Calo, A.; Getz, C.; Ory, J.; Munden-Dixon, K.; Galt, R.; Melone, B.; et al. Transitioning to sustainable agriculture requires growing and sustaining an ecologically skilled workforce. Front. Sustain. Food Syst. 2019, 3, 96.
- McNeill, D. The contested discourse of sustainable agriculture. Glob. Policy 2019, 10 (Suppl. 1), 16–27.
- Hertoge, K. Mals/Malles Venosta Referendum. 2014. Available online: http://www.marcozullo.it/wp-content/uploads/Malles-Venosta-Referendum.pdf (accessed on 22 May 2022).
- Damalas, C.A.; Koutroubas, S.D. Current status and recent development in biopesticides use. Agriculture 2018, 8, 13.
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sust. Food Syst. 2021, 5, 619058.
- Garrett, K.A. Climate change and plant disease risk. In Global Climate Change and Extreme Weather Events: Understanding the Contributions to Infectious Disease Emergence; Relman, D.A., Hamburg, M.A., Choffnes, E.R., Mack, A., Eds.; National Academies Press: Washington, DC, USA, 2008; pp. 143–155.
- Juroszek, P.; Von Tiedemann, A. Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol. 2011, 60, 100–112.
- Soussana, J.-F.; Graux, A.-I.; Tubiello, F.N. Improving the use of modelling for projections of climate change impacts on crops and pastures. J. Exp. Bot. 2010, 61, 2217–2228.
- Zhan, J.; Thrall, P.H.; Burdon, J.J. Achieving sustainable plant disease management through evolutionary principles. Trends Plant Sci. 2014, 19, 570–575.
- Leung, K.; Ras, E.; Ferguson, K.B.; Ariens, S.; Babendreier, D.; Bijma, P.; Bourtzis, K.; Brodeur, J.; Bruins, M.A.; Centurion, A.; et al. Next-generation biological control: The need for integrating genetics and genomics. Biol. Rev. 2020, 95, 1838–1854.
- Fenu, G.; Malloci, F.M. Forecasting plant and crop disease: An explorative study on current algorithms. Big Data Cogn. Comput. 2021, 5, 2.
- Fontana, D.C.; De Paula, S.; Torres, A.G.; Moura de Souza, V.H.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic fungi: Biological control and induced resistance to phytopathogens and abiotic stresses. Pathogens 2021, 10, 570.
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological control of plant diseases: An evolutionary and eco-economics consideration. Pathogens 2021, 10, 1311.
- Ferrante, P.; Scortichini, M. Frost promotes the pathogenicity of Pseudomonas syringae pv. actinidiae in Actinidia chinensis and A. deliciosa plants. Plant Pathol. 2014, 63, 12–19.
- Nuttall, J.G.; Perry, E.M.; Delahunty, A.J.; O’Leary, G.J.; Barlow, K.M.; Wallace, A.J. Frost response in wheat and early detection using proximal sensors. J. Agron. Crop Sci. 2019, 205, 220–234.
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the impact of heat and frost events on wheat crop production: A review. Field Crop Res. 2015, 171, 109–119.
- Haworth, M.; Marino, G.; Brunetti, C.; Killi, D.; De Carlo, A.; Centritto, M. The impact of the heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europea L.)—A case study of the 2017 heat wave. Plants 2018, 7, 76.
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: An overview. Sci. Food Agric. 2021, 101, 27–43.
- Macholdt, J.; Honermeier, B. Importance of variety choice: Adapting to climate change in organic and conventional farming system in Germany. Outlook Agric. 2017, 46, 178–184.
- Hulme, P.E. Unwelcome exchange: International trade as direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679.
- Garbelotto, M.; Pautasso, M. Impacts of exotic forest pathogens on Mediterranean ecosystems: Four case studies. Eur. J. Plant Pathol. 2012, 133, 101–116.
- European Commission. Proposal for a regulation of the european parliament and of the council establishing rules on support for strategic plans to be drawn up by Member States under the Common agricultural policy (CAP Strategic Plans) and financed by the European Agricultural Guarantee Fund (EAGF) and by the European Agricultural Fund for Rural Development (EAFRD) and repealing Regulation (EU); No 1305/2013 of the European Parliament and of the Council and Regulation (EU) No 1307/2013 of the European Parliament and of the Council; COM/2018/392 final—2018/0216 (COD). 2018.
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20.
- Peeters, A.; Ambhul, E.; Barberi, P.; Migliorini, P.; Ostermann, O.; Goris, M.; Donham, J.; Wezel, A.; Batello, C. Integrating Agroecology into European Agricultural Policies. Position Paper and Recommendations to the European Commission on Eco-Schemes. 2021. Available online: https://www.agroecology-europe.org/wp-content/uploads/2021/07/AEEU_Positionpaper_Ecoschemes_FINAL_english.pdf (accessed on 22 May 2022).
- Larkin, R.P.; Lynch, R.P. Use and effects of different Brassica and other rotation crops on soilborne diseases and yield of potato. Horticulturae 2018, 4, 37.
- Muscolo, A.; Sidari, M.; Nardi, S. Humic substance: Relationships between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 2013, 129, 57–63.
- Rillig, M.C.; Sosa-Hernandez, M.A.; Roy, J.; Aguilar-Trigueros, C.A.; Valyi, K.; Lehmann, A. Towards an integrated mycorrhizal technology: Harnessing mycorrhiza for sustainable intensification in agriculture. Front. Plant Sci. 2016, 7, 1625.
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslikova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanism of action, and roadmap to commercialization of biostimulant for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473.
- Semida, W.M.; Beheiry, H.R.; Sétamou, M.; Simpson, C.R.; Abd El-Mageed, T.A.; Rady, M.M.; Nelson, S.D. Biochar implications for sustainable agriculture and environment: A review. S. Afr. J. Bot. 2019, 127, 333–347.
- Seenivasagan, R.; Babalola, O.O. Utilization of microbial consortia as biofertilizers and biopesticides for the production of feasible agricultural product. Biology 2021, 10, 1111.
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792.
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48.
- Suarez-Estrella, F.; Arcos-Nievas, M.A.; Lopez, M.J.; Vargas-Garcia, M.C.; Moreno, J. Biological control of plant pathogens by microorganisms isolated from agro-industrial composts. Biol. Control 2013, 67, 509–515.
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. Chemosphere 2020, 255, 127006.
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46.
- Michael, C.; Gil, E.; Gallart, M.; Stavrinides, M.C. Influence of spray technology and application rate on leaf deposit and ground losses in mountain viticulture. Agriculture 2020, 10, 615.
- Gamliel, A. Application of soil solarization in the open field. In Soil Solarization THEORY and Practice; Gamliel, A., Katan, J., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2017; pp. 175–180.
- Van Bruggen, A.H.C.; Gamliel, A.; FinckH, M.R. Plant disease management in organic farming systems. Pest Sci. Manag. 2016, 72, 30–44.
- Hansen, Z.R.; Everts, K.L.; Fry, W.E.; Gevens, A.J.; Grunwald, N.J.; Gugino, B.K.; Johnson, D.A.; Johnson, S.B.; Knaus, B.J.; McGrath, M.T.; et al. Genetic variation within clonal lineages of Phytophthora infestans revealed through genotyping-by-sequencing, and implications for late blight epidemiology. PLoS ONE 2016, 11, e065690.
- Cohen, Y.; Ben Naim, Y.; Falach, L.; Rubin, A.E. Epidemiology of basil downy mildew. Phytopathology 2017, 107, 1149–1160.
- Newberry, A.A.; Babu, B.; Roberts, P.D.; Dufault, N.S.; Goss, E.M.; Jones, J.B.; Paret, M.L. Molecular epidemiology of Pseudomonas syringae pv. syringae causing bacterial leaf spot of watermelon and squash in Florida. Plant Dis. 2018, 102, 511–518.
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The epidemiology of Fusarium wilt of banana. Front. Plant Sci. 2019, 10, 1395.
- Pruvost, O.; Boyer, K.; Ravigné, V.; Richard, D.; Vernière, C. Deciphering how plant pathogenic bacteria disperse and meet: Molecular epidemiology of Xanthomonas citri pv. citri at a microgeographic scales in a tropical area of Asiatic citrus canker endemicity. Evol. Appl. 2019, 12, 1523–1538.
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Environ. Microbiol. 2020, 80, 81–102.
- Ostos, E.; Garcia-Lopez, M.T.; Porras, R.; Lopez-Escudero, F.J.; Trapero-Casas, A.; Michaelides, T.J.; Moral, J. Effect of cultivar resistance and soil management on spatial-temporal development of Verticillium wilt of olive: A long-term study. Front. Plant Sci. 2020, 11, 584496.
- Caffi, T.; Rossi, V. Fungicide models are components of multiple modeling approaches for decision-making in crop protection. Phytopathol. Medit. 2018, 57, 153–169.
- Kim, K.-H.; Jung, I. Development of a daily epidemiological model for rice blast tailored for seasonal disease early warning in South Korea. Plant Pathol. J. 2020, 36, 406–417.
- He, X.; Fu, P.; Aker, W.G.; Hwang, H.-M. Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J. Environ. Sci. Health Part C 2018, 36, 21–42.
- Gusberti, M.; Klemm, U.; Meier, M.S.; Maurhofer, M.; Hunger-Glaser, I. Fire blight control: The struggle goes on. A comparison of different fire blight control methods in Switzerland with respect to biosafety, efficacy and durability. Int. J. Environ. Res. Public Health 2015, 12, 11422–11447.
- Moser, R.; Pertot, I.; Elad, Y.; Raffaelli, R. Farmers’ attitudes toward the use of biocontrol agents in IPM strawberry production in three countries. Biol. Control 2008, 47, 125–132.
- Barzmann, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215.
- Jorgensen, L.N.; Hovmoller, M.S.; Hansen, J.G.; Lassen, P.; Clark, B.; Bayles, R.; Rodemann, B.; Flath, K.; Jahn, M.; Goral, T.; et al. IPM strategies and their dilemmas including an introduction to www.eurowheat.org. J. Integr. Agric. 2014, 13, 265–281.
- Berlin, A.; Nordström Kälström, H.; Lindgren, A.; Olson, A. Scientific evidence for sustainable plant disease protection strategies for the main arable crops in Sweden. A systematic map protocol. Environ. Evid. 2018, 7, 31.
- Holb, I.J.; Abpnyi, F.; Bourma, J.; Heijne, B. On-farm and on-station evaluations of three orchard management approaches against apple scab and apple powdery mildew. Crop Prot. 2017, 97, 109–118.
- Shipp, L.; Elliott, D.; Gillespie, D.; Brodeur, J. From chemical to biological control in Canadian greenhouse crops. In Biological Control: A Global Perspective; Vincent, C., Goettel, M.S., Lazarovitis, C., Eds.; CABI: Wallingford, UK, 2007; pp. 118–127.
- Jacobsen, B.J.; Zidack, N.K.; Larson, B.J. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology 2004, 94, 1272–1275.
- Galletti, S.; Paris, R.; Cianchetta, S. Selected isolates of Trichoderma gamsii induce different pathways of systemic resistance in maize upon Fusarium verticillioides challenge. Microbiol. Res. 2020, 233, 126406.
- Muneret, L.; Mitchell, M.; Seufert, V.; Aviron, S.; Djoud, E.A.; Pétillon, J.; Plantegenest, M.; Thiéry, D.; Rusch, A. Evidence that organic farming promotes pest control. Nat. Sustain. 2018, 1, 361–368.
- Baker, B.P.; Green, T.A.; Cooley, D.; Futrell, S.; Garling, L.; Gershuny, G.; Moyer, J.; Rajotte, E.G.; Seaman, A.J.; Young, S.L. Organic Agriculture and Integrated Pest Management: A Synergistic Partnership to Improve Sustainable Agriculture and Food Systems. 2015. Available online: https://organicipmwg.files.wordpress.com/2015/07/white-paper.pdf (accessed on 22 May 2022).
- Rossi, V.; Salinari, F.; Poni, S.; Caffi, T.; Bettati, T. Addressing the implementation problem in decision support systems: The example of vite.net®. Comput. Electron. Agric. 2014, 100, 88–99.
- Minchinton, E.J.; Auer, D.P.F.; Thomson, F.M.; Trapnell, L.; Petkowski, J.E.; Galea, V.J.; Faggian, R.; Kita, N.; Murdoch, C.; Kennedy, R. Evaluation of the efficacy and economics of irrigation management, plant resistance and BrassicaspotTM models for management of white blister on Brassica crops. Austalasian Plant Pathol. 2013, 42, 169–178.
- Pavan, W.; Fraisse, C.W.; Peres, N.A. Development of a web-based disease forecasting system for strawberry. Comput. Electron. Agric. 2011, 75, 169–175.
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845.
- Sund, I.; Eilenberg, J. Why has the authorization of microbial biocontrol agents been slower in the EU than in comparable jurisdictions? Pest Manag. Sci. 2021, 77, 2170–2178.
- Smith, D.; Hinz, H.; Mulema, J.; Weyl, P.; Ryan, M.J. Biological control and the Nagoya Protocol on access and benefit sharing-a case of effective due diligence. Biocontrol Sci. Technol. 2018, 28, 914–926.
- Pliego, C.; Ramos, C.; De Vicente, A.; Cazorla, F.M. Screening for candidate bacterial biocontrol agents against soilborne fungal pathogens. Plant Soil 2011, 340, 505–520.
- Ajouz, S.; Nicot, P.C.; Bardin, M. Adaptation to pyrrolnitrin of Botrytis cinerea and cost of resistance. Plant Pathol. 2010, 59, 556–566.
- Wei, W.; Xu, Y.; Li, S.; Zhu, L.; Song, J. Developing suppressive soil for root disease of soybean with continuous long-term cropping of soybean in black soils of Northeast China. Acta Agric. Scand. B Soil Plant Sci. 2015, 65, 279–285.
- Milgroom, M.G.; Cortesi, P. Biological control of chestnut blight with hypovirulence: A critical analysis. Annu. Rev. Phytopathol. 2004, 42, 311–338.
- Nicot, P.C.; Bardin, M.; Alabouvette, C.; Köhl, J.; Ruocco, M. Potential of biological control based on published research. 1. Protection against plant pathogens of selected crops. In Classical and Augmentative Biological Control against Diseases and Pests: Critical Status Analysis and Review of Factors Influencing Their Success; Nicot, P.C., Ed.; IOBC/WPRS, 2011; pp. 1–11.
- Moore, L.W.; Warren, G. Agrobacterium radiobacter strain K84 and biological control of crown gall. Annu. Rev. Phytopathol. 1979, 17, 163–179.
- Köhl, J.; Scheer, C.; Holb, I.J.; Masny, S.; Molhoek, W.M.L. Toward an integrated use of biological control of Cladosporium cladosporioides H39 in apple scab (Venturia inaequalis) management. Plant Dis. 2015, 99, 535–543.
- Haddoudi, I.; Cabrefiga, J.; Mora, I.; Mhadhbi, H.; Montesinos, E.; Mrabet, M. Biological control of fusarium wilt caused by Fusarium equiseti in Vicia faba with broad spectrum antifungal plant-associated Bacillus spp. Biol. Control 2021, 160, 104671.
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126.
- Sood, M.; Kapoor, D.; Sheteiwy, M.; Ramakhrisnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762.
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25.
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178.
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell Infect. Microbiol. 2020, 10, 604293.
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10.
- Höfte, M. The use of Pseudomonas spp. as bacterial biocontrol agents to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, V., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; p. 74.
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapon for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 3.




