
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fulvia Chiampo | -- | 1971 | 2022-06-26 08:17:19 | | | |
| 2 | Lindsay Dong | Meta information modification | 1971 | 2022-06-27 04:59:34 | | |
Video Upload Options
The tannery industry is characterized by the consumption of a large quantity of water, around 30–40 m3 for processing 1000 kg of hide or skin. This amount becomes wastewater, containing about 300 kg of different chemicals, mainly refractory organic compounds, with high chemical oxygen demand (COD), total dissolved salts (TDS), chromium, and evolution of toxic gases, such as ammonia and sulfides, etc. The remaining tanning chemicals are released as effluent having high resistance against biological degradation, becoming a serious environmental issue. The Zero Liquid Discharge (ZLD) system serves to ensure zero water emission, as well as treatment facilities by recycling, recovery, and reuse of the treated wastewater using advanced cleanup technology. The international scenario shows the implementation of ZLD thanks to pressure from regulatory agencies. The ZLD system consists of a pre-treatment system with conventional physicochemical treatment, tertiary treatment, softening of the treated effluent, reverse osmosis (RO) treatment for desalination, and thermal evaporation of the saline reject from RO to separate the salts. By adopting this system, water consumption is reduced. Moreover, ZLD also becomes effective in disaster mitigation in areas where the tannery industry is a strong economic actor.
1. Introduction
2. Tanning Process and Related Environmental Issues
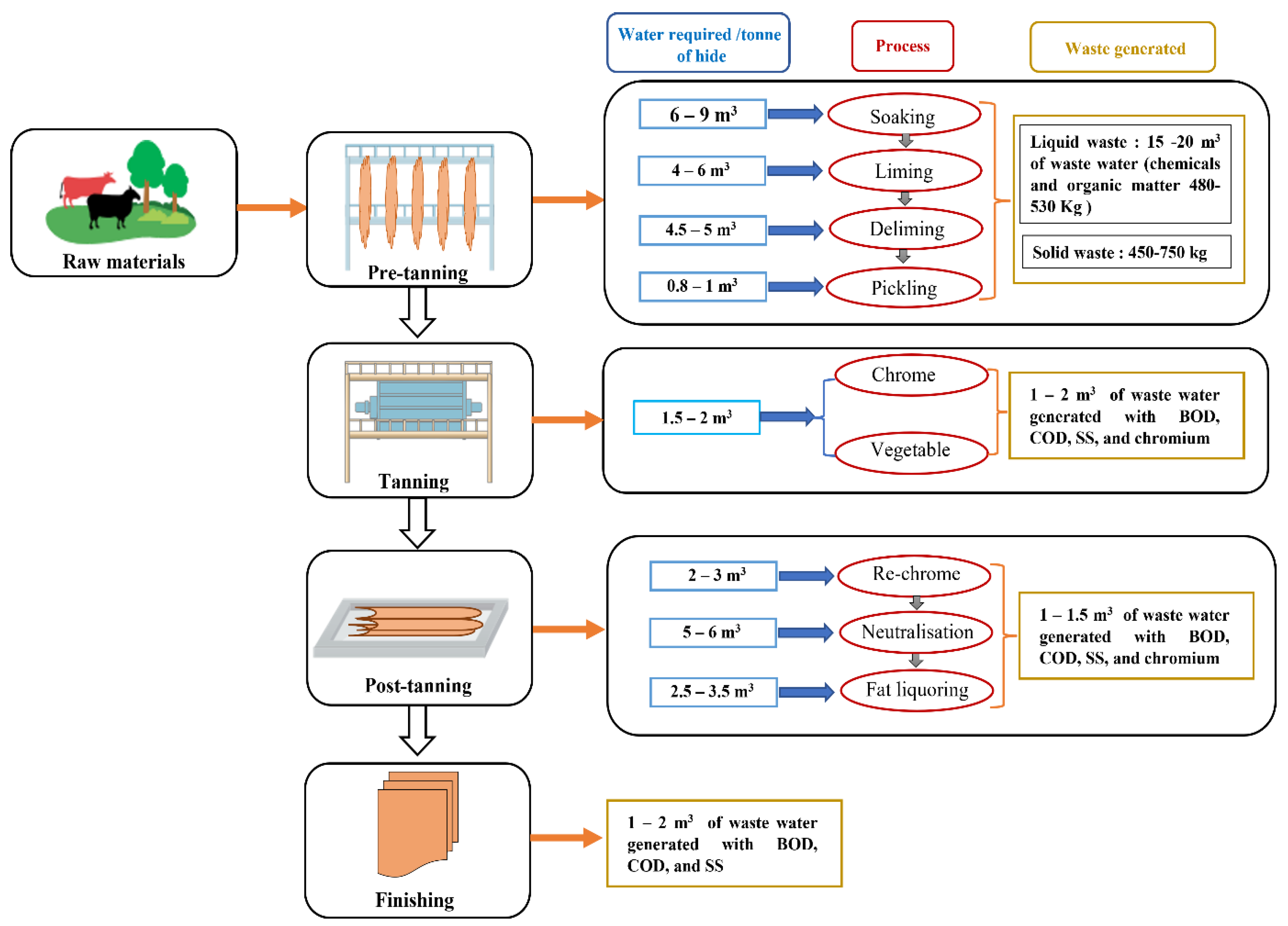
3. Clean Technology Approach in Tanneries
-
Use of enzymes in the dehairing bath: enzymes are added in the soaking phase to improve the water uptake and to degrade the proteins and fats present in the skin [16]. This approach reduces the processing time.
-
Precipitation of chrome: to recover and reuse chrome in spent liquor by raising the pH to minimize the solubility of chromium in the liquor [17].
-
Recycling the dehairing bath: instead of discharging it to the treatment plant after a single use, it can be reused after a simple filtration [18].
-
Recycling the chrome tanning bath (can reduce chrome use by 20%): reusing the contents in the tanning bath after a simple filtration process [19].
4. Need for Water Recycling in Tanneries
5. Zero Liquid Discharge system
5.1. Thermal-Based ZLD Systems
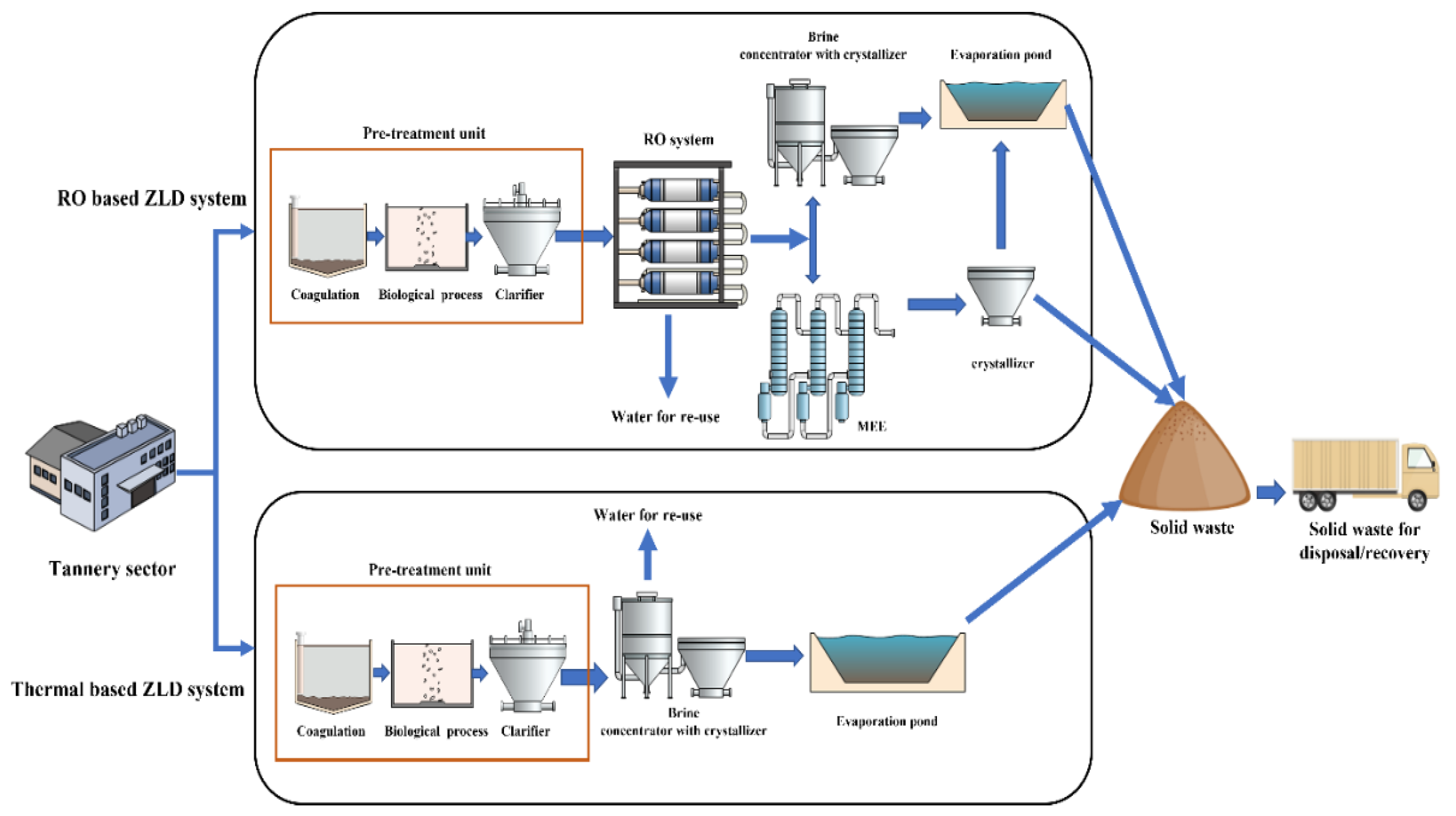
5.2. RO-Based ZLD Systems
6. Critical Assessment of ZLD Economics
References
- Sivaram, N.M.; Barik, D. Toxic waste from leather industries. In Energy from Toxic Organic Waste for Heat and Power Generation; Woodhead Publishing: Cambridge, UK, 2018; pp. 55–67. ISBN 9780081025284.
- Saran, S.; Chib, S.; Saxena, R.K. Biotechnology of leather: An alternative to conventional leather processing. A Handb. High Value Ferment. Prod. Hum. Welf. 2019, 2, 23–47.
- Saranya, D.; Shanthakumar, S. Opportunities for phycoremediation approach in tannery effluent: A treatment perspective. Environ. Prog. Sustain. Energy 2019, 38, e13078.
- Suman, H.; Sangal, V.K.; Vashishtha, M. Treatment of tannery industry effluent by electrochemical methods: A review. Mater. Today Proc. 2021, 47, 1438–1444.
- Rajamani, S. Sustainable Environmental Technologies Including Water Recovery for Reuse from Tannery and Industrial Wastewater–Indian and Asian Scenario. Ann. Univ. Oradea Fascicle Text. Oradea Rom. 2017, 173–179.
- Doble, M.; Kruthiventi, A.K. Industrial Examples. In Green Chemistry and Engineering; Academic Press: Burlington, ON, Canada, 2007; pp. 245–296. ISBN 978-0-12-372532-5.
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281.
- Famielec, S. Chromium concentrate recovery from solid tannery waste in a thermal process. Materials 2020, 13, 1533.
- Sundar, V.J.; Ramesh, R.; Rao, P.S.; Saravanan, P.; Sridharnath, B.; Muralidharan, C. Water management in leather industry. J. Sci. Ind. Res. 2001, 60, 443–450.
- Sivakumar, V.; Swaminathan, G.; Rao, P.G.; Ramasami, T. Influence of ultrasound on diffusion through skin/leather matrix. Chem. Eng. Process. Process Intensif. 2008, 47, 2076–2083.
- Thazeem, B.; Umesh, M.; Mani, V.M.; Beryl, G.P.; Preethi, K. Biotransformation of bovine tannery fleshing into utilizable product with multifunctionalities. Biocatal. Biotransformation 2021, 39, 81–99.
- Saravanabhavan, S.; Thanikaivelan, P.; Rao, J.R.; Nair, B.U.; Ramasami, T. Reversing the conventional leather processing sequence for cleaner leather production. Environ. Sci. Technol. 2006, 40, 1069–1075.
- Karuppiah, K.; Sankaranarayanan, B.; Ali, S.M.; Jabbour, C.J.C.; Bhalaji, R.K.A. Inhibitors to circular economy practices in the leather industry using an integrated approach: Implications for sustainable development goals in emerging economies. Sustain. Prod. Consum. 2021, 27, 1554–1568.
- Morera, J.M.; Bacardit, A.; Ollé, L.; Bartolí, E.; Borràs, M.D. Minimization of the environmental impact of chrome tanning: A new process with high chrome exhaustion. Chemosphere 2007, 69, 1728–1733.
- Bacardit, A.; Morera, J.M.; Ollé, L.; Bartolí, E.; Dolors Borràs, M. High chrome exhaustion in a non-float tanning process using a sulphonic aromatic acid. Chemosphere 2008, 73, 820–824.
- Singhania, R.R.; Patel, A.K.; Thomas, L.; Goswami, M.; Giri, B.S.; Pandey, A. Industrial Enzymes. In Industrial Biorefineries and White Biotechnology; Pandey, A., Höfer, R., Taherzadeh, M., Nampoothiri, K.M., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 473–497. ISBN 9780444634535.
- Kanagaraj, J.; Chandra Babu, N.K.; Mandal, A.B. Recovery and reuse of chromium from chrome tanning waste water aiming towards zero discharge of pollution. J. Clean. Prod. 2008, 16, 1807–1813.
- Collivignarelli, C.; Barducci, G. Waste recovery from the tanning industry. Waste Manag. Res. 1984, 2, 265–278.
- Blackman, A. Adoption of Clean Leather-Tanning Technologies in Mexico. Discussion Papers 10881, Resources for the Future; Washington, DC, USA, 2005; DP 05-38; Available online: https://ageconsearch.umn.edu/record/10881 (accessed on 22 May 2022).
- Molden, D.; Amarasinghe, U.; Hussain, I. Water for Rural Development—Background Paper on Water for Rural Development; International Water Management Institute: Anand, India, 2001; Volume 32, ISBN 9290904593.
- Raghava Rao, J.; Chandrababu, N.K.; Muralidharan, C.; Nair, B.U.; Rao, P.G.; Ramasami, T. Recouping the wastewater: A way forward for cleaner leather processing. J. Clean. Prod. 2003, 11, 591–599.
- Zhao, C.; Chen, W. A review for tannery wastewater treatment: Some thoughts under stricter discharge requirements. Environ. Sci. Pollut. Res. 2019, 26, 26102–26111.
- Ahamed, M.I.N.; Kashif, P.M. Safety Disposal of Tannery Effluent Sludge: Challenges to Researchers- a Review. Int. J. Pharm. Sci. Res. 2014, 5, 733–736.
- Sinha, S.; Singh, S.; Mallick, S. Comparative growth response of two varieties of Vigna radiata L. (var. PDM 54 and var. NM 1) grown on different tannery sludge applications: Efects of treated wastewater and ground water used for irrigation. Environ. Geochem. Health 2008, 30, 407–422.
- Suthanthararajan, R.; Ravindranath, E.; Chitra, K.; Umamaheswari, B.; Ramesh, T.; Rajamani, S. Membrane application for recovery and reuse of water from treated tannery wastewater. Desalination 2004, 164, 151–156.
- Byers, B. Zero discharge: A systematic approach to water reuse. Chem. Eng. 1995, 102, 96.
- Yang, F.; Huang, Z.; Huang, J.; Wu, C.; Zhou, R.; Jin, Y. Tanning wastewater treatment by ultrafiltration: Process efficiency and fouling behavior. Membranes 2021, 11, 461.
- Liang, Y.; Lin, X.; Kong, X.; Duan, Q.; Wang, P.; Mei, X.; Ma, J. Making waves: Zero liquid discharge for sustainable industrial effluent management. Water 2021, 13, 2852.
- Qiblawey, H.M.; Banat, F. Solar thermal desalination technologies. Desalination 2008, 220, 633–644.
- Bostjancic, J.; Ludlum, R. Getting to Zero Discharge: How to Recycle That Last Bit of Really Bad Wastewater A Brief History of Evaporation. In Proceedings of the International Water Conference, Engineers Society of Western Pennsylvania, West Chester, PA, USA, 21–24 April 1996; Volume 57, pp. 290–295.
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563.
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23.
- Boopathy, R.; Karthikeyan, S.; Mandal, A.B.; Sekaran, G. Characterisation and recovery of sodium chloride from salt-laden solid waste generated from leather industry. Clean Technol. Environ. Policy 2013, 15, 117–124.
- Al-Karaghouli, A.; Kazmerski, L.L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356.
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717.
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283.
- Almulla, A.; Eid, M.; Côté, P.; Coburn, J. Developments in high recovery brackish water desalination plants as part of the solution yp water quantity problems. Desalination 2003, 153, 237–243.
- Li, W.; Krantz, W.B.; Cornelissen, E.R.; Post, J.W.; Verliefde, A.R.D.; Tang, C.Y. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Appl. Energy 2013, 104, 592–602.
- Robertson, A.; Nghiem, L.D. Treatment of High TDS Liquid Waste: Is Zero Liquid Discharge Feasible? J. Water Sustain. 2011, 1, 1–11.
- Younos, T. The Economics of Desalination. J. Contemp. Water Res. Educ. 2009, 132, 39–45.
- Mickley, M.C. Membrane Concentrate Disposal: Practices and Regulation; US Department of the Interior, Bureau of Reclamation, Technical Service: Denver, CO, USA, 2006; Volume 123, ISBN 1856173895.
- Loganathan, K.; Chelme-Ayala, P.; Gamal El-Din, M. Pilot-scale study on the treatment of basal aquifer water using ultrafiltration, reverse osmosis and evaporation/crystallization to achieve zero-liquid discharge. J. Environ. Manag. 2016, 165, 213–223.
- Pancharatnam, S. Transient Behavior of a Solar Pond and Prediction of Evaporation Rates. Ind. Eng. Chem. Process Des. Dev. 1972, 11, 287–292.
- Ahmed, M.; Shayya, W.H.; Hoey, D.; Mahendran, A.; Morris, R.; Al-Handaly, J. Use of evaporation ponds for brine disposal in desalination plants. Desalination 2000, 130, 155–168.
- Rajkumar, R.; Sathish, S.; Senthilkumar, P. Studies on enhancing the efficiency of ZLD plant for tannery effluent by implementing Low-Cost ambient air evaporator system. Rasayan J. Chem. 2018, 11, 13–17.
- Martínez, J.; León, E.; Baena-Moreno, F.M.; Rodríguez-Galán, M.; Arroyo-Torralvo, F.; Vilches, L.F. Techno-economic analysis of a membrane-hybrid process as a novel low-energy alternative for zero liquid discharge systems. Energy Convers. Manag. 2020, 211, 112783.
- Buljan, J.; Emmanuel, K.V.; Viswanathan, M.; Bosnić, M.; Král, I. Analysis of flow and energy aspects of Zero Liquid Discharge (ZLD) technology in treatment of tannery effluents in Tamil Nadu, India. In Proceedings of the 34th IULTCS Congress: Science and Technology for Sustainability of Leather, Chennai, India, 6 February 2017; pp. 244–259.




