Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisa Casaglia | -- | 2964 | 2022-06-23 14:59:32 | | | |
| 2 | Peter Tang | -1 word(s) | 2963 | 2022-06-24 03:04:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Figorilli, M.; Lanza, G.; Congiu, P.; Lecca, R.; Casaglia, E.; Mogavero, M.P.; Puligheddu, M.; Ferri, R. Rapid Eye Movement Sleep Behavior Disorder. Encyclopedia. Available online: https://encyclopedia.pub/entry/24398 (accessed on 07 February 2026).
Figorilli M, Lanza G, Congiu P, Lecca R, Casaglia E, Mogavero MP, et al. Rapid Eye Movement Sleep Behavior Disorder. Encyclopedia. Available at: https://encyclopedia.pub/entry/24398. Accessed February 07, 2026.
Figorilli, Michela, Giuseppe Lanza, Patrizia Congiu, Rosamaria Lecca, Elisa Casaglia, Maria P. Mogavero, Monica Puligheddu, Raffaele Ferri. "Rapid Eye Movement Sleep Behavior Disorder" Encyclopedia, https://encyclopedia.pub/entry/24398 (accessed February 07, 2026).
Figorilli, M., Lanza, G., Congiu, P., Lecca, R., Casaglia, E., Mogavero, M.P., Puligheddu, M., & Ferri, R. (2022, June 23). Rapid Eye Movement Sleep Behavior Disorder. In Encyclopedia. https://encyclopedia.pub/entry/24398
Figorilli, Michela, et al. "Rapid Eye Movement Sleep Behavior Disorder." Encyclopedia. Web. 23 June, 2022.
Copy Citation
Rapid eye movement (REM) sleep without atonia (RSWA) is the polysomnographic (PSG) hallmark of REM sleep behavior disorder (RBD), a feature essential for the diagnosis of this condition.
REM sleep behavior disorder
REM sleep without atonia
polysomnography
neurophysiology
electroencephalography
transcranial magnetic stimulation
vestibular evoked myogenic potentials
1. Introduction
For the International Classification of Sleep Disorders 3rd Edition (ICSD-3) [1], rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia manifested by vivid, often frightening dreams accompanied by simple or complex motor behaviors during REM sleep. Patients seem to “enact their dreams” with their behaviors, probably mirroring the dream content [2]. Idiopathic (or isolated) RBD (iRBD) is an established early manifestation of a neurodegenerative disease, especially synucleinopathy [3]. RBD can be found in a large proportion of both children and adults with narcolepsy, representing a form of REM sleep motor behavior dyscontrol [4][5], and it seems to be a phenotype distinct from iRBD, with less marked sex predominance, more elementary and less complex movements and less violent behavior in REM sleep, younger age of onset, and orexin deficiency (a feature of narcolepsy type 1) [6][7].
The polysomnographic (PSG) hallmark of RBD is decreased muscle atonia during REM sleep, also called REM sleep without atonia (RSWA), because of increased electromyographic (EMG) activity during this stage, and this is a feature essential for the diagnosis of this condition, which is thus based on both clinical and laboratory findings [1]. The search for reliable biomarkers of RBD and its evolution into an overt synucleinopathy is a very lively field of interest, with many studies being published [8]. However, RSWA remains the most important neurophysiological aspect, being necessary for the diagnosis of RBD [9].
2. REM Sleep Network and REM Atonia Neurophysiopathology
Knowledge about the neural circuitry underling REM sleep physiology and mechanisms causing RSWA mostly arises from animal, post-mortem, and radiological studies. Brainstem lesions due to neurodegeneration, demyelination, tumors, or ischemic injury may cause RSWA and lead to secondary RBD [10][11][12][13].
Early animal studies showed that a state characterized by muscle atonia and rapid eye movement persists following decortication, or brainstem transections rostral to the pons in Jouvet’s “pontine cat” [14]. In addition, the sublaterodorsal tegmental nucleus (SLD), also called the subcoeruleus nucleus (SubC), contains many neurons that show tonic firing selective to the paradoxical (or REM) sleep (PS) state, thus called “PS-on” neurons [15]. Although initial studies indicated that the nature of SLD or SubC PS-on neurons was cholinergic [16], further research demonstrated that vesicular glutamate transporter 2 (vGlut2), a specific marker of glutamatergic neurons, was expressed in c-Fos-positive neurons localized in the SLD or SubC after PS hypersomnia [17]. Moreover, genetic inactivation of glutamatergic transmission in SLD or SubC neurons induces a 30% decrease in PS quantities and the occurrence of RBD, confirming the glutamatergic nature of SLD or SubC neurons generating PS [18]. Tract-tracing data also revealed that SLD or SubC glutamatergic PS-on neurons send descending projections to the gamma-amyno-butyric acid (GABA) and glycinergic neurons located in the nucleus raphe magnus, as well as the ventral alpha gigantocellular and lateral paragigantocellular reticular nuclei, inducing muscle atonia, but not to the intralaminar thalamus neurons known to mediate the activation of the cortex during REM sleep [18]. Conversely, cholinergic and noncholinergic neurons, located in the SLD or SubC, pedunculopontine, and laterodorsal tegmental nuclei, and glutamatergic neurons in the reticular formation projecting to the thalamus and hypothalamus (that seem to be firing both during waking and PS) are believed to play a role in cortical activation during REM sleep. Concerning the limbic cortical structures, only the dentate gyrus, anterior cingulate, and retrosplenial and medial entorhinal cortices seem to be activated during PS [19].
Recent findings suggest that the onset of a REM sleep episode might be due to a complex and still largely unknown mechanism implicating the activation of PS-on melanin-concentrating hormone and GABAergic neurons in the lateral hypothalamus [20]. This would remove the GABAergic tone of PS-off neurons located in the ventrolateral part of the periaqueductal gray (vlPAG) and the adjacent dorsal part of the deep mesencephalic nucleus (dDPMe), which gate PS by tonically inhibiting the PS-on neurons of the SLD or SubC during wakefulness and slow-wave sleep [21][22], combined with the continuous presence of a glutamatergic input. PS-on GABAergic neurons localized in the lateral hypothalamus, dorsal paragigantocellular nuclei, and vlPAG also seem to inactivate the PS-off orexin and aminergic neurons during REM sleep [23]. Conversely, the activation of arousal systems, reciprocally inhibiting the GABAergic PS-on neurons, might determine the exit from a REM sleep state [19], allegedly forming a “flip-flop” switch model for REM sleep.
REM sleep atonia (see Figure 1) is basically due to both the inhibition and decreased activation [24] of cranial and spinal skeletal motor neurons, which exhibit a tonic hyperpolarization during physiological REM sleep [25]. In fact, blockage of both the GABA and glycine motor neuron receptors prevents muscle paralysis during REM sleep [26]. During REM sleep, cranial and spinal motor neurons are very likely to be inhibited by the GABA and glycinergic neurons located in the ventromedial medulla (VMM). Additionally, the spinal motor neurons seem to also be inhibited by the spinal interneurons [26][27]. Moreover, decreased or lost glutamatergic, noradrenergic, dopaminergic, and hypocretinergic activity may contribute to reduce motor neuron excitability, thus strengthening muscle atonia during REM sleep [28][29][30]. The VMM and spinal interneurons are under the direct control of a group of glutamatergic neurons located within a small reticular region of the pontine tegmentum, namely the SLD or SubC, which act as an REM sleep generator and controller (REM-on cells), which is thought to be necessary and sufficient for generating REM sleep atonia [13][31][32][33]. SLD or SubC glutamatergic REM-on cells and VMM GABA and glycinergic neurons constitute the main REM atonia circuitry [34].
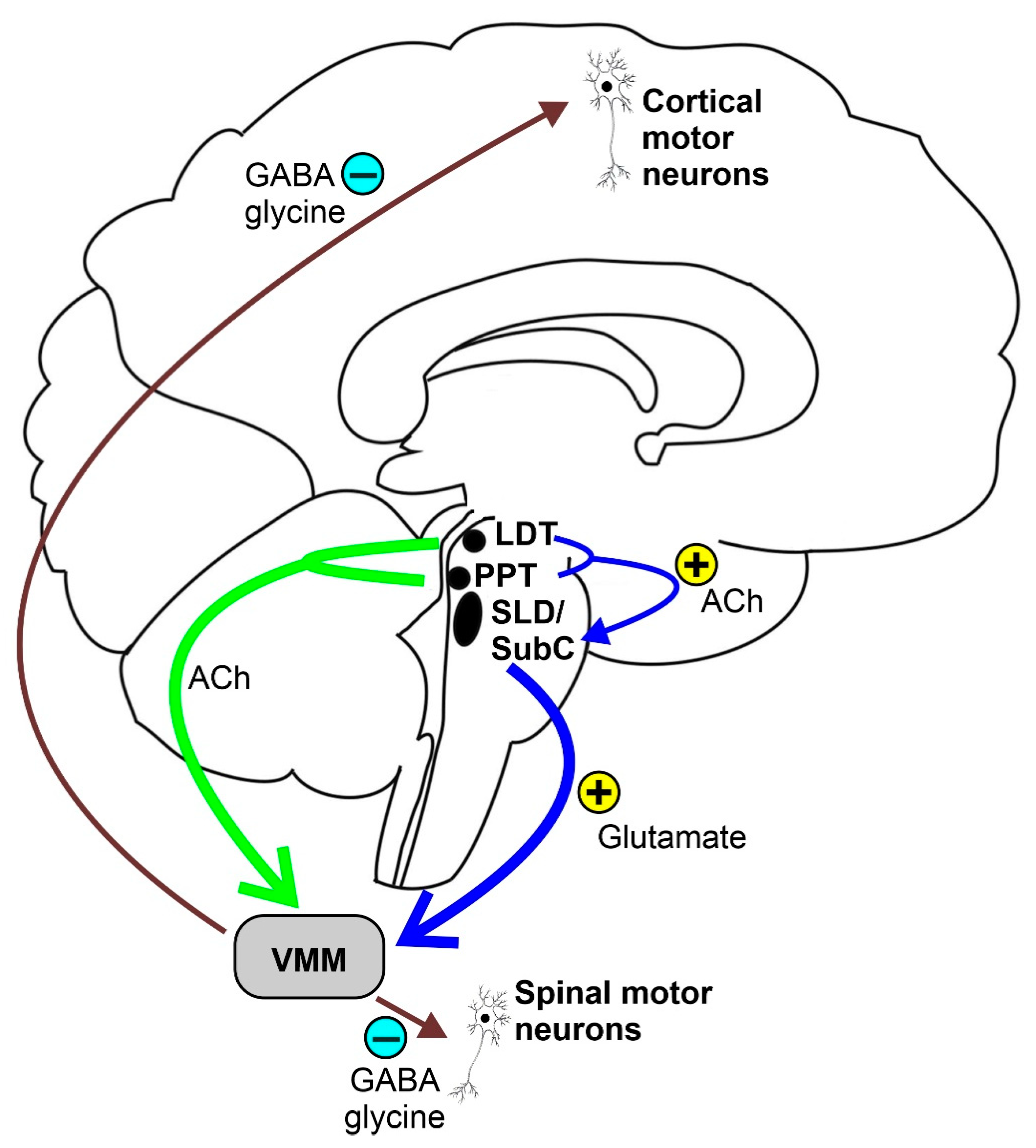
Figure 1. Schematic representation of the REM sleep atonia circuitry. Ach = acetylcholine, LDT = laterodorsal tegmental nucleus, PPT = pedunculopontine nucleus, SLD = sublaterodorsal tegmental nucleus, SubC = subcoeruleus nucleus, and VMM = ventromedial medulla.
3. REM Sleep without Atonia (RSWA)
3.1. Polysomnographic Features
Physiological REM sleep is characterized by complete muscle atonia with a markedly decreased amplitude for the EMG signal. As said above, RSWA is the neurophysiological and polysomnographic hallmark of RBD, and it is represented by a persistent muscle tone during REM sleep, resulting in either sustained (tonic) excessive activity during REM sleep in the chin EMG, intermittent (phasic) excessive activity during REM sleep in the chin or limb EMG, or both. The tonic and phasic components of RSWA can be present and isolated from each other or in combination, such as bursts of phasic EMG activity superimposed on a tonically increased muscle tone. According to the latest American Academy of Sleep Medicine (AASM) scoring manual [35], a 30-s epoch of REM sleep is defined as tonic when at least 50% of its duration contains a chin EMG with an amplitude greater than the minimum amplitude observed in non-REM sleep. On the other hand, the same scoring manual defines the phasic components of RSWA as excessive transient muscle activity bursts on the chin and limb EMG channels lasting 0.1–5.0 s with an amplitude at least 4 times as high as the background EMG activity. Moreover, a 30-s REM sleep epoch, subdivided into 3-s mini-epochs, is considered to have excessive phasic EMG activity when at least 50% of the 3-s mini-epochs contain bursts of transient EMG activity [35].
RSWA must be differentiated from increased muscle tone related to other different activities (artifacts), such as respiratory arousals, snoring, limb movements, and body movements. Furthermore, RSWA can be triggered or exacerbated by different drugs, such as tricyclic antidepressants, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, mirtazapine, venlafaxine, and betablockers. However, further studies are needed to assess cause–effect relationships [36][37][38][39][40][41]. Although the quantification of RSWA is mandatory for the diagnosis of RBD, both the AASM scoring manual and the ICSD-3 do not provide established cut-off values for RSWA, nor do they say how many tonic phasic epochs are needed to define RSWA [1][35].
3.2. Visual Scoring
Different visual scoring methods to detect and quantify RSWA have been developed and validated. However, there is no consensus on which method is more efficient or which muscle group is better suited to identify or quantify RSWA and distinguish RBD from healthy subjects. It is important to note that here, the researchers refer to phasic or tonic EMG activations during REM sleep that should not be confused with the phasic or tonic subtypes of REM sleep, usually identified on the basis of the presence or absence of eye movement activity during this sleep stage.
The first and the most widely used visual method for scoring RSWA was developed in 1992 by Lapierre and Montplaisir [42], here referred to as the “Montreal” method. According to this method, a 20-s REM sleep epoch is defined as tonic when sustained chin EMG lasts more than 50% of the epoch duration, with an amplitude at least twice the background EMG activity or greater than 10 µV. The percentage of tonic REM sleep epochs defines the tonic EMG density. Phasic activity is defined as bursts of EMG activity with an amplitude exceeding 4 times the background and lasting 0.1–10 s. Phasic EMG activity can occur in either atonic or tonic REM sleep epochs and is scored by dividing a 20-s [42] or a 30-s [43] REM sleep epoch into 2-s mini-epochs. The percentage of 2-s mini-epochs containing phasic chin EMG activity defines the phasic chin EMG density. The authors validated this method in a population of 80 subjects with clinical diagnoses of RBD, identifying RSWA if there was a tonic EMG density ≥30% or phasic chin EMG density ≥15% [43][44].
The Barcelona Innsbruck group (SINBAR) validated their visual scoring methods in 30 RBD patients (15 iRBD and 15 associated with Parkinson’s Disease (PD)) and 30 control subjects, analyzing REM sleep EMG activity in 11 different muscles, including the mentalis muscle and the flexor digitorum superficialis muscle (FDS), bilaterally in the upper limbs [45]. Tonic chin EMG activity was scored in 30-s epochs only in the mentalis muscle according to the Montreal method [44]. Phasic EMG events were scored separately in each EMG channel in 3-s mini-epochs as bursts of EMG activity lasting 0.1–5 s with an amplitude at least twice the background. Each 3-s mini-epoch was scored as having or not having phasic EMG activity. When phasic EMG activity bursts emerged in 3-s mini-epochs with tonic EMG activity, the amplitude of the phasic activity had to be at least twice the tonic background EMG activity. Moreover, each 3-s mini-epoch was scored as having or not having any EMG activity, containing irrespectively tonic or phasic activity or a combination of them [45]. The SINBAR group suggested the following cut-off values with the best specificity and sensitivity to identify RBD: >16.3% of the 3-s mini-epochs containing phasic chin EMG activity, >18% of the 3-s mini-epochs having any chin EMG activity, >32% of the 3-s mini-epochs having any chin EMG activity combined with bilateral FDS phasic EMG activity, and >27% of the 30-s epochs having any chin EMG activity combined with bilateral FDS phasic EMG activity [45]. The latter cut-off value was suggested as the most reliable evidence-based cut-off to distinguish RBD patients from their controls in the ICSD-3 [1].
Another group developed a visual scoring method for short-duration phasic EMG activity during REM sleep, known as the phasic EMG metric (PEM), in the submentalis, tibialis anterior, and brachioradialis muscles for the identification of RBD [46]. The PEM was defined as the percentage of 2.5-s intervals containing any discrete bursts of EMG activity lasting ≥100 ms with an amplitude at least 4 times higher than the pre-sleep background activity. The RBD patients showed higher levels of PEM activity in the mentalis and brachioradialis muscles than their controls [46].
Another visual scoring method was developed by McCarter et al. [47] by combining the tonic, phasic, and any EMG activity in the submentalis and tibialis anterior muscles, which was validated in a cohort of 20 RBD patients with PD, 20 patients with obstructive sleep apnea, and 20 patients with snoring. The tonic EMG activity was scored in each 30-s REM sleep epoch according to the Montreal method [44]. Phasic and any EMG activity were scored in 3-s mini-epochs and identified as any activity lasting 0.1–14.9 s with an amplitude 4 times greater than the background [47]. The authors provided the following cut-off values for the definition of RSWA using 3-s mini-epochs: >15.5% phasic, 21.6% any, and 1.2% tonic submentalis EMG activity; 30.2% for phasic and any tibialis anterior EMG activity; and 37.9% phasic and 43.4% any EMG activity in the combined submentalis and tibialis anterior muscles [47]. However, the same authors further validated their method in a population of 45 consecutive patients, including 15 with iRBD, finding very similar RSWA diagnostic cut-off values [48].
Recently, a comparative study has shown a very high degree of agreement between the two visual scoring methods (i.e., the Montreal and SINBAR methods) when considering tonic or any EMG activity in a large cohort of PD patients with and without RBD [49].
Further larger studies are needed to assess a reliable and usable visual scoring method for quantifying RSWA in RBD or both iRBD and association with alpha-synucleinopathies and to assess which muscle groups or combinations of them are most reliable to identify RSWA.
3.3. Automatic Scoring
Visual scoring of RSWA is a time-consuming process and may be challenging even for expert scorers. Therefore, multiple efforts have been made to develop computerized methods for the quantification and detection of RSWA [50][51].
The method that, to date, counts the highest number of published articles is the so-called REM Atonia Index (RAI), proposed by Ferri et al. in 2008 [52][53][54]. It is based on the analysis of the amplitude of the rectified submentalis EMG signal in 1-s mini-epochs. Theoretically, the RAI varies from 0 (total loss of atonia) to 1 (complete atonia), and the threshold for definite RSWA is below 0.8 [53]. This method has shown a relatively low night-to-night variability [55], good sensitivity and specificity, and good correlation to the visual methods [43]. The RAI was validated for patients with iRBD [53] and PD-RBD [49][56], and it is also reliable in patients with RBD and comorbid OSA [48] and for the detection of RSWA in narcolepsy [57][58], including children [4].
All automatic methods described here have been reported to have acceptable sensitivity and specificity in their studies of presentation. However, their comparison showed variable performance, making it impossible to assess the optimal method [59], although the RAI seemed to be the most reliable in the identification of RBD. Further comparative studies on larger numbers of recordings are required to identify the most reliable algorithm in order to introduce it in the scoring guidelines for everyday clinical practice. However, automatic methods seem to perform with an accuracy comparable to that of visual methods.
4. Other Neurophysiological Features
4.1. Electroencephalography during Wakefulness and Sleep
Patients with iRBD show some electroencephalographic (EEG) features that have also been reported in neurodegenerative disorders, such as PD and dementia with Lewy bodies (DLB) [60]. These changes typically include diffuse EEG slowing during wakefulness, although this is more evident over the occipital scalp areas [61]. Notably, this cortical slowdown has been associated with cognitive decline, thus suggesting a parallel between neuropsychological and electrophysiological impairment. It should be remarked that higher absolute delta and theta power in all cortical regions were able to identify iRBD patients with a higher risk of short-term progression into alpha-synucleinopathy [61].
4.2. Transcranial Magnetic Stimulation
Non-invasive brain stimulation techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation, are widely used to functionally investigate the neural pathways and brain network in vivo, also providing prognostic measures and neuromodulatory activity [62][63][64][65][66][67][68]. Accordingly, the data from EEG and functional neuroimaging, during both wakefulness and sleep, have suggested the involvement of different transmission systems which seem to be impaired in synucleinopathy, including PD, multiple system atrophy, and DLB [69][70].
Coherently, a number of studies have been carried out to evaluate the electrophysiological pattern of cortical excitability, neural plasticity, and functional connectivity to TMS in different sleep disorders [71][72][73][74]. In particular, most of the evidence converged on changes to both short-latency intracortical inhibition (SICI) and intracortical facilitation (ICF) in patients with PD, including those in the early stages, in terms of decreased SICI and reduced ICF. Translationally, this would indicate a disinhibition and a hypofacilitation of the motor cortex that are compatible with an impaired GABA and glutamate neurotransmission, respectively [75][76][77][78][79][80][81].
On the contrary, to date, only two TMS studies have been performed with iRBD patients. The first [82] reported an impairment of the short-latency afferent inhibition (SAI) that may support the hypothesis of cholinergic dysfunction in subjects developing cognitive impairment. This result was confirmed in a second study by the same research group on patients with RBD associated to PD, which can be viewed as the correlate of a cholinergic involvement at the basis of the cognitive decline observed in these subjects [83]. The authors concluded that cholinergic degeneration significantly contributes to non-motor Parkinsonian features, also raising the possibility that RBD increases the risk of cognitive impairment in PD [82]. Taken together, these findings may help to achieve an early recognition of the cognitive decline in PD and stimulate future “TMS-targeted” treatments [69]. The second very recent study [84] found that in patients still asymptomatic for a neurodegenerative disorder, changes in ICF and, to a lesser extent, SICI might precede the onset of future neurodegeneration. Furthermore, SICI correlated with the muscle tone alteration during REM sleep, possibly supporting the proposed RBD model of retrograde influence on the cortex from the brainstem [84].
Overall, although still limited, TMS studies provide novel insights into the mechanisms underlying cortical dysfunction in PD and RBD and might open future therapeutic avenues. When integrated with clinical, neuroimaging, and sleep-related data, TMS findings are suggestive of an electrocortical imbalance with multiple neurotransmission pathways involved in RBD. Longitudinal studies are required to verify whether the abnormalities detected at this early stage do correlate with the clinical progression of RBD.
4.3. Vestibular Evoked Myogenicpotentials
The brainstem has been identified as a key player in the pathophysiological process leading to the development of iRBD. Vestibular evoked myogenic potentials (VEMPs) (cervical (cVEMP), masseter (mVEMP), and ocular (oVEMP) VEMPs) are a group of neurophysiological tools for the extensive and indirect assessment of the brainstem’s function along its whole length, thus also allowing the identification of subclinical brainstem abnormalities in clinically and radiologically silent regions [85].
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014.
- Schenck, C.H.; Bundlie, S.R.; Ettinger, M.G.; Mahowald, M.W. Chronic behavioral disorders of human REM sleep: A new category of parasomnia. Sleep 1986, 9, 293–308.
- Dauvilliers, Y.; Schenck, C.H.; Postuma, R.B.; Iranzo, A.; Luppi, P.H.; Plazzi, G.; Montplaisir, J.; Boeve, B. REM sleep behaviour disorder. Nat. Rev. Dis. Primers 2018, 4, 19.
- Antelmi, E.; Pizza, F.; Vandi, S.; Neccia, G.; Ferri, R.; Bruni, O.; Filardi, M.; Cantalupo, G.; Liguori, R.; Plazzi, G. The spectrum of REM sleep-related episodes in children with type 1 narcolepsy. Brain 2017, 140, 1669–1679.
- Antelmi, E.; Pizza, F.; Donadio, V.; Filardi, M.; Sosero, Y.L.; Incensi, A.; Vandi, S.; Moresco, M.; Ferri, R.; Marelli, S.; et al. Biomarkers for REM sleep behavior disorder in idiopathic and narcoleptic patients. Ann. Clin. Transl. Neurol. 2019, 6, 1872–1876.
- Dauvilliers, Y.; Jennum, P.; Plazzi, G. Rapid eye movement sleep behavior disorder and rapid eye movement sleep without atonia in narcolepsy. Sleep Med. 2013, 14, 775–781.
- Antelmi, E.; Pizza, F.; Franceschini, C.; Ferri, R.; Plazzi, G. REM sleep behavior disorder in narcolepsy: A secondary form or an intrinsic feature? Sleep Med. Rev. 2020, 50, 101254.
- Miglis, M.G.; Adler, C.H.; Antelmi, E.; Arnaldi, D.; Baldelli, L.; Boeve, B.F.; Cesari, M.; Dall’Antonia, I.; Diederich, N.J.; Doppler, K.; et al. Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol. 2021, 20, 671–684.
- Cesari, M.; Heidbreder, A.; St Louis, E.K.; Sixel-Doring, F.; Bliwise, D.L.; Baldelli, L.; Bes, F.; Fantini, M.L.; Iranzo, A.; Knudsen-Heier, S.; et al. Video-polysomnography procedures for diagnosis of rapid eye movement sleep behavior disorder (RBD) and the identification of its prodromal stages: Guidelines from the International RBD Study Group. Sleep 2021.
- Manni, R.; Ratti, P.L.; Terzaghi, M. Secondary “incidental” REM sleep behavior disorder: Do we ever think of it? Sleep Med. 2011, 12 (Suppl. S2), S50–S53.
- Zambelis, T.; Paparrigopoulos, T.; Soldatos, C.R. REM sleep behaviour disorder associated with a neurinoma of the left pontocerebellar angle. J. Neurol. Neurosurg. Psychiatry 2002, 72, 821–822.
- Provini, F.; Vetrugno, R.; Pastorelli, F.; Lombardi, C.; Plazzi, G.; Marliani, A.F.; Lugaresi, E.; Montagna, P. Status dissociatus after surgery for tegmental ponto-mesencephalic cavernoma: A state-dependent disorder of motor control during sleep. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 719–723.
- McKenna, D.; Peever, J. Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 636–644.
- Jouvet, M. Recherches sur les structures nerveuses et les mecanismes responsables des differentes phases du sommeil physiologique. Arch. Ital. Biol. 1962, 100, 125–206.
- Sakai, K.; Koyama, Y. Are there cholinergic and non-cholinergic paradoxical sleep-on neurones in the pons? Neuroreport 1996, 7, 2449–2453.
- Vanni-Mercier, G.; Sakai, K.; Lin, J.S.; Jouvet, M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch. Ital. Biol. 1989, 127, 133–164.
- Clement, O.; Sapin, E.; Berod, A.; Fort, P.; Luppi, P.H. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic. Sleep 2011, 34, 419–423.
- Valencia Garcia, S.; Libourel, P.A.; Lazarus, M.; Grassi, D.; Luppi, P.H.; Fort, P. Genetic inactivation of glutamate neurons in the rat sublaterodorsal tegmental nucleus recapitulates REM sleep behaviour disorder. Brain 2017, 140, 414–428.
- Luppi, P.H.; Fort, P. Sleep-wake physiology. Handb. Clin. Neurol. 2019, 160, 359–370.
- Varin, C.; Luppi, P.H.; Fort, P. Melanin-concentrating hormone-expressing neurons adjust slow-wave sleep dynamics to catalyze paradoxical (REM) sleep. Sleep 2018, 41.
- Sapin, E.; Lapray, D.; Berod, A.; Goutagny, R.; Leger, L.; Ravassard, P.; Clement, O.; Hanriot, L.; Fort, P.; Luppi, P.H. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS ONE 2009, 4, e4272.
- Hayashi, Y.; Kashiwagi, M.; Yasuda, K.; Ando, R.; Kanuka, M.; Sakai, K.; Itohara, S. Cells of a common developmental origin regulate REM/non-REM sleep and wakefulness in mice. Science 2015, 350, 957–961.
- Luppi, P.H.; Clement, O.; Sapin, E.; Gervasoni, D.; Peyron, C.; Leger, L.; Salvert, D.; Fort, P. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med. Rev. 2011, 15, 153–163.
- McGinty, D.J.; Harper, R.M. Dorsal raphe neurons: Depression of firing during sleep in cats. Brain Res. 1976, 101, 569–575.
- Nakamura, Y.; Goldberg, L.J.; Chandler, S.H.; Chase, M.H. Intracellular analysis of trigeminal motoneuron activity during sleep in the cat. Science 1978, 199, 204–207.
- Brooks, P.L.; Peever, J.H. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J. Neurosci. 2012, 32, 9785–9795.
- Soja, P.J.; Lopez-Rodriguez, F.; Morales, F.R.; Chase, M.H. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: Effect of strychnine on motoneuron properties. J. Neurosci. 1991, 11, 2804–2811.
- Burgess, C.; Lai, D.; Siegel, J.; Peever, J. An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle. J. Neurosci. 2008, 28, 4649–4660.
- Lai, Y.Y.; Kodama, T.; Siegel, J.M. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: An in vivo microdialysis study. J. Neurosci. 2001, 21, 7384–7391.
- Fenik, V.B.; Davies, R.O.; Kubin, L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am. J. Respir. Crit. Care Med. 2005, 172, 1322–1330.
- Peever, J.; Luppi, P.H.; Montplaisir, J. Breakdown in REM sleep circuitry underlies REM sleep behavior disorder. Trends Neurosci. 2014, 37, 279–288.
- Fraigne, J.J.; Torontali, Z.A.; Snow, M.B.; Peever, J.H. REM Sleep at its Core—Circuits, Neurotransmitters, and Pathophysiology. Front. Neurol. 2015, 6, 123.
- Boissard, R.; Gervasoni, D.; Schmidt, M.H.; Barbagli, B.; Fort, P.; Luppi, P.H. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: A combined microinjection and functional neuroanatomical study. Eur. J. Neurosci. 2002, 16, 1959–1973.
- Peever, J.; Fuller, P.M. Neuroscience: A Distributed Neural Network Controls REM Sleep. Curr. Biol. 2016, 26, R34–R35.
- Berry, R.B.; Quan, S.F.; Abreu, A.R.; Bibbs, M.L.; DelRosso, L.; Harding, S.M.; Mao, M.-M.; Plante, D.T.; Pressman, M.R.; Troester, M.R.; et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6; American Academy of Sleep Medicine: Darien, IL, USA, 2020.
- Hoque, R.; Chesson, A.L., Jr. Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: Literature review, qualitative scoring, and comparative analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2010, 6, 79–83.
- Postuma, R.B.; Gagnon, J.F.; Tuineaig, M.; Bertrand, J.A.; Latreille, V.; Desjardins, C.; Montplaisir, J.Y. Antidepressants and REM sleep behavior disorder: Isolated side effect or neurodegenerative signal? Sleep 2013, 36, 1579–1585.
- Lee, K.; Baron, K.; Soca, R.; Attarian, H. The Prevalence and Characteristics of REM Sleep without Atonia (RSWA) in Patients Taking Antidepressants. J. Clin. Sleep Med. 2016, 12, 351–355.
- McCarter, S.J.; St Louis, E.K.; Sandness, D.J.; Arndt, K.; Erickson, M.; Tabatabai, G.; Boeve, B.F.; Silber, M.H. Antidepressants Increase REM Sleep Muscle Tone in Patients with and without REM Sleep Behavior Disorder. Sleep 2015, 38, 907–917.
- Iranzo, A.; Santamaria, J. Bisoprolol-induced rapid eye movement sleep behavior disorder. Am. J. Med. 1999, 107, 390–392.
- Ferri, R.; Mogavero, M.P.; Bruni, O.; Plazzi, G.; Schenck, C.H.; DelRosso, L.M. Increased Chin Muscle Tone during All Sleep Stages in Children Taking SSRI Antidepressants and in Children with Narcolepsy Type 1. Sleep 2021, 44, zsab147.
- Lapierre, O.; Montplaisir, J. Polysomnographic features of REM sleep behavior disorder: Development of a scoring method. Neurology 1992, 42, 1371–1374.
- Ferri, R.; Gagnon, J.F.; Postuma, R.B.; Rundo, F.; Montplaisir, J.Y. Comparison between an automatic and a visual scoring method of the chin muscle tone during rapid eye movement sleep. Sleep Med. 2014, 15, 661–665.
- Montplaisir, J.; Gagnon, J.F.; Fantini, M.L.; Postuma, R.B.; Dauvilliers, Y.; Desautels, A.; Rompre, S.; Paquet, J. Polysomnographic diagnosis of idiopathic REM sleep behavior disorder. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 2044–2051.
- Frauscher, B.; Iranzo, A.; Gaig, C.; Gschliesser, V.; Guaita, M.; Raffelseder, V.; Ehrmann, L.; Sola, N.; Salamero, M.; Tolosa, E.; et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep 2012, 35, 835–847.
- Bliwise, D.L.; Rye, D.B. Elevated PEM (phasic electromyographic metric) rates identify rapid eye movement behavior disorder patients on nights without behavioral abnormalities. Sleep 2008, 31, 853–857.
- McCarter, S.J.; St Louis, E.K.; Duwell, E.J.; Timm, P.C.; Sandness, D.J.; Boeve, B.F.; Silber, M.H. Diagnostic thresholds for quantitative REM sleep phasic burst duration, phasic and tonic muscle activity, and REM atonia index in REM sleep behavior disorder with and without comorbid obstructive sleep apnea. Sleep 2014, 37, 1649–1662.
- McCarter, S.J.; St Louis, E.K.; Sandness, D.J.; Duwell, E.J.; Timm, P.C.; Boeve, B.F.; Silber, M.H. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med. 2017, 33, 23–29.
- Figorilli, M.; Ferri, R.; Zibetti, M.; Beudin, P.; Puligheddu, M.; Lopiano, L.; Cicolin, A.; Durif, F.; Marques, A.; Fantini, M.L. Comparison Between Automatic and Visual Scorings of REM Sleep Without Atonia for the Diagnosis of REM Sleep Behavior Disorder in Parkinson Disease. Sleep 2017, 40.
- Frauscher, B.; Ehrmann, L.; Hogl, B. Defining muscle activities for assessment of rapid eye movement sleep behavior disorder: From a qualitative to a quantitative diagnostic level. Sleep Med. 2013, 14, 729–733.
- Neikrug, A.B.; Ancoli-Israel, S. Diagnostic tools for REM sleep behavior disorder. Sleep Med. Rev. 2012, 16, 415–429.
- Ferri, R.; Manconi, M.; Plazzi, G.; Bruni, O.; Vandi, S.; Montagna, P.; Ferini-Strambi, L.; Zucconi, M. A quantitative statistical analysis of the submentalis muscle EMG amplitude during sleep in normal controls and patients with REM sleep behavior disorder. J. Sleep Res. 2008, 17, 89–100.
- Ferri, R.; Rundo, F.; Manconi, M.; Plazzi, G.; Bruni, O.; Oldani, A.; Ferini-Strambi, L.; Zucconi, M. Improved computation of the atonia index in normal controls and patients with REM sleep behavior disorder. Sleep Med. 2010, 11, 947–949.
- Ferri, R.; Bruni, O.; Fulda, S.; Zucconi, M.; Plazzi, G. A quantitative analysis of the submentalis muscle electromyographic amplitude during rapid eye movement sleep across the lifespan. J. Sleep Res. 2012, 21, 257–263.
- Ferri, R.; Marelli, S.; Cosentino, F.I.; Rundo, F.; Ferini-Strambi, L.; Zucconi, M. Night-to-night variability of automatic quantitative parameters of the chin EMG amplitude (Atonia Index) in REM sleep behavior disorder. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2013, 9, 253–258.
- Ferri, R.; Fulda, S.; Cosentino, F.I.; Pizza, F.; Plazzi, G. A preliminary quantitative analysis of REM sleep chin EMG in Parkinson’s disease with or without REM sleep behavior disorder. Sleep Med. 2012, 13, 707–713.
- Ferri, R.; Franceschini, C.; Zucconi, M.; Vandi, S.; Poli, F.; Bruni, O.; Cipolli, C.; Montagna, P.; Plazzi, G. Searching for a marker of REM sleep behavior disorder: Submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy. Sleep 2008, 31, 1409–1417.
- Olesen, A.N.; Cesari, M.; Christensen, J.A.E.; Sorensen, H.B.D.; Mignot, E.; Jennum, P. A comparative study of methods for automatic detection of rapid eye movement abnormal muscular activity in narcolepsy. Sleep Med. 2018, 44, 97–105.
- Cesari, M.; Christensen, J.A.E.; Kempfner, L.; Olesen, A.N.; Mayer, G.; Kesper, K.; Oertel, W.H.; Sixel-Doring, F.; Trenkwalder, C.; Sorensen, H.B.D.; et al. Comparison of computerized methods for rapid eye movement sleep without atonia detection. Sleep 2018, 41, zsy133.
- Aarsland, D. Cognitive impairment in Parkinson’s disease and dementia with Lewy bodies. Parkinsonism Relat. Disord. 2016, 22 (Suppl. S1), S144–S148.
- Ferini-Strambi, L.; Fasiello, E.; Sforza, M.; Salsone, M.; Galbiati, A. Neuropsychological, electrophysiological, and neuroimaging biomarkers for REM behavior disorder. Expert Rev. Neurother. 2019, 19, 1069–1087.
- Agarwal, S.; Koch, G.; Hillis, A.E.; Huynh, W.; Ward, N.S.; Vucic, S.; Kiernan, M.C. Interrogating cortical function with transcranial magnetic stimulation: Insights from neurodegenerative disease and stroke. J. Neurol. Neurosurg. Psychiatry 2019, 90, 47–57.
- Di Lazzaro, V.; Bella, R.; Benussi, A.; Bologna, M.; Borroni, B.; Capone, F.; Chen, K.S.; Chen, R.; Chistyakov, A.V.; Classen, J.; et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2021, 132, 2568–2607.
- Nardone, R.; Sebastianelli, L.; Versace, V.; Brigo, F.; Golaszewski, S.; Pucks-Faes, E.; Saltuari, L.; Trinka, E. Effects of repetitive transcranial magnetic stimulation in subjects with sleep disorders. Sleep Med. 2020, 71, 113–121.
- Lanza, G. Repetitive TMS for sleep disorders: Are we ready? Sleep Med. 2020, 71, 111–112.
- Lanza, G. Repetitive TMS for the “cognitive tsunami” of sleep deprivation. Sleep Med. 2021, 77, 279–280.
- Cantone, M.; Lanza, G.; Vinciguerra, L.; Puglisi, V.; Ricceri, R.; Fisicaro, F.; Vagli, C.; Bella, R.; Ferri, R.; Pennisi, G.; et al. Age, Height, and Sex on Motor Evoked Potentials: Translational Data From a Large Italian Cohort in a Clinical Environment. Front. Hum. Neurosci. 2019, 13, 185.
- Fisicaro, F.; Lanza, G.; Cantone, M.; Ferri, R.; Pennisi, G.; Nicoletti, A.; Zappia, M.; Bella, R.; Pennisi, M. Clinical and Electrophysiological Hints to TMS in De Novo Patients with Parkinson’s Disease and Progressive Supranuclear Palsy. J. Pers. Med. 2020, 10, 274.
- Nardone, R.; Brigo, F.; Versace, V.; Holler, Y.; Tezzon, F.; Saltuari, L.; Trinka, E.; Sebastianelli, L. Cortical afferent inhibition abnormalities reveal cholinergic dysfunction in Parkinson’s disease: A reappraisal. J. Neural Transm. 2017, 124, 1417–1429.
- Lanza, G.; Ferri, R. The neurophysiology of hyperarousal in restless legs syndrome: Hints for a role of glutamate/GABA. Adv. Pharmacol. 2019, 84, 101–119.
- Lanza, G.; Lanuzza, B.; Arico, D.; Cantone, M.; Cosentino, F.I.I.; Bella, R.; Pennisi, G.; Ferri, R.; Pennisi, M. Impaired short-term plasticity in restless legs syndrome: A pilot rTMS study. Sleep Med. 2018, 46, 1–4.
- Magalhaes, S.C.; Kaelin-Lang, A.; Sterr, A.; do Prado, G.F.; Eckeli, A.L.; Conforto, A.B. Transcranial magnetic stimulation for evaluation of motor cortical excitability in restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2015, 16, 1265–1273.
- Cantone, M.; Lanza, G.; Ranieri, F.; Opie, G.M.; Terranova, C. Editorial: Non-invasive Brain Stimulation in the Study and Modulation of Metaplasticity in Neurological Disorders. Front. Neurol. 2021, 12, 721906.
- Lanza, G.; Scalise, A. The present and the future of Transcranial Magnetic Stimulation in Restless Legs Syndrome. Sleep Med. 2020, 71, 122–123.
- Bares, M.; Kanovsky, P.; Klajblova, H.; Rektor, I. Intracortical inhibition and facilitation are impaired in patients with early Parkinson’s disease: A paired TMS study. Eur. J. Neurol. 2003, 10, 385–389.
- Lefaucheur, J.P. Motor cortex dysfunction revealed by cortical excitability studies in Parkinson’s disease: Influence of antiparkinsonian treatment and cortical stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2005, 116, 244–253.
- Vacherot, F.; Attarian, S.; Vaugoyeau, M.; Azulay, J.P. A motor cortex excitability and gait analysis on Parkinsonian patients. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 2747–2755.
- Kacar, A.; Filipovic, S.R.; Kresojevic, N.; Milanovic, S.D.; Ljubisavljevic, M.; Kostic, V.S.; Rothwell, J.C. History of exposure to dopaminergic medication does not affect motor cortex plasticity and excitability in Parkinson’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2013, 124, 697–707.
- Leon-Sarmiento, F.E.; Rizzo-Sierra, C.V.; Bayona, E.A.; Bayona-Prieto, J.; Doty, R.L.; Bara-Jimenez, W. Novel mechanisms underlying inhibitory and facilitatory transcranial magnetic stimulation abnormalities in Parkinson’s disease. Arch. Med Res. 2013, 44, 221–228.
- Kobayashi, M.; Ohira, T.; Mihara, B.; Fujimaki, T. Changes in intracortical inhibition and clinical symptoms after STN-DBS in Parkinson’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 2031–2037.
- Fernandez-Lago, H.; Bello, O.; Mora-Cerda, F.; Montero-Camara, J.; Fernandez-Del-Olmo, M.A. Treadmill Walking Combined With Anodal Transcranial Direct Current Stimulation in Parkinson Disease: A Pilot Study of Kinematic and Neurophysiological Effects. Am. J. Phys. Med. Rehabil. 2017, 96, 801–808.
- Nardone, R.; Bergmann, J.; Kunz, A.; Christova, M.; Brigo, F.; Tezzon, F.; Trinka, E.; Golaszewski, S. Cortical afferent inhibition is reduced in patients with idiopathic REM sleep behavior disorder and cognitive impairment: A TMS study. Sleep Med. 2012, 13, 919–925.
- Nardone, R.; Bergmann, J.; Brigo, F.; Christova, M.; Kunz, A.; Seidl, M.; Tezzon, F.; Trinka, E.; Golaszewski, S. Functional evaluation of central cholinergic circuits in patients with Parkinson’s disease and REM sleep behavior disorder: A TMS study. J. Neural Transm. 2013, 120, 413–422.
- Lanza, G.; Arico, D.; Lanuzza, B.; Cosentino, F.I.I.; Tripodi, M.; Giardina, F.; Bella, R.; Puligheddu, M.; Pennisi, G.; Ferri, R.; et al. Facilitatory/inhibitory intracortical imbalance in REM sleep behavior disorder: Early electrophysiological marker of neurodegeneration? Sleep 2020, 43, zsz242.
- Magnano, I.; Pes, G.M.; Cabboi, M.P.; Pilurzi, G.; Ginatempo, F.; Achene, A.; Salis, A.; Conti, M.; Deriu, F. Comparison of brainstem reflex recordings and evoked potentials with clinical and MRI data to assess brainstem dysfunction in multiple sclerosis: A short-term follow-up. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2016, 37, 1457–1465.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
24 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




