Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Serena Balducci | -- | 2432 | 2022-06-23 18:57:00 | | | |
| 2 | Lindsay Dong | Meta information modification | 2432 | 2022-06-24 03:42:40 | | | | |
| 3 | Lindsay Dong | -21 word(s) | 2411 | 2022-06-24 03:43:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Galimberti, S.; Balducci, S.; Guerrini, F.; Re, M.D.; Cacciola, R. Digital Droplet PCR in Hematologic Malignancies. Encyclopedia. Available online: https://encyclopedia.pub/entry/24413 (accessed on 08 February 2026).
Galimberti S, Balducci S, Guerrini F, Re MD, Cacciola R. Digital Droplet PCR in Hematologic Malignancies. Encyclopedia. Available at: https://encyclopedia.pub/entry/24413. Accessed February 08, 2026.
Galimberti, Sara, Serena Balducci, Francesca Guerrini, Marzia Del Re, Rossella Cacciola. "Digital Droplet PCR in Hematologic Malignancies" Encyclopedia, https://encyclopedia.pub/entry/24413 (accessed February 08, 2026).
Galimberti, S., Balducci, S., Guerrini, F., Re, M.D., & Cacciola, R. (2022, June 23). Digital Droplet PCR in Hematologic Malignancies. In Encyclopedia. https://encyclopedia.pub/entry/24413
Galimberti, Sara, et al. "Digital Droplet PCR in Hematologic Malignancies." Encyclopedia. Web. 23 June, 2022.
Copy Citation
Digital Droplet Polymerase Chain Reaction (ddPCR) is a specific, accurate and time-saving technique capables of accurately quantifying gene expression or detecting point mutations applicable in several hematologic disorders, such as leukemias, lymphomas, myeloma, and chronic myeloproliferative neoplasms, and in transplant field. The ddPCR might provide useful informations in prognostic and therapeutic setting.
digital PCR
quantitative PCR
multiplexing PCR
clonality
point mutations
hematology
1. Digital PCR
Digital Droplet Polymerase Chain Reaction (ddPCR) technique is a recent “version” of quantitative PCR (QT-PCR), based on the partition of sample into several thousand droplets, so that—at least nominally—one single DNA/cDNA copy is partitioned into a single droplet. After the end-point amplification phase, an appropriate software counts and quantifies the numbers of droplets containing the amplified products, applying the Poisson’s correction (it would be possible that any DNA/cDNA molecule enters a droplet or that two or more DNA/cDNA copies would be co-present in a single droplet) (Figure 1) [1].
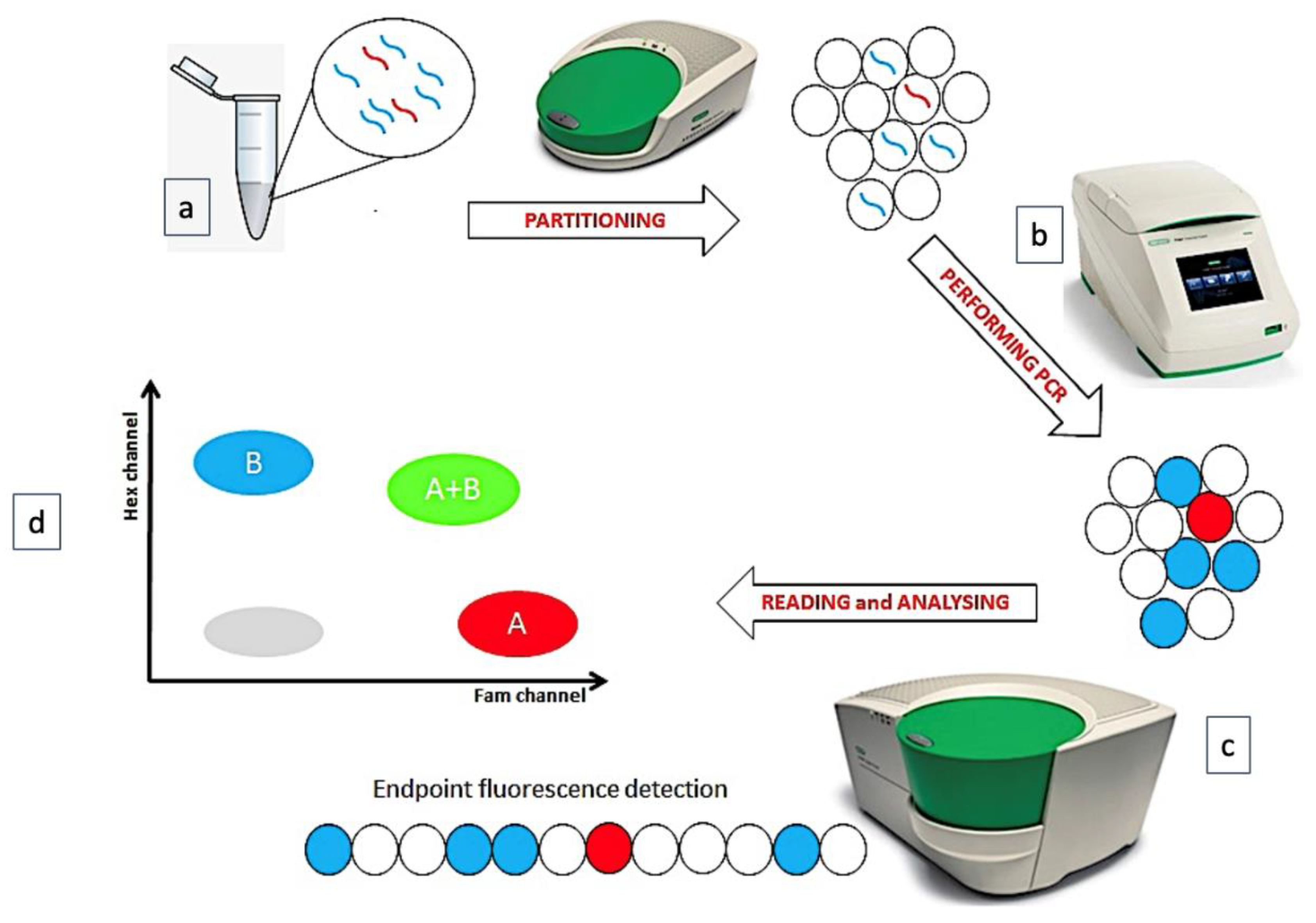
Figure 1. The phases of ddPCR technique. (a) The sample is partitioned in many thousands of droplets. (b) In each droplet a target is amplified. (c) The endpoint amplification results are analyzed. (d) A plot is generated, reading 2 fluorescence channels.
More recently, ddPCR has been used with success during the Coronavirus pandemics: with a minimum cutoff of 0.04 copies/μL, ddPCR was able to quantify the Coronavirus genome with a sensitivity and specificity of 97.6% and 100%, respectively. Interestingly, in 12 out of 18 patients who converted back to Coronavirus positivity after a negative phase, only ddPCR—and not QT-PCR—still detected viral genome, so reducing the diagnostic error during the recovery phase from the SARS-CoV-2 infection [2]. Moreover, ddPCR accurately quantified Coronavirus genome from crude lysate, with high concordance with measures from purified RNA, thus making more rapid and simpler the viral genome detection [3].
The principal distinctive feature of ddPCR in respect of QT-PCR is that the former does not require a reference standard curve, because the number of “amplified” droplets are divided by the total number of droplets giving an absolute percentage (for example: if 1000 droplets are “positive” among 20,000 total droplets: 1000/20,000 = 0.05%). This is relevant, making possible to quantitate new genes or mutations without need of cloning sequences into “ad hoc” plasmids whose presence in a laboratory significantly increases the probability of environmental contamination.
In terms of sensitivity, ddPCR is at least comparable to QT-PCR, and probably even higher, as shown in several different contexts. In non-Hodgkin’s lymphomas (NHLs) Dr. Drandi and coworkers showed a higher sensitivity for ddPCR (up to one log), especially in samples with very low tumor infiltration [4]. In Waldenstrom’s Macroglobulinemia (WM), it has been shown that ddPCR reached a sensitivity of 5 × 10−5, 1.5 log higher than that offered by the Allele-Specific Oligonucleotide PCR (ASO-PCR), the technique classically used for quantitating the rearrangement of the immunoglobulins heavy chain (IgH).
2. ddPCR Applications in Hematology
2.1. ddPCR in Acute Myeloid Leukemia and Myelodysplasias
Acute myeloid leukemia (AML) represents the prototype of a disease where the “target-therapy” is fundamental for improving patients’ survival [5]. In the recent years, the availability of the anti-CD33 monoclonal antibody gemtuzumab ozogamicin [6], of the FLT3 inhibitors (midostaurin and gilteritinib) [7][8] and of IDH1 and IDH2 inhibitors (ivosidenib and enasidenib) [9][10] significantly changed the therapeutic scenario. The increased probability of therapeutic success and the more defined disease genetic features prompted physicians to revise the WHO classification in 2016 [11] and to better define different prognostic classes (at low-, intermediate-, and poor-risk), with consequent different risk-adapted treatment strategies (chemotherapy only for low-risk patients, transplantation for high-risk cases and for those at intermediate-risk but still MRD-positive after the consolidation phase) [12]. Obviously, also the role of the MRD monitoring became more relevant and better standardized [13].
The acute promyelocytic leukemia (APL) was the first AML subtype where ddPCR played a relevant role: indeed, the positive clinical impact of restarting therapy at the re-appearance of the molecular transcript instead of at the hematological relapse is well known [14]. Consequently, the monitoring of PML-RAR transcript by a very sensitive technique represents a real clinical need.
Another ddPCR has been set for detection of PML-A216V mutation, already known to be responsible for the resistance to arsenic trioxide. Using ddPCR, 5/13 cases were recognized as mutated versus only 3 by Sanger sequencing; in addition, ddPCR anticipated the mutation appearance by 24, 3 and 4 months compared to Sanger sequencing [15].
In other AML types, ddPCR has been used at diagnosis for distinguishing between two “not otherwise specified” (NOS) forms: the expression levels of the ANXA3 and S100A9 genes were increased, whereas those of WT1 were decreased in the AML-M2 (according to the previous FAB classification) in respect of AML-M1. Moreover, STMN1 and ABL1 were upregulated in AMLs with FLT3 mutations, while CAT was over-expressed in the FLT3-wild-type cases [16].
In the complex genomic scenario characterizing AML, ddPCR was also used to track DNMT3A, IDH1 and IDH2 mutations: indeed, one third of patients resulted positive for at least one mutation (DNMT3A > IDH2 > IDH1), even in hematological complete remission (CR). Moreover, among relapsing patients, 78% resulted ddPCR positive 60 days before, while 75% of patients who remained disease-free were persistently unmutated [17].
2.2. Digital PCR in Acute Lymphoblastic Leukemia
Acute Lymphoblastic Leukemia (ALL) is the most frequent childhood neoplasia with a dismal outcome when diagnosed in adults; in the last few years a better knowledge of B and T-ALL genetic landscape and advanced tools for MRD monitoring allowed to refine indications for alloSCT and ameliorate prognosis [18][19].
MRD monitoring in chromosome Philadelphia-positive (Ph’-positive) B-ALL is based on quantification of BCR-ABL1 transcript using QT-PCR; a recent Italian study applied ddPCR to patients enrolled into the GIMEMA LAL2116 trial, showing optimal sensitivity (1 × 10−5–5 × 10−6) and specificity (near to 100%). In follow-up samples, ddPCR was able to reduce the proportion of positive-not-quantifiable (PNQ) cases, which represent a grey zone in the clinical practice, significantly increasing the proportion of quantifiable samples. Therefore, of the 5 cases that were negative by QT-PCR and positive by ddPCR during follow-up, 4/5 experienced a relapse, confirming the clinical relevance of a deeper MRD monitoring [20].
In Ph’-positive neoplasms, the emergence of point mutations in Tyrosine Kinase Inhibitors (TKIs)-ligand domain of ABL1 may represent a major barrier for success of TKIs, with T315I mutations rendering cells sensible only to the 3rd generation TKI, ponatinib. If the role of ABL1 point mutations is well-recognized in Chronic Myeloid Leukemia (CML), in ALL the prognostic relevance of detecting small clones of T315I-mutated cells at diagnosis remains to be fully elucidated [21].
In Ph’-negative ALLs (B or T), which represent most ALLs in childhood, MRD detection is more complicated and based on the Immunoglobulin IgH, Ig, Ig and TCRs rearrangement analysis; nevertheless, a consistent fraction of samples with very-low MRD levels cannot be properly quantified and must be scored as positive not quantifiable (PNQ), that represent a clinical dilemma (are they MRD positive or negative? Is it worth to proceed with transplantation or not?) [22]. Trying to address this issue, Dr. Della Starza and coworkers proposed ddPCR as an alternative method for MRD monitoring [23][24] in samples from patients enrolled in the GIMEMA LAL1308 and in the AIEOP-BFM ALL 2000 trials, finding a concordance rate of 70% between QT-PCR and ddPCR. The greater accuracy of ddPCR allowed to quantitate samples defined as PNQ by QT-PCR in a quarter of cases. To allow a better standardization, the group also proposed a fixed-threshold of positive-droplet number to define a sample as negative, PNQ or positive [24].
2.3. Digital PCR in Lymphoproliferative Disorders (Lymphomas and Multiple Myeloma)
The BRAF V600E mutation interests 70–100% of hairy cell leukemias (HCL) patients [25], but also up to 50% of cases of Langerhans cell histiocytosis, especially those with skin or central nervous system involvement [26]. The clinical translation of this finding is the possibility of administering vemurafenib, already employed in melanoma [27] and in non-small lung cell cancer [28], to relapsed/refractory hematological patients carrying the B-RAF mutation.
In chronic lymphocytic leukemia (CLL), identification of TP53 mutations, as well as of chromosome 17 deletions, that occur in about 5–10% of patients at diagnosis and more frequently at relapse, has a relevant clinical impact, making the chemo-immunotherapy not advisable for this kind of patients [29].
Another field where ddPCR resulted useful for identification of patients who might benefit of Bruton Kinase inhibitors [30] is that of lymphoproliferative disorders characterized by the presence of the MYD88L265P mutation. This genetic abnormality triggers the anti-apoptotic NF-kB pathway, activates the JAK-STAT3 and BTK signals, leading to the uncontrolled B cells proliferation. This mutation characterizes about 30% of Activated Diffuse Large B Cell Lymphomas (ABC-DLBCL), 52% of IgM monoclonal gammopathies (IgM-MGUS), 54% of the cutaneous DLBCL, 70% of primary DLBCL involving the central nervous system, and 90% of MW, while is absent in IgM multiple myeloma (MM) [31].
Because BCL2/JH rearrangement can be found only in 60% of FL and the assessment of IgH rearrangement in this lymphoma is often difficult due to its hypermutated status [32], the possibility of assessing different molecular markers is intriguing. Among them, mutations of EZH2 are becoming relevant, even from the clinical point of view, after the recent introduction in the therapeutic armamentarium of the oral EZH2 inhibitor. In patients with relapsed/refractory FL, tazemetostat offered to EZH2-mutated patients 69% of OR and 13% of CR vs. 35% of ORR and 4% of CR to the wild-type subgroup [33]. A ddPCR for detecting EZH2 mutations was set; interestingly, in a patient carrying two different mutations in different tumor sites, the analysis of ctDNA revealed both EZH2 genomic aberrations, so demonstrating the optimal representativeness of liquid biopsy [34]. Even in early-stage FL, ddPCR for BCL2/IgH rearrangement was compared to classical QT-PCR: the concordance between the two techniques amounted to 92%, and the fusion gene was recovered by ddPCR in 18% of cases otherwise negative by QT-PCR [35].
2.4. Digital PCR in Chronic Myeloid Leukemia
CML is a chronic myeloproliferative neoplasm characterized by the presence of Philadelphia chromosome (Ph’) and of BCR/ABL1 fusion gene originating from the t(9;22). TKIs (imatinib as first generation, dasatinib, nilotinib and bosutinib as second generation, ponatinib as third generation and asciminib, a new STAMP-inhibitor) are orally available drugs able to inhibit the chimeric protein function so leading to a long-term remission in more than 90% of patients [36]. Nevertheless, about one third of them must change TKI for scarce tolerability or treatment failure. In about 10% of cases, resistance to TKIs is due to point mutations in the kinase domain; among them, T315I confers resistance to all TKIs except for ponatinib and asciminib [37].
The correct management of CML patients is currently based on the serial quantitative molecular assessment of BCR-ABL1/ABL1 ratio, which results fundamental for continuing the same TKI (in patients with optimal response), changing drug (for failing cases) or for more strictly following cases with doubt or not stable response [38]. Nevertheless, in the last 10 years a great opportunity is opened for patients with deep and stable molecular response: the attempt of TKI discontinuation (treatment-free remission or TFR), that has success in about half of cases [39]. Many efforts have been made to correctly identify patients with high probability of TFR to reduce the failure occurrence [38][40]. Among them, it is necessary to correctly identify cases in real deep molecular response (because it is known that patients in less deep response are destined to rapidly fail TFR) [41][42]. In this context, ddPCR demonstrated e good correlation with QT-PCR (99.6%), but even a superiority in terms of LOD and level of quantification (LOQ) [40][43][44][45][46]. In addition to QT-PCR, the reproducibility of results was tested on the two different commercially available platforms: the QX200 Droplet Digital PCR System and the QuantStudio 3D Digital PCR System: the concordance raised to 98.7%, with consistent results [47].
2.5. Digital PCR in Chronic Myeloproliferative Neoplasms
The chronic myeloproliferative neoplasms (MPNs), including essential thrombocythemia (ET), polycythemia vera (PV) and myelofibrosis (MF), are frequently characterized by the JAK2 mutations [48][49]. Because the presence of these mutations (or, in unmutated cases, of mutations of Calreticulin (CALR) or MPL) is one of the diagnostic criteria [50], it is obvious that ddPCR was firstly set for the screening of JAK2 V617F mutation (that is more common than mutations at exon 12).
2.6. Digital PCR in Transplant and Immunoterapies
Allogeneic hematopoietic stem cell transplantation (AlloSCT) is a potentially curative therapeutic option for several high-risk hematological malignancies (AML, ALL, MDS, lymphomas), especially if performed in CR. After AlloSCT the follow-up is principally based on chimerism and, when possible, on disease specific MRD markers or persistence of mutations: all these strategies allow to promptly detect and treat graft rejection or disease relapse [51]. Nevertheless, the correct timing, samples source—PB or BM—and techniques for chimerism evaluation as well as the exact threshold to distinguish complete donor chimerism from mixed chimerism are still matters of debate [52][53][54].
Currently, the standard methods to measure chimerism are QT-PCR-based analysis of Short Tandem Repeats (STR), with a sensitivity between 5% and 1%, according to the diversity of donor/recipient fingerprint [55]. During the last few years, several studies tried to apply ddPCR to the chimerism assessment, even for levels <1% [56][57]. One of the proposed strategies for children who underwent transplantation for primary immunodeficiency diseases included ddPCR for SRY and RPP30 genes that allowed detect the male/female chimerism. This method revealed accurate and was able to analyze very small amount of genomic material (less than 10 ng) [57]. With a sensitivity of 8 × 10−5, the correlation between STR and ddPCR was higher than 99%, thus supporting the use of ddPCR also for the chimerism assessment [58].
3. Conclusions
Born about 20 years ago, ddPCR is a new version of QT-PCR, more sensitive, specific, and accurate. With a LOD ranging from 10−4 and 10−5 according to different assays, ddPCR allows to quantitate about one quarter of samples already defined as PNQ by QT-PCR, so making more easily the patients’ management and follow-up. The versatility of this technique makes it available for measuring gene expression (without the need of a standard curve or plasmids), but also for detecting single or multiple point mutations, either on cDNA but also on genomic DNA, both on bone marrow, peripheral blood or liquid biopsy.
As above reported, many are the hematological contexts where ddPCR has been used and implemented: acute leukemias, where it is able to quantitate NPM1 mutations but also WT1 expression; Ph’-positive leukemias, where it is used for measuring more accurately the BCR-ABL1/ABL1 ratio to also identify the patients best candidate to TFR but also for BCR-ABL1 mutations detection; the MPNs, where JAK2 and CALR mutations have a clear diagnostic role, and the lymphoma/myeloma setting, where IgH and TCR clonality can be combined with BCL2/JH and BCL1/JH fusion genes for assessing MRD. Finally, ddPCR can be used for chimerism determination and for monitoring immune reconstitution and CAR-T persistence in patients who receive transplantation or the new immunotherapies .
References
- Quan, P.L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271, PMCID:PMC5948698.
- Liu, C.; Shi, Q.; Peng, M.; Lu, R.; Li, H.; Cai, Y.; Chen, J.; Xu, J.; Shen, B. Evaluation of droplet digital PCR for quantification of SARS-CoV-2 Virus in discharged COVID-19 patients. Aging 2020, 12, 20997–21003.
- Vasudevan, H.N.; Xu, P.; Servellita, V.; Miller, S.; Liu, L.; Gopez, A.; Chiu, C.Y.; Abate, A.R. Digital droplet PCR accurately quantifies SARS-CoV-2 viral load from crude lysate without nucleic acid purification. Sci. Rep. 2021, 11, 780.
- Drandi, D.; Kubiczkova-Besse, L.; Ferrero, S.; Dani, N.; Passera, R.; Mantoan, B.; Gambella, M.; Monitillo, L.; Saraci, E.; Ghione, P.; et al. Minimal Residual Disease Detection by Droplet Digital PCR in Multiple Myeloma, Mantle Cell Lymphoma, and Follicular Lymphoma: A Comparison with Real-Time PCR. J. Mol. Diagn. 2015, 17, 652–660.
- DiNardo, C.D.; Wei, A.H. How I treat acute myeloid leukemia in the era of new drugs. Blood 2020, 135, 85–96.
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119.
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464.
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740.
- DiNardo, C.D.; Stein, A.S.; Stein, E.M.; Fathi, A.T.; Frankfurt, O.; Schuh, A.C.; Döhner, H.; Martinelli, G.; Patel, P.A.; Raffoux, E.; et al. Mutant Isocitrate Dehydrogenase 1 Inhibitor Ivosidenib in Combination with Azacitidine for Newly Diagnosed Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 57–65, Erratum in J. Clin. Oncol. 2021, 39, 341.
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731.
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405.
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447.
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291.
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T.; Lengfelder, E.; Döhner, H.; Burnett, A.K.; Chen, S.J.; et al. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 2019, 133, 1630–1643.
- Alfonso, V.; Iaccarino, L.; Ottone, T.; Cicconi, L.; Lavorgna, S.; Divona, M.; Cairoli, R.; Cristiano, A.; Ciardi, C.; Travaglini, S.; et al. Early and sensitive detection of PML-A216V mutation by droplet digital PCR in ATO-resistant acute promyelocytic leukemia. Leukemia 2019, 33, 1527–1530.
- Handschuh, L.; Kaźmierczak, M.; Milewski, M.C.; Góralski, M.; Łuczak, M.; Wojtaszewska, M.; Uszczyńska-Ratajczak, B.; Lewandowski, K.; Komarnicki, M.; Figlerowicz, M. Gene expression profiling of acute myeloid leukemia samples from adult patients with AML-M1 and -M2 through boutique microarrays, real-time PCR and droplet digital PCR. Int. J. Oncol. 2018, 52, 656–678.
- Brambati, C.; Galbiati, S.; Xue, E.; Toffalori, C.; Crucitti, L.; Greco, R.; Sala, E.; Crippa, A.; Chiesa, L.; Soriani, N.; et al. Droplet digital polymerase chain reaction for DNMT3A and IDH1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica 2016, 101, e157–e161.
- Marks, D.I.; Rowntree, C. Management of adults with T-cell lymphoblastic leukemia. Blood 2017, 129, 1134–1142, Erratum in Blood 2017, 129, 2204.
- Hein, K.; Short, N.; Jabbour, E.; Yilmaz, M. Clinical Value of Measurable Residual Disease in Acute Lymphoblastic Leukemia. Blood Lymphat. Cancer 2022, 12, 7–16.
- Ansuinelli, M.; Della Starza, I.; Lauretti, A.; Elia, L.; Siravo, V.; Messina, M.; De Novi, L.A.; Taherinasab, A.; Canichella, M.; Guarini, A.; et al. Applicability of droplet digital polymerase chain reaction for minimal residual disease monitoring in Philadelphia-positive acute lymphoblastic leukaemia. Hematol. Oncol. 2021, 39, 680–686.
- Soverini, S.; Vitale, A.; Poerio, A.; Gnani, A.; Colarossi, S.; Iacobucci, I.; Cimino, G.; Elia, L.; Lonetti, A.; Vignetti, M.; et al. Philadelphia-positive acute lymphoblastic leukemia patients already harbor BCR-ABL kinase domain mutations at low levels at the time of diagnosis. Haematologica 2011, 96, 552–557.
- van der Velden, V.H.; Cazzaniga, G.; Schrauder, A.; Hancock, J.; Bader, P.; Panzer-Grumayer, E.R.; Flohr, T.; Sutton, R.; Cave, H.; Madsen, H.O.; et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: Guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007, 21, 604–611.
- Della Starza, I.; Nunes, V.; Cavalli, M.; De Novi, L.A.; Ilari, C.; Apicella, V.; Vitale, A.; Testi, A.M.; Del Giudice, I.; Chiaretti, S.; et al. Comparative analysis between RQ-PCR and digital-droplet-PCR of immunoglobulin/T-cell receptor gene rearrangements to monitor minimal residual disease in acute lymphoblastic leukaemia. Br. J. Haematol. 2016, 174, 541–549.
- Della Starza, I.; Nunes, V.; Lovisa, F.; Silvestri, D.; Cavalli, M.; Garofalo, A.; Campeggio, M.; De Novi, L.A.; Soscia, R.; Oggioni, C.; et al. Droplet Digital PCR Improves IG-/TR-based MRD Risk Definition in Childhood B-cell Precursor Acute Lymphoblastic Leukemia. Hemasphere 2021, 5, e543.
- Maitre, E.; Cornet, E.; Troussard, X. Hairy cell leukemia: 2020 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 1413–1422.
- Rodriguez-Galindo, C.; Allen, C.E. Langerhans cell histiocytosis. Blood 2020, 135, 1319–1331.
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516.
- Mazieres, J.; Cropet, C.; Montané, L.; Barlesi, F.; Souquet, P.J.; Quantin, X.; Dubos-Arvis, C.; Otto, J.; Favier, L.; Avrillon, V.; et al. Vemurafenib in non-small-cell lung cancer patients with BRAFV600 and BRAFnonV600 mutations. Ann. Oncol. 2020, 31, 289–294.
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760.
- Gertz, M.A. Waldenström macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2021, 96, 258–269.
- Yu, X.; Li, W.; Deng, Q.; Li, L.; Hsi, E.D.; Young, K.H.; Zhang, M.; Li, Y. MYD88 L265P Mutation in Lymphoid Malignancies. Cancer Res. 2018, 78, 2457–2462.
- Galimberti, S.; Luminari, S.; Ciabatti, E.; Grassi, S.; Guerrini, F.; Dondi, A.; Marcheselli, L.; Ladetto, M.; Piccaluga, P.P.; Gazzola, A.; et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: The FIL FOLL05 trial. Clin. Cancer Res. 2014, 20, 6398–6405.
- von Keudell, G.; Salles, G. The role of tazemetostat in relapsed/refractory follicular lymphoma. Ther. Adv. Hematol. 2021, 12, 20406207211015882.
- Nagy, Á.; Bátai, B.; Balogh, A.; Illés, S.; Mikala, G.; Nagy, N.; Kiss, L.; Kotmayer, L.; Matolcsy, A.; Alpár, D.; et al. Quantitative Analysis and Monitoring of EZH2 Mutations Using Liquid Biopsy in Follicular Lymphoma. Genes 2020, 11, 785.
- Cavalli, M.; De Novi, L.A.; Della Starza, I.; Cappelli, L.V.; Nunes, V.; Pulsoni, A.; Del Giudice, I.; Guarini, A.; Foà, R. Comparative analysis between RQ-PCR and digital droplet PCR of BCL2/IGH gene rearrangement in the peripheral blood and bone marrow of early stage follicular lymphoma. Br. J. Haematol. 2017, 177, 588–596.
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2020, 95, 691–709.
- Smith, G.; Apperley, J.; Milojkovic, D.; Cross, N.C.P.; Foroni, L.; Byrne, J.; Goringe, A.; Rao, A.; Khorashad, J.; de Lavallade, H.; et al. A British Society for Haematology Guideline on the diagnosis and management of chronic myeloid leukaemia. Br. J. Haematol. 2020, 191, 171–193.
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984.
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757.
- Atallah, E.; Sweet, K. Treatment-Free Remission: The New Goal in CML Therapy. Curr. Hematol. Malig. Rep. 2021, 16, 433–439.
- Baccarani, M.; Abruzzese, E.; Accurso, V.; Albano, F.; Annunziata, M.; Barulli, S.; Beltrami, G.; Bergamaschi, M.; Binotto, G.; Bocchia, M.; et al. Managing chronic myeloid leukemia for treatment-free remission: A proposal from the GIMEMA CML WP. Blood Adv. 2019, 3, 4280–4290.
- Dragani, M.; Rege Cambrin, G.; Berchialla, P.; Dogliotti, I.; Rosti, G.; Castagnetti, F.; Capodanno, I.; Martino, B.; Cerrano, M.; Ferrero, D.; et al. A Retrospective Analysis about Frequency of Monitoring in Italian Chronic Myeloid Leukemia Patients after Discontinuation. J. Clin. Med. 2020, 9, 3692.
- Chung, H.J.; Hur, M.; Yoon, S.; Hwang, K.; Lim, H.S.; Kim, H.; Moon, H.W.; Yun, Y.M. Performance Evaluation of the QXDx BCR-ABL %IS Droplet Digital PCR Assay. Ann. Lab. Med. 2020, 40, 72–75.
- Wang, W.J.; Zheng, C.F.; Liu, Z.; Tan, Y.H.; Chen, X.H.; Zhao, B.L.; Li, G.X.; Xu, Z.F.; Ren, F.G.; Zhang, Y.F.; et al. Droplet digital PCR for BCR/ABL(P210) detection of chronic myeloid leukemia: A high sensitive method of the minimal residual disease and disease progression. Eur. J. Haematol. 2018, 101, 291–296.
- Franke, G.N.; Maier, J.; Wildenberger, K.; Cross, M.; Giles, F.J.; Müller, M.C.; Hochhaus, A.; Niederwieser, D.; Lange, T. Comparison of Real-Time Quantitative PCR and Digital Droplet PCR for BCR-ABL1 Monitoring in Patients with Chronic Myeloid Leukemia. J. Mol. Diagn. 2020, 22, 81–89.
- Cortés, A.A.; Olmedillas, S.; Serrano-López, J.; Lainez-González, D.; Castaño, T.; Iñiguez, R.; Lopez-Lorenzo, J.L.; García, A.; Atance, M.; Sánchez, R.N.S.; et al. Comparison of Droplet Digital PCR versus qPCR Measurements on the International Scale for the Molecular Monitoring of Chronic Myeloid Leukemia Patients. Mol. Diagn. Ther. 2020, 24, 593–600.
- Fava, C.; Bernardi, S.; Gottardi, E.M.; Lorenzatti, R.; Galeotti, L.; Ceccherini, F.; Cordoni, F.; Daraio, F.; Giugliano, E.; Jovanovski, A.; et al. Alignment of Qx100/Qx200 Droplet Digital (Bio-Rad) and QuantStudio 3D (Thermofisher) Digital PCR for Quantification of BCR-ABL1 in Ph+ Chronic Myeloid Leukemia. Diseases 2021, 9, 35.
- Tefferi, A.; Barbui, T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 1599–1613.
- Tefferi, A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2021, 96, 145–162.
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018, 8, 15.
- Baron, F.; Sandmaier, B.M. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia 2006, 20, 1690–1700.
- Bader, P.; Niethammer, D.; Willasch, A.; Kreyenberg, H.; Klingebiel, T. How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transplant. 2005, 35, 107–119.
- Antin, J.H.; Childs, R.; Filipovich, A.H.; Giralt, S.; Mackinnon, S.; Spitzer, T.; Weisdorf, D. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: Recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2001, 7, 473–485.
- Mika, T.; Baraniskin, A.; Ladigan, S.; Wulf, G.; Dierks, S.; Haase, D.; Schork, K.; Turewicz, M.; Eisenacher, M.; Schmiegel, W.; et al. Digital droplet PCR-based chimerism analysis for monitoring of hematopoietic engraftment after allogeneic stem cell transplantation. Int. J. Lab. Hematol. 2019, 41, 615–621.
- Alizadeh, M.; Bernard, M.; Danic, B.; Dauriac, C.; Birebent, B.; Lapart, C.; Lamy, T.; Le Prisé, P.Y.; Beauplet, A.; Bories, D.; et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 2002, 99, 4618–4625.
- Stahl, T.; Böhme, M.U.; Kröger, N.; Fehse, B. Digital PCR to assess hematopoietic chimerism after allogeneic stem cell transplantation. Exp. Hematol. 2015, 43, 462–468.e1.
- Okano, T.; Tsujita, Y.; Kanegane, H.; Mitsui-Sekinaka, K.; Tanita, K.; Miyamoto, S.; Yeh, T.W.; Yamashita, M.; Terada, N.; Ogura, Y.; et al. Droplet Digital PCR-Based Chimerism Analysis for Primary Immunodeficiency Diseases. J. Clin. Immunol. 2018, 38, 300–306.
- Kliman, D.; Castellano-Gonzalez, G.; Withers, B.; Street, J.; Tegg, E.; Mirochnik, O.; Lai, J.; Clancy, L.; Gottlieb, D.; Blyth, E. Ultra-Sensitive Droplet Digital PCR for the Assessment of Microchimerism in Cellular Therapies. Biol. Blood Marrow Transplant. 2018, 24, 1069–1078.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
24 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




