You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kafayat Aderonke Yusuf | -- | 2006 | 2022-06-25 07:01:24 | | | |
| 2 | Jessie Wu | -31 word(s) | 1975 | 2022-06-27 04:58:03 | | | | |
| 3 | Jessie Wu | Meta information modification | 1975 | 2022-06-27 05:00:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yusuf, K.; Saha, S.; Umar, S. Health Benefits of Dietary Fiber. Encyclopedia. Available online: https://encyclopedia.pub/entry/24472 (accessed on 23 December 2025).
Yusuf K, Saha S, Umar S. Health Benefits of Dietary Fiber. Encyclopedia. Available at: https://encyclopedia.pub/entry/24472. Accessed December 23, 2025.
Yusuf, Kafayat, Subhrajit Saha, Shahid Umar. "Health Benefits of Dietary Fiber" Encyclopedia, https://encyclopedia.pub/entry/24472 (accessed December 23, 2025).
Yusuf, K., Saha, S., & Umar, S. (2022, June 25). Health Benefits of Dietary Fiber. In Encyclopedia. https://encyclopedia.pub/entry/24472
Yusuf, Kafayat, et al. "Health Benefits of Dietary Fiber." Encyclopedia. Web. 25 June, 2022.
Copy Citation
Inflammatory bowel disease (IBD) is a severe and painful gastrointestinal tract disease that affects children and adults. IBD is a complex disease that has a detrimental effect on people’s quality of life. Due to the severe inflammation associated with IBD, the symptoms and associated morbidity are always prevalent. Despite substantial research, there is no permanent cure for IBD, and patients face an increased risk of colon cancer. Dietary fiber’s health advantages have been thoroughly investigated, and it is recommended for its enormous health benefits.
inflammatory bowel disease
high-fiber diet
gut
gut microbiome
colitis
crohn's disease
IBD
dietary fiber
1. A High-Fiber Diet Can Help Minimize Inflammation
One hallmark of IBD is the persistence of chronic inflammation in the gut of affected patients. Moreover, resolution of gut inflammation in IBD and a complete mucosal healing despite being the ultimate goal remains a therapeutic challenge. Swann and colleagues recently reported that a high-fiber diet might help reduce inflammation by altering the pH and permeability of the gut [1]. Clinical trials on the anti-inflammatory effect of a high fiber diet have also shown promising results in patients with IBD.
In 2011, Benjamin and associates found that patients with active Crohn’s disease who received 15 g of fructo-oligosaccharides (FOS) daily had lower proportions of interleukin (IL-6)-positive lamina propria dendritic cells (DC), higher levels of IL-10 staining in the DC, and no change in IL-12p40 production [2]. Despite affecting DC function, the group concluded that no therapeutic benefit was achieved in patients with active Crohn’s disease; one reason for this could be the amount of dietary fiber, the short trial duration, which lasted only four weeks, and the use of FOS as the only treatment.
Another pilot study evaluated the effect of germinated barley foodstuff (GBF) on serum TNF-α, IL-6, and IL-8 levels in UC patients who were in remission. During the two-month study, patients in the treatment group received 30 g of GBF daily by oral administration and standard drug therapy. TNF-α, IL-6, and IL-8 levels all decreased in the GBF group compared to controls throughout the study [3]. In 2014, the same researchers conducted a follow-up trial to see how (GBF) supplementation affected serum c-reactive protein (CRP) levels and clinical symptoms in patients with UC. According to the findings, serum CRP in the GBF group reduced considerably, and combining GBF with existing medications may effectively reduce inflammation and clinical symptoms in UC patients [4].
A group of researchers also explored a semi-vegetarian diet (SVD), specifically a lacto-ovo-vegetarian diet, for patients with inflammatory bowel disease. The SVD contained 32.4 g of dietary fiber. The study discovered that for newly diagnosed CD patients, the remission rate with combined medication (infliximab) and SVD was 100 percent. At two years, 92% of patients maintained remission on SVD without scheduled maintenance therapy with biological drugs. These excellent outcomes were attributed in part to SVD, which prompted the researchers to recommend a high fiber intake for the treatment of CD [5].
A more recent crossover study of 17 individuals with UC who were either in remission or had minimal disease revealed similar findings. Patients were assigned to a low-fat, high-fiber diet or an improved standard American diet. The results indicated that the low-fat, high-fiber diet decreased indicators of inflammation and dysbiosis [6].
The microbial metabolism of dietary fibers can also lead to the release of ferulic acid (FA) by commensal bacteria [7]. FA exhibits antioxidant and anti-inflammatory characteristics in the digestive tract and could be evaluated as a potential therapeutic treatment for a variety of chronic diseases [7]. FA was found to exert protective anti-inflammatory effects in a trinitrobenzene sulfonic acid (TNBS) induced colitis model. The researchers suggested that FA’s mechanism of action is by regulating oxido-nitrosative stress, apoptosis, pro-inflammatory cytokine release, and COX-2 generation [8].
Additionally, anti-inflammatory properties of high fiber supplementation have been demonstrated extensively using preclinical animal models mimicking human inflammatory bowel disease. These studies discovered that fiber supplements reduced pro-inflammatory markers and repaired the epithelial barrier alongside preventing mucosal damage [9][10][11], (Figure 1).
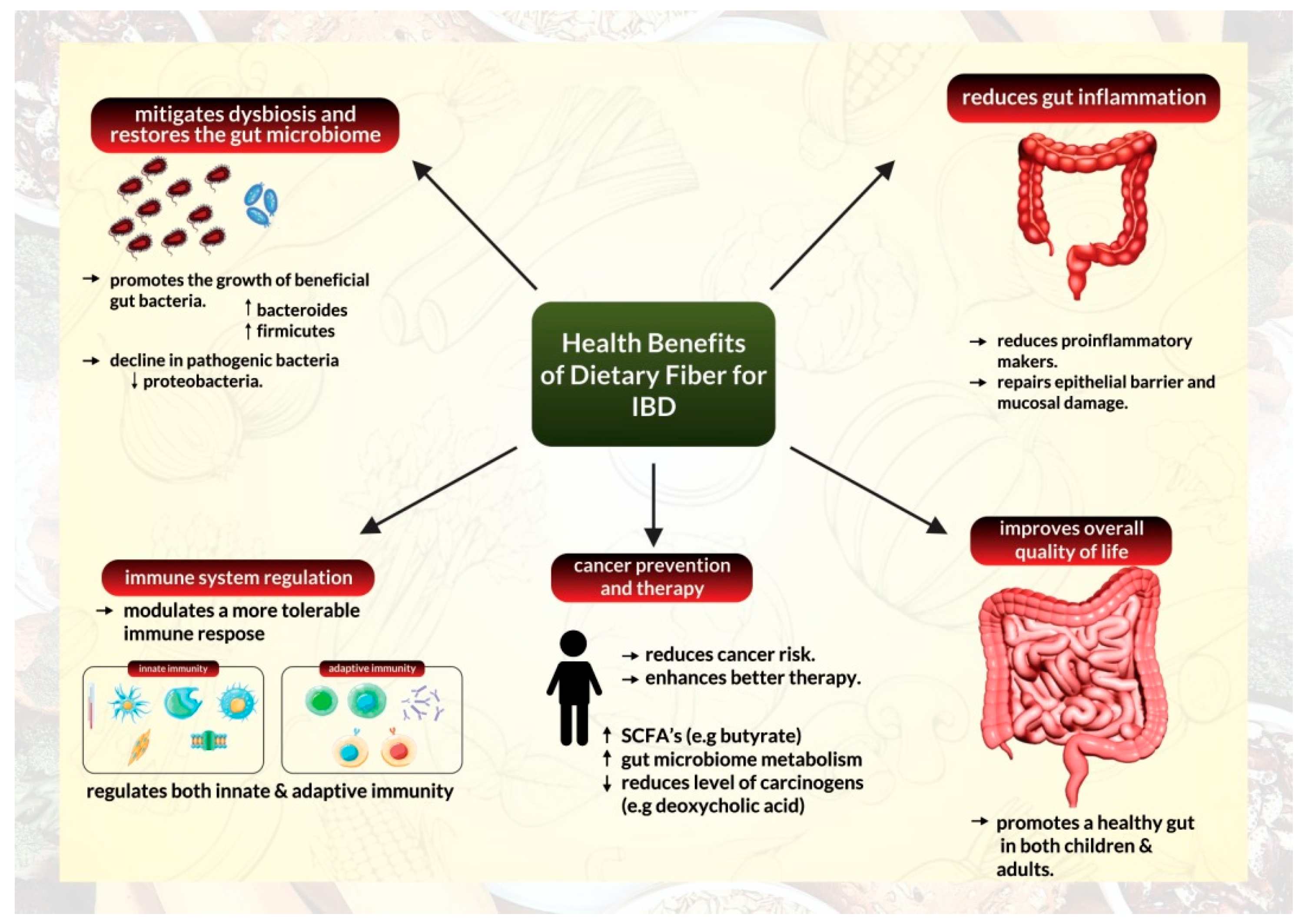
Figure 1. Health benefits of a high-fiber diet for management of inflammatory bowel disease.
2. A High-Fiber Diet Can Help Mitigate Dysbiosis by Restoring the Gut Microbiome
The relationship between the gut microbiome and gut health has been a topic of extensive study in recent times [12][13][14][15]. The human gut microbiota is a distinct habitat that differs significantly from person to person. It is made up of trillions of non-pathogenic microorganisms, most of which are bacterial in origin [12]. The microbiota collaborates with the host’s immune system to prevent disease colonization, gut infiltration from pathogens, and intestinal epithelial damage [16]. Digestion, metabolite synthesis, and immune system conditioning are all aspects of the microbiome that help modify inflammatory processes [17]. The variety and quantity of bacterial species can vary dramatically depending on various circumstances, including health state, with a microbiome with a higher diversity of microorganisms associated with better health [17].
Gut dysbiosis occurs when the composition and function of the gut microbiota are disturbed, resulting in the loss of intestinal homeostasis and inappropriate immune activation [12][18]. Gut dysbiosis has been recognized as a hallmark of IBD, characterized by a loss of microbial diversity, a decline in helpful anaerobic bacteria populations, and an increase in unfavorable adherent and invasive pathogenic bacteria [18]. Patients with inflammatory bowel disease have a decreased diversity of anaerobic bacteria such as Firmicutes but an increased population of Proteobacteria [12][13]. Gut dysbiosis may also damage the intestinal epithelial barrier, promoting heightened immunological responses and persistent inflammation [12]. The ability of commensal gut microbes to degrade fiber into short-chain fatty acids makes a high-fiber diet beneficial for gut microbiome regulation [19][20][21]. SCFAs can include acetate, propionate, and butyrate. They serve as energy sources for colonic epithelial cells and help maintain intestinal homeostasis [12].
Reports have illustrated that a high-fiber diet can transform the microbiome composition of patients with IBD (Figure 1). According to data from a crossover research of UC remission patients who followed a low-fat, high-fiber diet, the relative abundance of Actinobacteria in feces declined following the diet, but the relative abundance of Bacteroidetes increased. After four weeks on the diet, the relative abundance of Faecalibacterium prausnitzii was similarly increased. According to the researchers, patients on the diet also had higher fecal levels of SCFA acetate [6]. Another study on CD patients indicated that the fecal bacterial count of F. prausnitzii in patients with CD was lower than those of healthy controls [5]. Similarly, Bolte and colleagues discovered that human food patterns could influence the general ability to develop protective gut bacteria or foster an abundance of pathogenic and harmful bacteria [22].
Similar findings have been described in experimental mouse models of colitis. Tian and colleagues demonstrated that insoluble dietary fiber from barley leaves alleviated Dextran sulfate sodium (DSS) induced colitis in mice by modulating the gut microbiota. The study found that supplementing with dietary fiber significantly decreased the abundance of Akkermansia and increased the number of Parasutterella, Erysipelatoclostridium, and Alistipes. These effects were eliminated when antibiotics were used to reduce the gut flora [23]. Another DSS-induced colitis model used fermented barley and soybean as a dietary fiber substitute. The study demonstrated that the barley soybean combination reduced epithelial barrier failure, increased tight junction protein levels in colon tissues, and inhibited FITC–dextran permeability. Additionally, the diet boosted Lactobacilli and Bacteroides levels [24]. Furthermore, by rebuilding the microbiome, pectin and tributyrin diets were able to alleviate colitis in mice [25]. Mice fed with pectin showed a decrease in the abundance of Proteobacteria and an increase in the population of Bacteroidetes and Firmicutes [25]. Another study revealed that a lack of dietary fiber alters the gut microbiota composition, promotes neutrophil recruitment, and aggravates colitis in mice [26].
Extensive research into the benefits of healthy gut microbiota has led to the utilization of fecal microbiota transplantations from healthy donors as a therapeutic avenue for patients with IBD [27][28].
3. Mechanism of Dietary Fiber Action
Extensive studies have been conducted on dietary fiber interventions’ impact on modifying the gastrointestinal tract [29][30]. To fully comprehend the overall health advantages of dietary fiber, it is necessary to understand the mechanism by which different forms of dietary fiber work on different individuals and how the makeup of individuals influences the effect they receive from fiber consumption.
3.1. Physicochemical Properties of Dietary Fiber
Diverse types of fiber have different impacts on the body, and from a functional standpoint, not all fibers have the same benefits; as this relies on the quantity and fiber types [31].
Soluble fibers are highly fermentable, allowing beneficial bacteria to flourish in the colon. Fiber fermentation enables the formation of SCFAs, amines, ammonium, gases, and phenols, which affects the gut microbiota diversity and composition [20][31]. The immune barrier of the GI tract is also preserved by the bloom of beneficial microbiota during fiber degradation in the colon. Fermentation products, primarily SCFAs, interact with the immune cells of the small intestine before being degraded by microbial enzymes, resulting in these positive effects [32].
Due to their inability to retain water, insoluble dietary fibers, like lignin and cellulose, are less fermentable by the gut microbiome [31]. Insoluble dietary fibers have a fiber matrix that holds water, increases stool mass, alleviates constipation, and improves bowel regularity by providing a bulking effect. Additionally, insoluble fibers have been linked to a reduction in intestinal transit time, which can help avoid and alleviate constipation [31]
Psyllium and other gel-forming fibers thicken the contents of the intestinal lumen and impede the migration of nutrients to the intestinal walls. As a result, they can lower cholesterol, sugar, and other nutrient absorption. Fiber can also help with bile salt reabsorption from the small intestine, which is another component that contributes to lower cholesterol levels [20].
3.2. Protection of the Host’s GI Tract
The mucus layer in the GI tract covers and protects the gut epithelium, keeping pathogens away from the mucosa [7]. One of the host’s defenses against microbial invasions and infection is maintaining a well-structured and undamaged mucus layer. The gut microbiota and diet have been found to be two essential contributors to maintaining normal intestinal mucus structure and production [7]. Dietary fibers can promote mucus secretion by mechanically stimulating the intestinal epithelium [33]. Dietary fibers and SCFAs (such as acetate and butyrate) also boosts mucus synthesis and secretion by increasing goblet cell differentiation and mucin gene expression [7].
On the other hand, a low-fiber diet alters the gut microbiota. It promotes severe mucus layer degeneration, increasing susceptibility to infections and the development of chronic inflammatory conditions [34][35]. Low dietary fiber intake reduces microbial diversity and SCFA generation. It changes gut microbial metabolism toward less favorable substrates, such as dietary and endogenously provided proteins and host mucins, which is eventually harmful to the host [34][35]. Reduced fiber fermentation or increased protein fermentation reduces total SCFAs and butyrate production and increases the production of potentially harmful metabolites derived from amino acid fermentation [36]. These compounds’ cytotoxic and pro-inflammatory properties lead to the development of chronic illnesses, including colorectal cancer [37]. The switch in metabolism explains many diseases associated with a low-fiber diet. It also explains why microbial diversity is reduced in both humans and mice on a low-fiber diet [7].
3.3. Individuals May React Differently to the Same Fiber Intake
Despite multiple discoveries regarding the significance of fiber in inflammatory bowel disease, investigations have also revealed that the response to dietary fiber in IBD is variable [38]. Some comprehensive studies have also found no association between fiber consumption and IBD [39][40]. A possible explanation for this variation is the prevalence of inter-individual heterogeneity in the gut microbiota [41]. This heterogeneity has been causally related to varying effects of dietary fiber on host metabolic phenotypes, suggesting that a one-size-fits-all fiber supplementation strategy to improve health is unlikely to generate consistent outcomes across individuals [41].
Lancaster and colleagues found a similar result in a small crossover experiment with 18 participants using different fiber compositions. The researchers discovered that responses to different fiber types were not consistent across the participants, inferring that each person’s microbiome may influence their responses [42].
These findings suggest a complex link that exists between the effects originating from fiber, host gut microbiome, and host metabolism.
References
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411.
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929.
- Faghfoori, Z.; Navai, L.; Shakerhosseini, R.; Somi, M.H.; Nikniaz, Z.; Norouzi, M.F. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and -8 in patients with ulcerative colitis. Ann. Clin. Biochem. 2011, 48, 233–237.
- Faghfoori, Z.; Shakerhosseini, R.; Navai, L.; Somi, M.H.; Nikniaz, Z.; Abadi, A. Effects of an Oral Supplementation of Germinated Barley Foodstuff on Serum CRP Level and Clinical Signs in Patients with Ulcerative Colitis. Health Promot. Perspect. 2014, 4, 116–121.
- Chiba, M.; Tsuji, T.; Nakane, K.; Komatsu, M. High Amount of Dietary Fiber Not Harmful But Favorable for Crohn Disease. Perm. J. 2015, 19, 58–61.
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1189–1199.e30.
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715.
- Sadar, S.S.; Vyawahare, N.S.; Bodhankar, S.L. Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, iNOs, COX-2, and apoptosis in laboratory rats. EXCLI J. 2016, 15, 482–499.
- Araki, Y.; Kanauchi, O.; Sugihara, H.; Fujiyama, Y.; Hattori, T. Germinated barley foodstuff suppresses dextran sulfate experimental colitis in rats: The role of mast cells. Int. J. Mol. Med. 2007, 19, 257–262.
- Panasevich, M.R.; Allen, J.M.; Wallig, M.A.; Woods, J.A.; Dilger, R.N. Moderately Fermentable Potato Fiber Attenuates Signs and Inflammation Associated with Experimental Colitis in Mice. J. Nutr. 2015, 145, 2781–2788.
- Rodríguez-Cabezas, M.E.; Camuesco, D.; Arribas, B.; Garrido-Mesa, N.; Comalada, M.; Bailón, E.; Cueto-Sola, M.; Utrilla, P.; Guerra-Hernández, E.; Pérez-Roca, C.; et al. The combination of fructooligosaccharides and resistant starch shows prebiotic additive effects in rats. Clin. Nutr. 2010, 29, 832–839.
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537.
- Ceballos, D.; Hernández-Camba, A.; Ramos, L. Diet and microbiome in the beginning of the sequence of gut inflammation. World J. Clin. Cases 2021, 9, 11122–11147.
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665.
- Amamou, A.; O’Mahony, C.; Leboutte, M.; Savoye, G.; Ghosh, S.; Marion-Letellier, R. Gut Microbiota, Macrophages and Diet: An Intriguing New Triangle in Intestinal Fibrosis. Microorganisms 2022, 10, 490.
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191.
- Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients 2021, 13, 3208.
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2020, 13, 135.
- Akbar, A.; Shreenath, A. High Fiber Diet. In StatPearls ; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Dai, F.J.; Chau, C.F. Classification and regulatory perspectives of dietary fiber. J. Food Drug Anal. 2017, 25, 37–42.
- Cui, S.W.; Nie, S.; Roberts, K.T. Functional Properties of Dietary Fiber. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 517–525.
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298.
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley Leaf Insoluble Dietary Fiber Alleviated Dextran Sulfate Sodium-Induced Mice Colitis by Modulating Gut Microbiota. Nutrients 2021, 13, 846.
- Woo, J.K.; Choi, S.; Kang, J.H.; Kim, D.E.; Hurh, B.S.; Jeon, J.E.; Kim, S.Y.; Oh, S.H. Fermented barley and soybean (BS) mixture enhances intestinal barrier function in dextran sulfate sodium (DSS)-induced colitis mouse model. BMC Complement. Altern. Med. 2016, 16, 498.
- Ahmed, I.; Yusuf, K.; Roy, B.C.; Stubbs, J.; Anant, S.; Attard, T.M.; Sampath, V.; Umar, S. Dietary Interventions Ameliorate Infectious Colitis by Restoring the Microbiome and Promoting Stem Cell Proliferation in Mice. Int. J. Mol. Sci. 2021, 23, 339.
- Shen, S.; Prame Kumar, K.; Wen, S.W.; Shim, R.; Wanrooy, B.J.; Stanley, D.; Moore, R.J.; Van, T.T.H.; Robert, R.; Hickey, M.J.; et al. Deficiency of Dietary Fiber Modulates Gut Microbiota Composition, Neutrophil Recruitment and Worsens Experimental Colitis. Front. Immunol. 2021, 12, 619366.
- Tan, P.; Li, X.; Shen, J.; Feng, Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 2020, 11, 574533.
- Lopez, J.; Grinspan, A. Fecal Microbiota Transplantation for Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 374–379.
- Wong, C.; Harris, P.J.; Ferguson, L.R. Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2016, 17, 919.
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116.
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354.
- Breton, J.; Plé, C.; Guerin-Deremaux, L.; Pot, B.; Lefranc-Millot, C.; Wils, D.; Foligné, B. Intrinsic immunomodulatory effects of low-digestible carbohydrates selectively extend their anti-inflammatory prebiotic potentials. BioMed Res. Int. 2015, 2015, 162398.
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264.
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21.
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e4.
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078.
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196.
- Armstrong, H.; Mander, I.; Zhang, Z.; Armstrong, D.; Wine, E. Not All Fibers Are Born Equal; Variable Response to Dietary Fiber Subtypes in IBD. Front. Pediatr. 2020, 8, 620189.
- Wedlake, L.; Slack, N.; Andreyev, H.J.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel. Dis. 2014, 20, 576–586.
- Andersen, V.; Chan, S.; Luben, R.; Khaw, K.T.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Grip, O.; Bergmann, M.M.; Boeing, H.; et al. Fibre intake and the development of inflammatory bowel disease: A European prospective multi-centre cohort study (EPIC-IBD). J. Crohns Colitis 2018, 12, 129–136.
- Murga-Garrido, S.M.; Hong, Q.; Cross, T.L.; Hutchison, E.R.; Han, J.; Thomas, S.P.; Vivas, E.I.; Denu, J.; Ceschin, D.G.; Tang, Z.Z.; et al. Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome 2021, 9, 117.
- Lancaster, S.M.; Lee-McMullen, B.; Abbott, C.W.; Quijada, J.V.; Hornburg, D.; Park, H.; Perelman, D.; Peterson, D.J.; Tang, M.; Robinson, A.; et al. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 2022.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
27 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




