Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samuel Álvarez-Almazán | -- | 1838 | 2022-06-25 05:04:01 | | | |
| 2 | Amina Yu | + 3 word(s) | 1841 | 2022-06-27 04:28:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Filisola-Villaseñor, J.G.; Aranda-Barradas, M.E.; Miranda-Castro, S.P.; Mendieta-Wejebe, J.E.; Guerrero, A.S.V.; Castro, S.A.G.; Castillo, M.M.; Tamay-Cach, F.; Álvarez-Almazán, S. Symmetrical Compounds in Insulin-Sensitizing Treatments for Type 2 Diabetes. Encyclopedia. Available online: https://encyclopedia.pub/entry/24470 (accessed on 04 March 2026).
Filisola-Villaseñor JG, Aranda-Barradas ME, Miranda-Castro SP, Mendieta-Wejebe JE, Guerrero ASV, Castro SAG, et al. Symmetrical Compounds in Insulin-Sensitizing Treatments for Type 2 Diabetes. Encyclopedia. Available at: https://encyclopedia.pub/entry/24470. Accessed March 04, 2026.
Filisola-Villaseñor, Jessica Georgina, María E. Aranda-Barradas, Susana Patricia Miranda-Castro, Jessica Elena Mendieta-Wejebe, Amaranta Sarai Valdez Guerrero, Selene Amasis Guillen Castro, Macario Martínez Castillo, Feliciano Tamay-Cach, Samuel Álvarez-Almazán. "Symmetrical Compounds in Insulin-Sensitizing Treatments for Type 2 Diabetes" Encyclopedia, https://encyclopedia.pub/entry/24470 (accessed March 04, 2026).
Filisola-Villaseñor, J.G., Aranda-Barradas, M.E., Miranda-Castro, S.P., Mendieta-Wejebe, J.E., Guerrero, A.S.V., Castro, S.A.G., Castillo, M.M., Tamay-Cach, F., & Álvarez-Almazán, S. (2022, June 25). Symmetrical Compounds in Insulin-Sensitizing Treatments for Type 2 Diabetes. In Encyclopedia. https://encyclopedia.pub/entry/24470
Filisola-Villaseñor, Jessica Georgina, et al. "Symmetrical Compounds in Insulin-Sensitizing Treatments for Type 2 Diabetes." Encyclopedia. Web. 25 June, 2022.
Copy Citation
Type 2 diabetes is a complex disease that involves damage to multiple signaling pathways. Through various pathways, chronic high blood glucose generates or aggravates insulin resistance. The drugs most widely used to decrease insulin resistance without producing hypoglycemia are biguanides (e.g., metformin) and thiazolidinediones (TZDs, such as pioglitazone). Symmetrical molecules have been a focus of research due to their unique structural characteristics, including stability and internal balance. Some advantages and disadvantages of utilizing symmetrical and asymmetrical thiazolidinediones as insulin sensitizers (or even antioxidant molecules) are mentioned.
symmetrical molecule
type 2 diabetes
insulin resistance
PPAR
1. Advantages of Molecular Symmetry
The harmonious structure of symmetrical molecules, which is identical on both sides of its axis (Figure 1), furnishes them with internal balance and stability [1]. Given these qualities, it is important to examine their conformation, since it defines their biochemical and biophysical characteristics. Hence, the interaction of symmetrical molecules with receptors is herein analyzed with docking studies in order to compare the different responses of new molecular designs with respect to the desired biological activity [2]. An appropriate chemical structure depends on the particular physicochemical processes of the organism being targeted. A characteristic that is advantageous for a compound in one treatment could be a disadvantage in another.
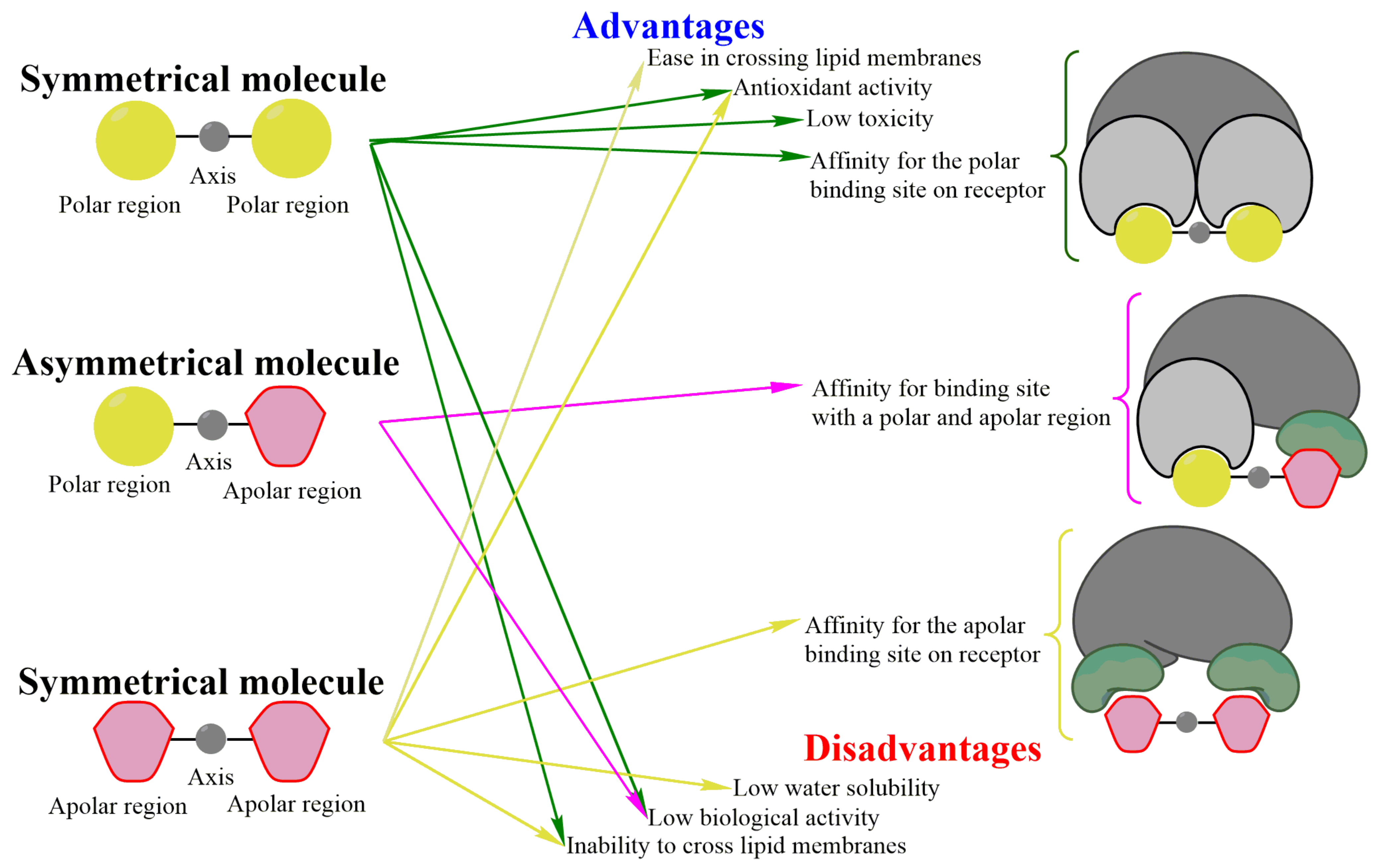
Figure 1. General scheme of the advantages and disadvantages of molecules with or without symmetry. Symmetrical molecules have two equal regions joined by an axis of symmetry (left). Their advantages include low toxicity, antioxidant capacity, and the possibility (for non-polar symmetrical molecules) of crossing lipid membranes. Polar symmetrical molecules bind with high affinity (right) at the polar site of their receptors, whereas nonpolar symmetrical compounds show high affinity for the apolar region. If a receptor contains a polar and a non-polar region in the binding site, greater affinity will exist for asymmetrical molecules with a polar and a non-polar region compared to symmetrical polar and non-polar molecules. Among the disadvantages of symmetrical molecules are low biological activity, the inability to cross cell membranes, and low water solubility (the latter applies to nonpolar molecules).
1.1. Low Toxicity and Good Antioxidant Activity
Some symmetrical molecules can serve as antioxidants because they have oxidation-reduction properties and are safe and non-cytotoxic [3][4][5][6]. Intrinsic antioxidant capacity has been related to a chemical structure with conjugated double bonds, amino groups, and/or hydrogen atoms available for donation [5][7]. For example, compound 1G [(5Z,5′Z)-5,5′-((oxybis(4,1-phenylene))bis-(methanylylidene))bis(thiazolidine-2,4-dione)] has a series of conjugated double bonds that run throughout the molecule, which may give it greater antioxidant capacity than symmetrical tetradentate Schiff base complexes of oxovanadium(IV) [4] or bisferrocenyl bisthiourea analogs [5]. For the last two types of compounds, conjugated double bonds do not extend throughout the molecule.
Among molecules with similar structures capable of inhibiting oxidation, those with symmetry (versus asymmetry) will have a greater antioxidant capacity [5], since both regions contribute to the same property. On the other hand, this could be a disadvantage because the high oxidative stress in diabetes [8] might limit recognition of these compounds by the corresponding receptor, and thus, decrease the intended pharmacological effect. Additionally, symmetrical molecules possibly trigger signaling pathways for the activation of endogenous antioxidant molecules. It has been hypothesized that the binding of 1G to its receptor induces the expression of glutathione peroxidase (GSHpx), superoxide dismutase (SOD), and catalase (CAT) [3], but further research is required to confirm this notion.
1.2. Affinity of Ligands for Their Receptors
The affinity of a ligand depends largely on its polarity and the nature of the target receptor. Whereas polar molecules are more suitable for kidney therapy, nonpolar compounds tend to be able to cross the blood-brain barrier (BBB) and reach the brain [9][10].
Adequately designed symmetrical molecules can bind to a given receptor and cause the desired response. If the binding site of a receptor has enough space and the proper electrostatic characteristics, it will accept a symmetrical molecule with either ionizable groups or hydrophobic ligands. In contrast, asymmetrical molecules require a relatively large binding site or one with a combination of polar and nonpolar characteristics [11]. Polar symmetrical molecules interact with polar binding sites [3] but not nonpolar ones (or only with low affinity). Likewise, nonpolar symmetrical molecules interact with nonpolar sites [12]. In all cases, a molecule with adequate affinity interacts with amino acids crucial for receptor activation [4][5][8][13]. The peroxisome proliferator-activated receptor gamma (PPARγ) has a wide ligand binding pocket (LBP) with polar and non-polar properties. Consequently, it can interact with symmetrical polar molecules, such as 1G, and asymmetrical ones, such as pioglitazone (PIO), rosiglitazone (ROSI), and other recently designed ligands, including lobeglitazone [13].
The reason why a particular compound has multiple types of activity (e.g., anticancer, antibiotic, antifungal, antidiabetic and so on) may be due to its ability to bind to multiple receptors [12]. Whether a ligand is polar or nonpolar and symmetrical or asymmetrical might determine the nature of its activity as an inhibitor, partial agonist, or total agonist. Selectivity is stablished by the receptor, with PPARs being highly non-selective, interacting with a variety of fatty acids and exogenous ligands [4][5][8][11][13]. In contrast, enzymes are much more specific and therefore bind to a specific substrate, or at least, a very limited number of them [14].
Insulin-sensitizing agents have additional applications based on their affinity for other receptors. One example of the multiple-receptor hypothesis is the symmetrical compound presently denominated 1G. The structure of this compound, with an acidic head and tail region, perhaps decreases its affinity for PPARγ but favors binding to other proteins, which would account for its antioxidant, lipid-lowering, and anti-inflammatory effects. In contrast, multiple activity has not been found for PIO [3]. PIO and ROSI may have other activities dependent on their affinity for distinct proteins. In a docking and a half-maximal inhibitory concentration (IC50) study, ROSI displayed a higher affinity for the nutrient-deprivation autophagy factor-1 (NAF-1) protein. NAF-1 has been shown to participate in aging, cell proliferation, deafness, blindness, and diabetes [15], which confirms that the binding site of each protein has specific characteristics (size, polarity and so on) capable of determining its affinity for a certain ligand.
2. Disadvantages of Molecular Symmetry
Symmetrical molecules have certain disadvantages that must be considered when designing and synthesizing new compounds. For instance, molecules bearing ionizable groups have low lipophilicity and consequently cannot cross biological membranes, which lends itself to adverse effects or even tissue damage [1][5][8]. However, low lipophilicity could be considered suitable for avoiding the accumulation of these compounds in adipose tissue or passage through the BBB [9][10]. Asymmetrical molecules with a polar and nonpolar region (e.g., metformin) do not have the ability to cross the BBB, but perhaps their mimetic amino acid structure favors transport across this barrier, which could explain their association with neuroprotective effects [16].
Elaborating facile synthetic strategies for symmetrical molecules is a challenge [1]. In some cases, they turn out to be very toxic, causing mutagenic, teratogenic, or irritant effects [14]. Regarding the relationship of the antioxidant capacity of symmetrical molecules with low toxicity, it is necessary to carry out further studies to achieve greater congruency in the findings. Moreover, the response produced by symmetrical molecules is not always the desired one [4][6], possibly due to the formation of homo-oligomers [2]. This might explain the low solubility of symmetrical molecules, a subject in need of further research.
3. Symmetry: An Advantage or Disadvantage for Ligand-Receptor Interactions?
Symmetrical molecules may have the advantage of high affinity for receptors containing an LBP with similar polarity [3] or the disadvantage of low affinity for an LBP with distinct polarity. In the case of PPARγ, the LBP is Y-shaped or T-shaped and relatively large in size [11][13]. The main region (branch I) of LBP is polar, another region (branch II) is nonpolar, and the last one (branch III) is polar and nonpolar [17], which allows a symmetrical molecule like 1G to bind to PPARγ between branches I and III and act as a partial agonist. This could be advantageous given that partial agonists tend to have fewer side effects [13].
The affinity for a receptor is determined by the polarity, length, and number of rotatable bonds of the ligand. Compound 1G has four rotatable bonds and a binding affinity of −12.19 kcal/mol. PIO and ROSI have seven rotatable bonds and lower binding affinities (−10.164 kcal/mol and −10.106 kcal/mol, respectively). All three compounds can form five H-bonds [3]. The clear differences are the polarity and symmetry of 1G and the smaller number of rotatable bonds due to the double bond at carbon 5 (Figure 2). The probable greater stability of 1G favors its interaction with the receptor. Hence, 1G would be expected to have greater pharmacological activity than PIO. However, both treatments were found to have similar effects in a rat model of diabetes, evidencing partial agonist activity [3].
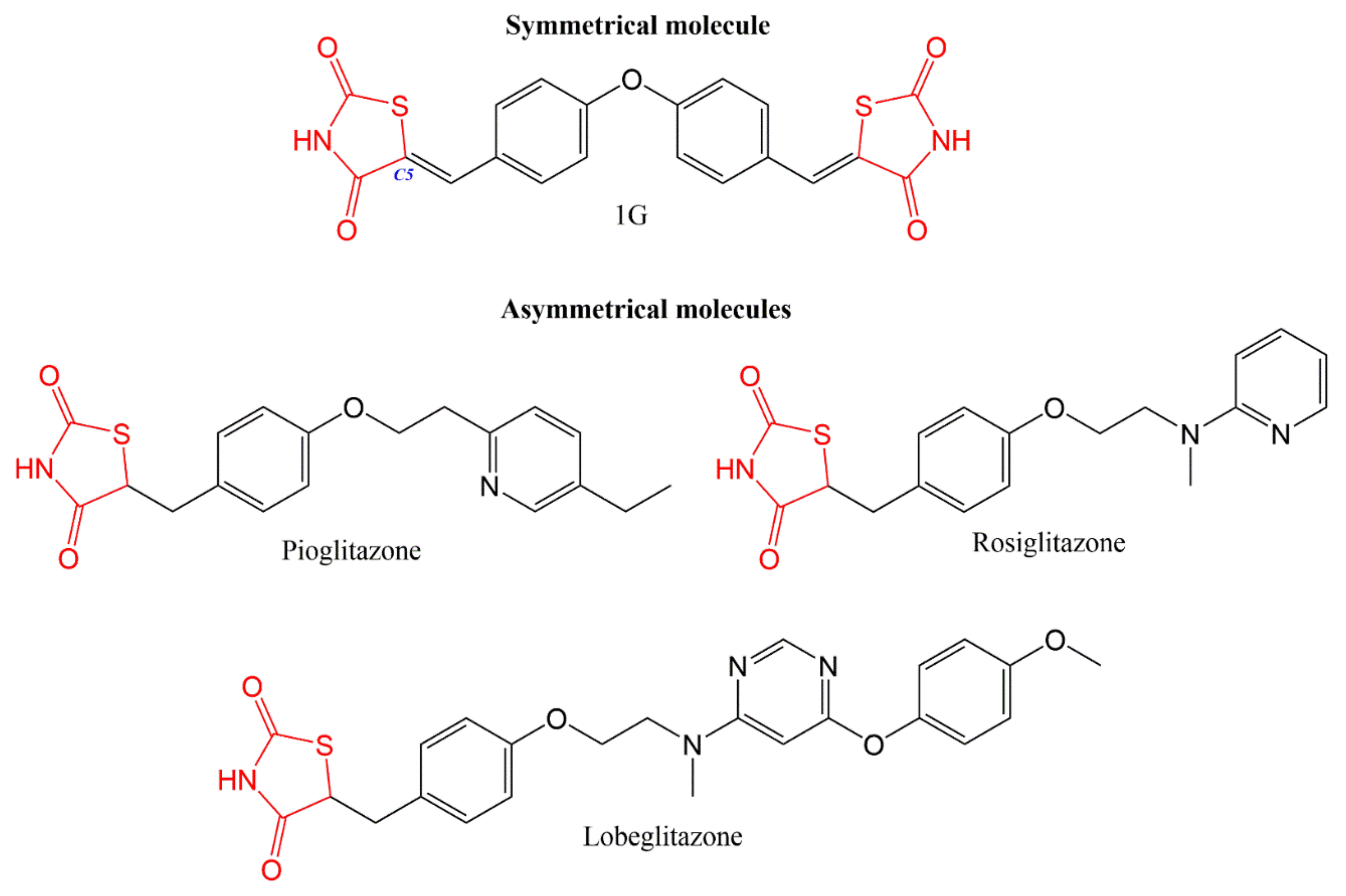
Figure 2. The chemical structure of symmetrical and asymmetrical thiazolidinediones. 1G, PIO, and ROSI are similar in length. The symmetrical regions of 1G have high polarity due to the thiazolidinedione ring (marked in red) that is unsaturated at carbon 5 (marked in blue), resulting in rigidity and fewer rotatable bonds. PIO and ROSI are asymmetrical molecules with more rotatable bonds than 1G. Lobeglitazone is an asymmetrical molecule that is longer and has more rotatable bonds than 1G, PIO, and ROSI.
Another possible explanation for the observed behavior is that 1G interacts with the receptor for a shorter time than PIO, owing to subsequent dissociation from the receptor resulting from the solubility of the ligand in the cytoplasm (polar medium). PIO has less polarity because it is an asymmetrical molecule with one polar region and another region with less polarity, which may favor a longer interaction with the receptor. In this case, molecular symmetry turns out to be a disadvantage for PPARγ binding. Perhaps a receptor exists with the necessary characteristics to bind 1G with high affinity (leading to favorable therapeutic activity). More research needed on plausible receptors for 1G.
The way a ligand binds to a receptor can mediate its interaction with other proteins. The capacity of cyclin-dependent kinase 5 (Cdk5) to inhibit PPARγ phosphorylation at Ser245 has been suggested to somehow enhance the pharmacological ability of compounds to lower blood glucose. Asymmetrical molecules might favor this inhibition. Lobeglitazone is a relatively long asymmetrical molecule capable of binding to the wide LBP of PPARγ. The binding site may be located in branch I and branch II of the receptor, allowing the ligand to act as a selective PPARγ modulator (SPPARγM) [13]. The ligand-dependent activating function (AF-2) of the receptor is in branch I, whereas Ser245 is located in beta sheet 1 of branch II (polar and non-polar region). Since this pattern of interaction is able to cause conformational changes, lobeglitazone has been suggested to block Cdk5-mediated phosphorylation of PPARγ. The polar head of this ligand generates the same interaction network with AF-2 as ROSI, thought weaker. Nevertheless, lobeglitazone binds with higher affinity to PPARγ than ROSI (−11.4 versus −9.6 kcal/mol, respectively). This might be explained by the hydrophobic interactions produced by the nonpolar region of the ligand, and the conformational change favored by interaction of the ether group of the p-methoxyphenol moiety with Arg280. These ligand-induced molecular changes in the receptor could be related to its efficacy for lowering hyperglycemia by means of blocking Ser245 phosphorylation [13].
References
- Bai, W.J.; Wang, X. Appreciation of symmetry in natural product synthesis. Nat. Prod. Rep. 2017, 34, 1345–1358.
- Lorieau, J.L. Partial alignment, residual dipolar couplings and molecular symmetry in solution NMR. J. Biomol. NMR 2019, 73, 477–491.
- Álvarez-Almazán, S.; Navarrete-Vázquez, G.; Padilla-Martínez, I.I.; Correa-Basurto, J.; Alemán-González-duhart, D.; Tamay-Cach, F.; Mendieta-Wejebe, J.E. A new symmetrical thiazolidinedione derivative: In silico design, synthesis, and in vivo evaluation on a streptozotocin-induced rat model of diabetes. Processes 2021, 9, 1294.
- Nejo, A.A.; Kolawole, G.A.; Opoku, A.R.; Wolowska, J.; O’Brien, P. Synthesis, characterization and preliminary insulin-enhancing studies of symmetrical tetradentate Schiff base complexes of oxovanadium(IV). Inorganica Chim. Acta 2009, 362, 3993–4001.
- Patujo, J.; Azeem, M.; Khan, M.; Muhammad, H.; Raheel, A.; Fatima, S.; Mirza, B.; Hussain, Z.; Badshah, A. Assessing the biological potential of new symmetrical ferrocene based bisthiourea analogues. Bioorg. Chem. 2021, 106, 104180.
- Naz, S.; Zahoor, M.; Umar, M.N.; Ali, B.; Ullah, R.; Shahat, A.A.; Mahmood, H.M.; Sahibzada, M.U.K. Enzyme inhibitory, antioxidant and antibacterial potentials of synthetic symmetrical and unsymmetrical thioureas. Drug Des. Dev. Ther. 2019, 13, 3485–3495.
- Alemán-González-Duhart, D.; Álvarez-Almazán, S.; Valdes, M.; Tamay-Cach, F.; Mendieta-Wejebe, J.E. In Vivo and Ex Vivo Evaluation of 1, 3-Thiazolidine-2, 4-Dione Derivatives as Euglycemic Agents. PPAR Res. 2021, 2021, 5100531.
- Álvarez-Almazán, S.; Filisola-Villaseñor, J.G.; Alemán-González-Duhart, D.; Tamay-Cach, F.; Mendieta-Wejebe, J.E. Current molecular aspects in the development and treatment of diabetes. J. Physiol. Biochem. 2020, 76, 13–35.
- Crommelin, D.J.A.; Sindelar, R.D.; Meibohm, B. Pharmaceutical Biotechnology. In Fundamentals and Applications, 5th ed.; Springer: Cham, Switzerland, 2019; ISBN 9783030007096.
- Shen, J.; Cheng, F.; Xu, Y.; Li, W.; Tang, Y. Estimation of ADME properties with substructure pattern recognition. J. Chem. Inf. Model. 2010, 50, 1034–1041.
- Álvarez-Almazán, S.; Bello, M.; Tamay-Cach, F.; Martínez-Archundia, M.; Alemán-González-Duhart, D.; Correa-Basurto, J.; Mendieta-Wejebe, J.E. Study of new interactions of glitazone’s stereoisomers and the endogenous ligand 15d-PGJ2 on six different PPAR gamma proteins. Biochem. Pharmacol. 2017, 142, 168–193.
- Iyer, P.; Bolla, J.; Kumar, V.; Gill, M.S.; Sobhia, M.E. In silico identification of targets for a novel scaffold, 2-thiazolylimino-5-benzylidin-thiazolidin-4-one. Mol. Divers. 2015, 19, 855–870.
- Jang, J.Y.; Bae, H.; Lee, Y.J.; Choi, Y.I.; Kim, H.J.; Park, S.B.; Suh, S.W.; Kim, S.W.; Han, B.W. Structural Basis for the Enhanced Anti-Diabetic Efficacy of Lobeglitazone on PPARγ. Sci. Rep. 2018, 8, 31.
- Shahzad, D.; Saeed, A.; Larik, F.A.; Channar, P.A.; Abbas, Q.; Alajmi, M.F.; Ifzan Arshad, M.; Erben, M.F.; Hassan, M.; Raza, H.; et al. Novel C-2 symmetric molecules as α-glucosidase and α-amylase inhibitors: Design, synthesis, kinetic evaluation, molecular docking and pharmacokinetics. Molecules 2019, 24, 1511.
- Geldenhuys, W.J.; Skolik, R.; Konkle, M.E.; Menze, M.A.; Long, T.E.; Robart, A.R. Binding of thiazolidinediones to the endoplasmic reticulum protein nutrient-deprivation autophagy factor-1. Bioorg. Med. Chem. Lett. 2019, 29, 901–904.
- Rotermund, C.; Machetanz, G.; Fitzgerald, J.C. The therapeutic potential of metformin in neurodegenerative diseases. Front. Endocrinol. 2018, 9, 400.
- Kroker, A.J.; Bruning, J.B. Review of the structural and dynamic mechanisms of PPAR γ partial agonism. PPAR Res. 2015, 2015, 816856.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
922
Revisions:
2 times
(View History)
Update Date:
27 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





