Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | LeiSun Lei Sun | -- | 1624 | 2022-06-23 12:09:14 | | | |
| 2 | Rita Xu | -3 word(s) | 1621 | 2022-06-24 03:35:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xu, R.; Hu, D.; Lin, J.; Tang, J.; Zhan, R.; Liu, G.L.G.; Sun, L.; Sun, L. Anthracycline Dutomycin. Encyclopedia. Available online: https://encyclopedia.pub/entry/24379 (accessed on 08 February 2026).
Xu R, Hu D, Lin J, Tang J, Zhan R, Liu GLG, et al. Anthracycline Dutomycin. Encyclopedia. Available at: https://encyclopedia.pub/entry/24379. Accessed February 08, 2026.
Xu, Ruoxuan, Dinghang Hu, Jinlian Lin, Jie Tang, Ruoting Zhan, Guiyou Liu Guiyou Liu, Lei Sun, Leisun Sun. "Anthracycline Dutomycin" Encyclopedia, https://encyclopedia.pub/entry/24379 (accessed February 08, 2026).
Xu, R., Hu, D., Lin, J., Tang, J., Zhan, R., Liu, G.L.G., Sun, L., & Sun, L. (2022, June 23). Anthracycline Dutomycin. In Encyclopedia. https://encyclopedia.pub/entry/24379
Xu, Ruoxuan, et al. "Anthracycline Dutomycin." Encyclopedia. Web. 23 June, 2022.
Copy Citation
Anthracycline dutomycin is a tetracyclic quinone glycoside produced by Streptomyces minoensis NRRL B-5482. SW91 is a C-12 demethylated dutomycin derivative. In vitro cytotoxicity and apoptosis assays of these two compounds were conducted to demonstrate their antiproliferation activities.
dutomycin
anthracycline
aromatic methyl group
DNA-binding agents
molecular docking
1. Background
Anthracycline quinones, discovered in the 1960s, are planar molecules consisting of a rigid hydrophobic tetracycline ring, with a daunosamine sugar attached through a glycosidic bond [1]. Due to the fact of their electrophilic properties, some natural quinones interact directly with cellular nucleophiles, including soluble and protein thiol groups, and may inhibit key processes in cells [2][3]. Quinones work in three main ways. Firstly, they intercalate between base pairs to block the DNA replication and RNA transcription [4]. Secondly, anthracycline quinones stabilize the binding of nuclear topoisomerase II to DNA, resulting in protein-associated DNA strand breaks [5]. Thirdly, cytotoxic quinones exert their effects by forming redox-reactive species [6]. Anthracyclines, such as doxorubicin [7][8], have been considered as antitumor components in clinical practice for more than 50 years [9]. Due to the fact of their success in treating various types of cancer, approximately 2000 anthracycline analogs have been developed as potential anticancer agents [10], including emerrubicin, epirubicin, kamycin, pirarubicin, amrubicin, valmycin, and zorubicin [11].
Anthracyclines show good antitumor activity, though it is believed to be the main cause of chemotherapy-induced cardiotoxicity [12][13][14]. The mechanisms of the cardiotoxicity of anthracyclines are still elusive. The effects of reactive oxygen species (ROS) and topoisomerase-II (Top II) are the key factors leading to myocardial injury. ROS form toxic free radicals and reactive nitrogen species to increase nitrosative stress and mitochondrial dysfunction, which is primarily related to cardiotoxicity. Oxidative stress also leads to activation of molecular pathways leading to cardiomyocyte loss through necrosis and apoptosis. The Top II–doxorubicin–DNA ternary cleavage complex induces DNA double-stranded breaks (DSBs), leading to cardiomyocyte death [15]. Top II also acts as a regulator of various gene expression, including those involved in mitochondrial biogenesis and antioxidant function [15]. In order to enhance pharmacological activity and reduce the cardiotoxicity of anthracyclines, many studies focused on structural modifications have been undertaken [16]. For example, doxorubicin is hydroxy-substituted at the C-14 position of the A ring, which is more effective in lymphoma, sarcoma, and broad-spectrum solid tumors [11]. Due to the glycosyl group, the 4’-deoxy analog (esorubicin) shows superior pharmacological properties compared to DOX containing daunorubicin [17][18]. In addition, there are a small number of examples of modification on the D ring [18]. Idarubicin and doxorubicin differ by a methoxy substitution at the C-4 position, DOX is mainly used in solid tumors and lymphomas, and IDA is mainly used in the treatment of acute leukemia [19].
It is known that the antitumor activity of anthracyclines is mainly through the insertion of the nearly planar aromatic ring skeleton between the base pairs of DNA. The intercalation binding force mainly comes from the hydrophobicity or embedded in the minor groove region of the DNA double helix. In this way, the drug–DNA complex breaks the DNA strands and destroys the replication template [17]. In addition, anthracyclines also interfere with Top II, resulting in DNA strand breaks and the formation of ternary drug–DNA–Top II complexes, thereby affecting the structure of DNA [20][21].
Anthracycline dutomycin was isolated from Streptomyces minoensis NRRL B-5482, with a tetracyclic quinone core structure and two sugar moieties, β-D-amylogenose and α-L-axenose (β-D-amicetose and α-L-axenose) (Figure 1) [22]. By knocking out the methyltransferase DutMT1, a C-12 demethylated dutomycin derivative (named SW91) was obtained (Figure 1). SW91 demonstrates higher anti-MRSA activity compared to dutomycin [23]. It has previously been reported that adding a methyl group to safinamide results in a more than 20-fold increase in potency and a nearly 1000-fold difference in selectivity [24]. Researchers found that the 12-methyl group of dutomycin had a critical effect on its antitumor activity.
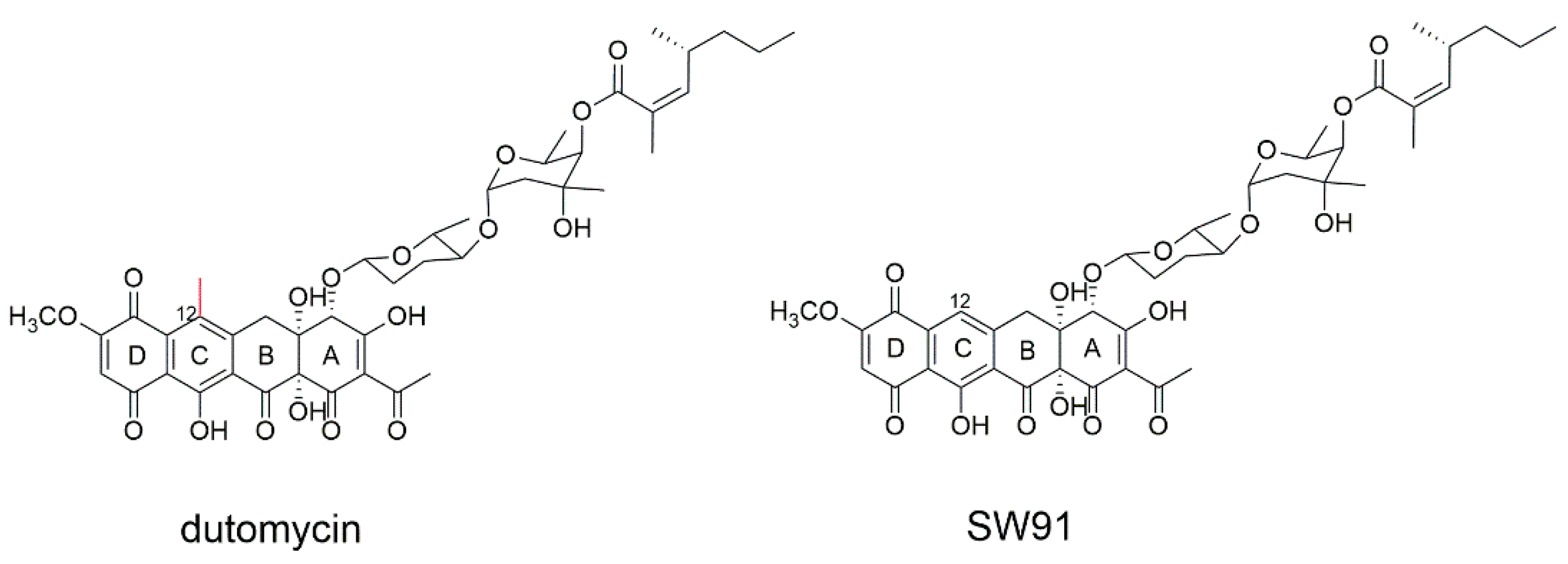
Figure 1. The structure of dutomycin and SW91.
2. Cytotoxicity Studies
HepG2 and Smmc-7721 were used for the in vitro assay of antiproliferative activity of dutomycin and SW91. In order to obtain the IC50 of the two compounds in different cell lines more accurately, researchers used different concentrations to study them. The dose-dependent tumor suppression is shown in the histograms of survival rate (p < 0.01). In HepG2 cells, the IC50 values of dutomycin and SW91 are 6.59 (Figure 2a) and 39.81 μM (Figure 2b), respectively. The corresponding IC50 values in Smmc-7721 cells were 7.58 (Figure 2c) and 33.20 μM (Figure 2d), respectively. The results show that both dutomycin and SW91 inhibit proliferation of tumor cells. The tumor suppression effect of dutomycin on cancer cells, Smmc-7721 or HepG2, is higher than that of SW91. Since SW91 is a C-12 demethylated dutomycin derivative, it implies that the presence of a methyl group at the C-12 position has a great impact on the antiproliferation bioactivity of dutomycin.
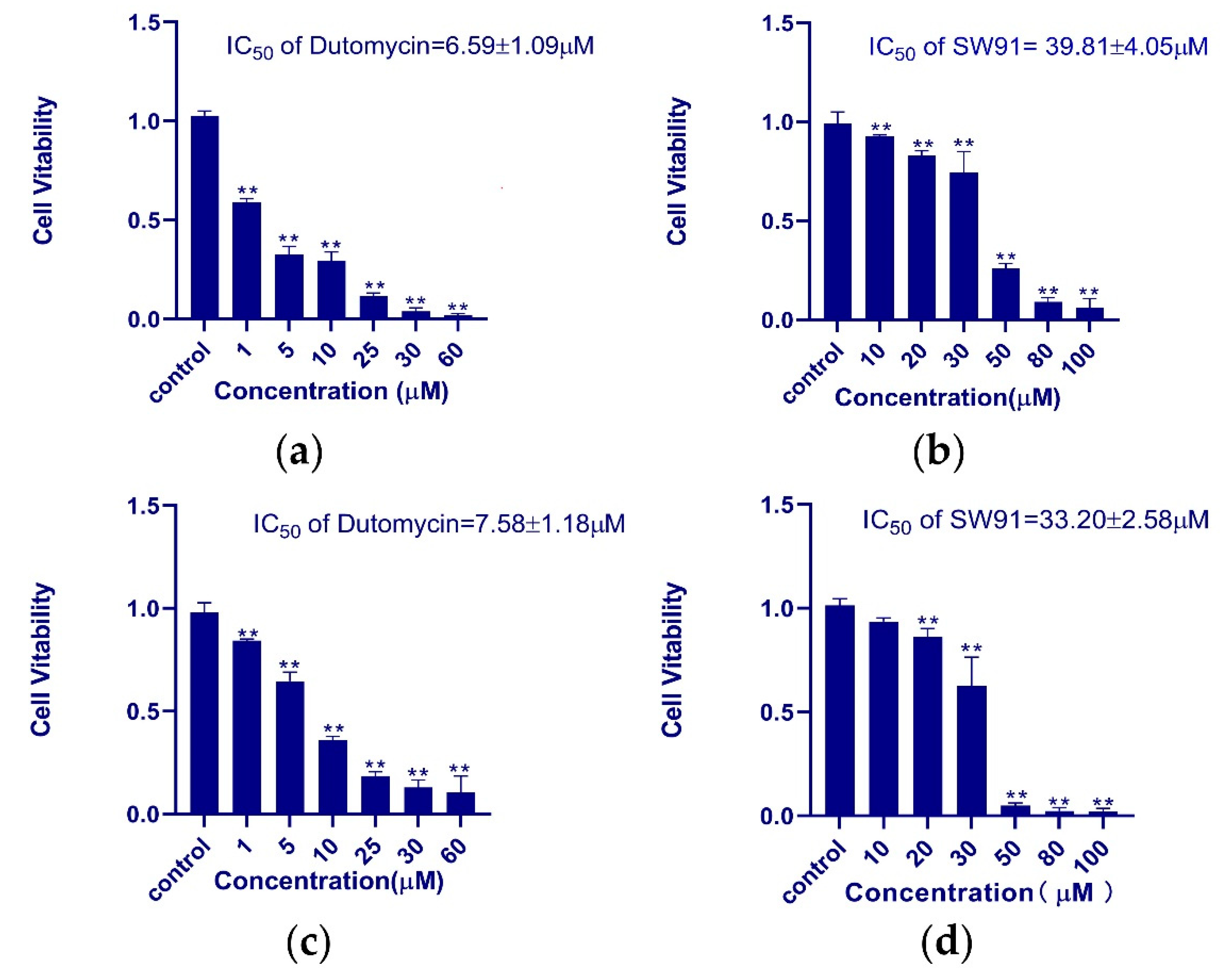
Figure 2. The histogram of the survival rate of dutomycin and SW91 in different cell lines: (a) cell survival rate in cancer cell HepG2 at different concentrations of dutomycin (μM); (b) cell survival rate under different concentrations of SW91 (μM) in cancer cell HepG2; (c) cell survival rate at different concentrations of dutomycin (μM) in cancer cell Smmc-7721; (d) cell survival rate at different concentrations of SW91 (μM) in cancer cell Smmc-7721. p-Values were considered significant compared to the control if they were less than 0.05, and they are indicated throughout using asterisks: ** p < 0.01.
3. Apoptosis Analysis
In order to demonstrate the morphology of cells, the fluorescent dye Hoechst 33342 was used for nucleus staining (Figure 3a). The cells treated with dutomycin (Figure 3b) or SW91 (Figure 3c), in the field of vision, were observed with few cell nucleus replications. While the blank control group had five cells with obvious mitosis in the field of view, which are marked with white arrows. This indicates that dutomycin and SW91 affect the nucleus replication and division. Since most anthracyclines demonstrate antitumor activity by inhibiting DNA replication, it is likely that dutomycin and its derivatives share similar mechanisms.
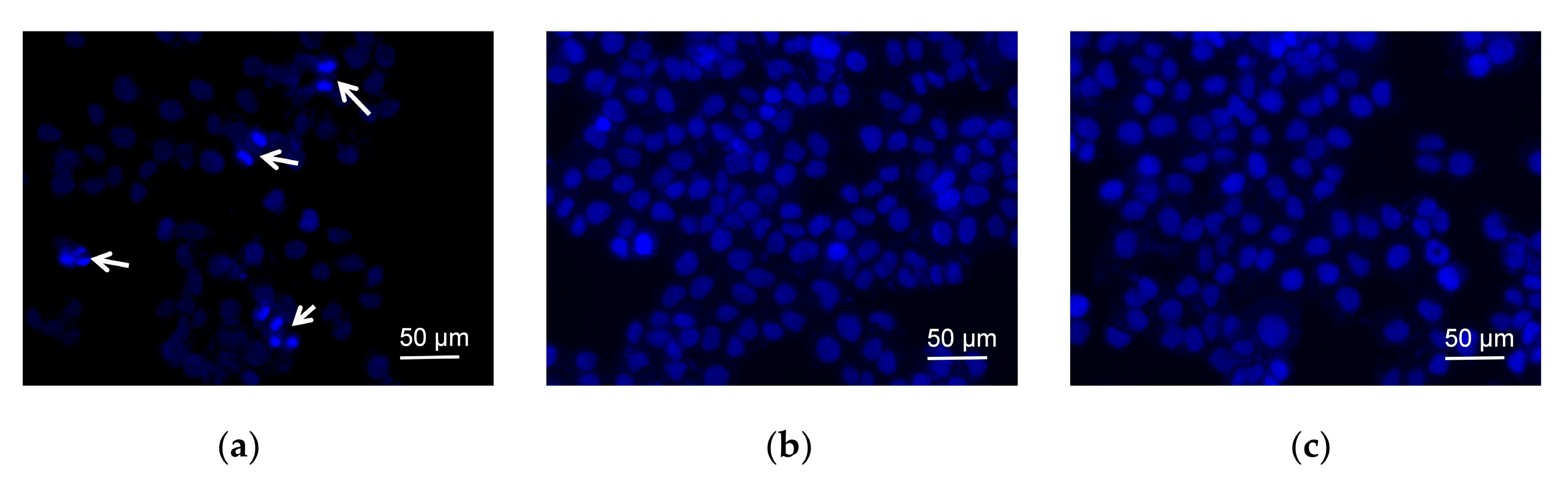
Figure 3. Fluorescent dye Hoechst-33342 was used for nuclear staining and changes were observed after adding different compounds; (a) negative control nucleus morphology and cell number; (b) nucleus morphology and cell number after adding SW91; (c) nucleus morphology and cell number after adding dutomycin.
Apoptotic cells were detected using FITC-labeled Annexin V (Annexin VFITC) to detect everted phosphatidylserine (PS). Propidium iodide (PI) fluoresces when it enters necrotic cells or cells lost in late apoptosis. The apoptosis analyzed using the software FlowJo7.6.5. The results show that cell apoptosis is dose-dependent, and the percentage of apoptosis between the two groups of compounds is statistically significant (p < 0.01). As shown in Table 1, when treated with 20 μM dutomycin or SW91, the apoptosis rate of Smmc-7721 cells was 76.37 ± 5.19% and 10.45 ± 0.52%, respectively. The apoptotic rate induced by dutomycin was 6.31 times higher than that induced by SW91, which demonstrates that dutomycin has much higher antiproliferative activity than SW91.
Table 1. Apoptosis degree of SW91 and dutomycin in cancer cell Smmc-7721.
| Concentration (μM) | 20 | 30 | 40 | 50 |
|---|---|---|---|---|
| Dutomycin | 76.37 ± 5.19 | 86.37 ± 0.25 | 93.43 ± 0.17 | 96.41 ± 0.47 |
| SW91 | 10.45 ± 0.52 | 23.70 ± 2.25 | 45.10 ± 7.05 | 65.10 ± 4.12 |
4. Cycle Assay
To show the effect on cell cycle caused by dutomycin or SW91, HepG2 and Smmc-7721 were synchronized in the G0/G1 period by starvation. A flow chart of cells over time after administration is shown in Figure 4. Compared to the control, there was a significant delay for the cells treated with drugs to start the G2 phase. The blank control starts DNA replication after 4 h incubation, whereas the drug-treated groups were detected at 12 h.
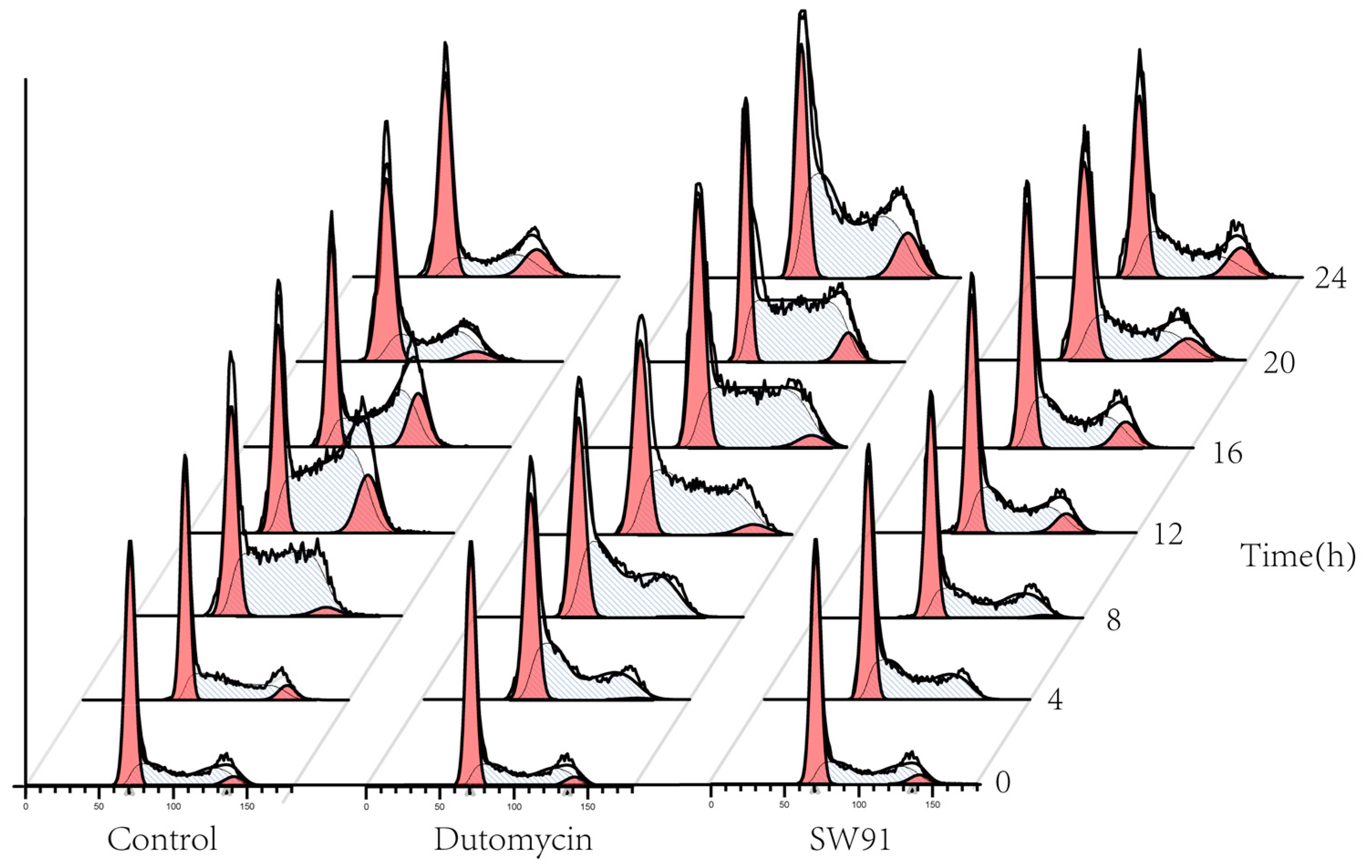
Figure 4. Graph of cell cycle changes for different compounds over time.
The DNA content was monitored every 4 h after synchronization release. In the control group, the content of G1 gradually decreased, while the S phase increased, the highest being 61.77% at 8 h and the lowest being 29.20% at 20 h, recovering to 38.16% at 24 h. In the G2 period, from 0.12% at the beginning, it gradually reached 15.48% at 16 h, and recovered to 8.26% at 24 h, and the proportions in each period returned to normal (Figure 5a). The change in the cycle after adding the drug dutomycin was different: The S phase reached the highest proportion of 61.25% at 20 h and decreased by 60.62% at 24 h. This phenomenon was more obvious in the G2 period: the proportion in the first 16 h was basically unchanged, and there was a significant increase at 20 h, which indicates that the S-phase block phenomenon had obviously occurred (Figure 5b). The change in the period after the addition of the drug SW91 was also different. The proportion of the S period remained basically unchanged within 24 h of the detection, with only a slight increase in 12 h, and a slight decrease in the subsequent period. Compared with the control, the change was more obvious in the G2 period, which has been showing a slow growth state, reaching the highest proportion of 13.77% in 24 h. The results show that, compared to dutomycin, SW91 has a weaker ability to block cells in the S phase, with some cells turning to the G2 phase within 8 h (Figure 5c).
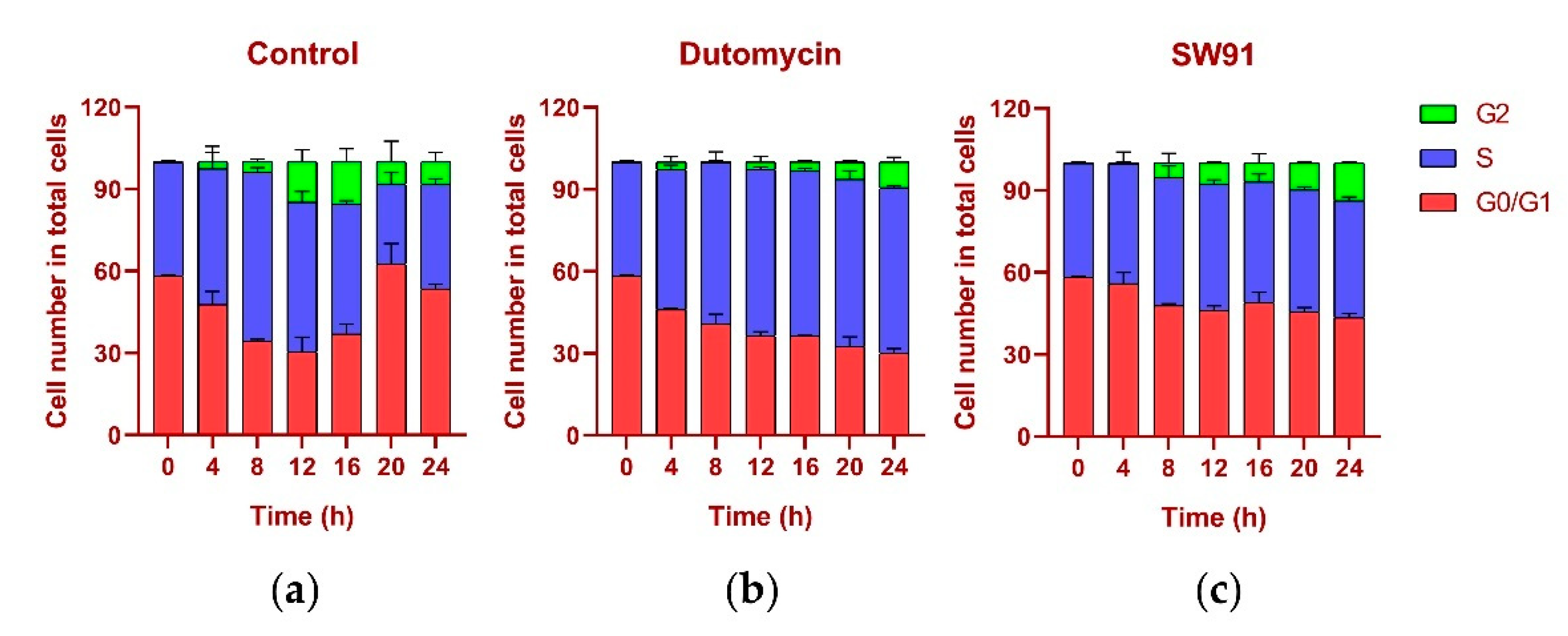
Figure 5. Cell cycle percentage stacking plots for different time periods 0-24 (h) to illustrate the cycles of cells treated with drugs at their IC50 concentrations: (a) plot of cell cycle percentage stacking at different time periods 0–24 (h) after addition of dutomycin; (b) plot of cell cycle percentage stacking at different time periods 0–24 (h) after addition of SW91; (c) plot of cell cycle percentage stacking at different time periods 0–24 (h) after addition of control.
In terms of cycle changes, dutomycin blocks cells in the S phase, so the proportion of the S phase is significantly increased, and the G2 phase is delayed. Compared to dutomycin, the ability of SW91 to block cells in the S phase was weaker, showing that the proportion of the S phase remained basically unchanged, and the increase in the G2 phase was faster but still lower than that of the control group. This part of the experiment showed that both act on the S phase of cells, and dutomycin is more efficient.
References
- Edwardson, D.W.; Narendrula, R.; Chewchuk, S.; Mispel-Beyer, K.; Mapletoft, J.P.J.; Parissenti, A.M. Role of drug metabolism in the cytotoxicity and clinical efficacy of anthracyclines. Curr. Drug Metab. 2015, 16, 412–426.
- Di Monte, D.; Ross, D.; Bellomo, G.; Eklöw, L.; Orrenius, S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch. Biochem. Biophys. 1984, 235, 334–342.
- Ross, D.; Thor, H.; Orrenius, S.; Moldeus, P. Interaction of menadione (2-methyl-1,4-naphthoquinone) with glutathione. Chem. Biol. Interact. 1985, 55, 177–184.
- Pigram, W.J.; Fuller, W.; Hamilton, L.D. Stereochemistry of intercalation: Interaction of daunomycin with DNA. Nat. New Biol. 1972, 235, 17–19.
- Pommier, Y.; Schwartz, R.E.; Zwelling, L.A.; Kohn, K.W. Effects of DNA intercalating agents on topoisomerase ii induced DNA strand cleavage in isolated mammalian cell nuclei. Biochemistry 1985, 24, 6406–6410.
- Verrax, J.; Beck, R.; Dejeans, N.; Glorieux, C.; Sid, B.; Pedrosa, R.C.; Benites, J.; Vásquez, D.; Valderrama, J.A.; Calderon, P.B. Redox-active quinones and ascorbate: An innovative cancer therapy that exploits the vulnerability of cancer cells to oxidative stress. Anti-Cancer Agents Med. Chem. 2011, 11, 213–221.
- Orsini, F.; Pavelic, Z.; Mihich, E. Increased primary cell-mediated immunity in culture subsequent to adriamycin or daunorubicin treatment of spleen donor mice. Cancer Res. 1977, 37, 1719–1726.
- Mattarollo, S.R.; Loi, S.; Duret, H.; Ma, Y.; Zitvogel, L.; Smyth, M.J. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011, 71, 4809–4820.
- Singal, P.K.; Iliskovic, N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905.
- Hortobágyi, G.N. Anthracyclines in the treatment of cancer. Overv. Drugs 1997, 54, 1–7.
- Martins-Teixeira, M.B.; Carvalho, I. Antitumour anthracyclines: Progress and perspectives. ChemMedChem 2020, 15, 933–948.
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225.
- Bhagat, A.; Kleinerman, E.S. Anthracycline-induced cardiotoxicity: Causes, mechanisms, and prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192.
- Henriksen, P.A. Anthracycline cardiotoxicity: An update on mechanisms, monitoring and prevention. Heart 2018, 104, 971–977.
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642.
- Sobczuk, P.; Czerwińska, M.; Kleibert, M.; Cudnoch-Jędrzejewska, A. Anthracycline-induced cardiotoxicity and renin-angiotensin-aldosterone system—From molecular mechanisms to therapeutic applications. Heart Fail. Rev. 2022, 27, 295–319.
- Temperini, C.; Cirilli, M.; Aschi, M.; Ughetto, G. Role of the amino sugar in the DNA binding of disaccharide anthracyclines: Crystal structure of the complex MAR70/d(CGATCG). Bioorg. Med. Chem. 2005, 13, 1673–1679.
- Wadler, S.; Fuks, J.Z.; Wiernik, P.H. Phase I and II agents in cancer therapy: I. Anthracyclines and related compounds. J. Clin. Pharmacol. 1986, 26, 491–509.
- Menna, P.; Recalcati, S.; Cairo, G.; Minotti, G. An introduction to the metabolic determinants of anthracycline cardiotoxicity. Cardiovasc. Toxicol. 2007, 7, 80–85.
- Zunino, F.; Pratesi, G.; Perego, P. Role of the sugar moiety in the pharmacological activity of anthracyclines: Development of a novel series of disaccharide analogs. Biochem. Pharmacol. 2001, 61, 933–938.
- Cipollone, A.; Berettoni, M.; Bigioni, M.; Binaschi, M.; Cermele, C.; Monteagudo, E.; Olivieri, L.; Palomba, D.; Animati, F.; Goso, C.; et al. novel anthracycline oligosaccharides: Influence of chemical modifications of the carbohydrate moiety on biological activity. Bioorg. Med. Chem. 2002, 10, 1459–1470.
- Xuan, L.J.; Xu, S.H.; Zhang, H.L.; Xu, Y.M.; Chen, M.Q. Dutomycin, a new anthracycline antibiotic from streptomyces. J. Antibiot. 1992, 45, 1974–1976.
- Sun, L.; Wang, S.; Zhang, S.; Shao, L.; Zhang, Q.; Skidmore, C.; Chang, C.-W.T.; Yu, D.; Zhan, J. Characterization of three tailoring enzymes in dutomycin biosynthesis and generation of a potent antibacterial analogue. ACS Chem. Biol. 2016, 11, 1992–2001.
- Leonetti, F.; Capaldi, C.; Pisani, L.; Nicolotti, O.; Muncipinto, G.; Stefanachi, A.; Cellamare, S.; Caccia, C.; Carotti, A. Solid-phase synthesis and insights into structure-activity relationships of safinamide analogues as potent and selective inhibitors of type b monoamine oxidase. J. Med. Chem. 2007, 50, 4909–4916.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
942
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
24 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




