Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vivey Mogotladi Phasha | -- | 1761 | 2022-06-22 12:40:49 | | | |
| 2 | Sirius Huang | Meta information modification | 1761 | 2022-06-23 02:52:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Use of Kojic Acid in Cosmetics. Encyclopedia. Available online: https://encyclopedia.pub/entry/24341 (accessed on 04 March 2026).
Phasha V, Senabe J, Ndzotoyi P, Okole B, Fouche G, Chuturgoon A. Use of Kojic Acid in Cosmetics. Encyclopedia. Available at: https://encyclopedia.pub/entry/24341. Accessed March 04, 2026.
Phasha, Vivey, Jeremiah Senabe, Phatheka Ndzotoyi, Blessed Okole, Gerda Fouche, Anil Chuturgoon. "Use of Kojic Acid in Cosmetics" Encyclopedia, https://encyclopedia.pub/entry/24341 (accessed March 04, 2026).
Phasha, V., Senabe, J., Ndzotoyi, P., Okole, B., Fouche, G., & Chuturgoon, A. (2022, June 22). Use of Kojic Acid in Cosmetics. In Encyclopedia. https://encyclopedia.pub/entry/24341
Phasha, Vivey, et al. "Use of Kojic Acid in Cosmetics." Encyclopedia. Web. 22 June, 2022.
Copy Citation
In 1907, Saito discovered Kojic Acid (KA), a natural product; it has since become one of the most investigated skin-lightening agents. KA inhibits tyrosinase and has been commonly researched in the cosmetic industry. It is incorporated in many kinds of cosmetic products.
Kojic acid
hyper-pigmentation
tyrosinase
melasma
cytotoxicity
sensitization
1. Introduction
Global researchers are exploring the development of various groups of tyrosinase inhibitors, as they have a huge impact on the cosmetic and pharmaceutical industries and the global economy [1]. One of the main considerations for tyrosinase inhibitors is safety, particularly when applied regularly and not considering the recommended dosages. Some challenges experienced with the use of these agents include high cytotoxicity and instability, thus necessitating additional research to improve their applications as ingredients in cosmetics [1][2].
KA inhibits tyrosinase and has been commonly researched in the cosmetic industry [1][2][3][4][5][6]. KA and its derivatives have radioprotective, skin-lightening, anti-inflammatory, anti-oxidant, and anti-proliferative properties [1][7]. Due to its tyrosinase inhibitory activity, KA can protect the skin from ultraviolet (UV) rays, reduce hyperpigmentation, and prevent melanin formation [1][8]. It is produced by several types of fungi, and it is also a by-product of the fermentation process of certain foods, such as soy sauce and sake [1].
KA is incorporated in many kinds of cosmetic products [9]. The CIR approved KA as safe at a concentration of 1% in cosmeceutical products [9]. The existing dermatological safety data also support the safety of KA at a concentration of 2% in cosmeceuticals, indicating that a limit of 2% might be applicable [9].
2. Physical and Chemical Properties of Kojic Acid
KA chemical structure is defined as 5-hydroxy-2-hydroxymethyl-γ-pyrone [10]. It is also known as 5-hydroxy-2-hydroxymethyl-4H-pyran-4-one and 5-hydroxy-2-hydroxymethyl-4-pyrone [10][11].
The crystals of KA are acicular and colorless, and they sublime in a vacuum with no variations. KA is soluble in some organic solvents, such as ethyl acetate, water, and ethanol. It is unlikely to dissolve in ether, alcohol ether mixture, chloroform, and pyridine [10][12].
The melting point of KA lies between 151–154 degrees Celsius (°C) [10][12]. According to Cryoscopy Technique, the molecular weight of KA is 142.1, and its maximum peak of UV Absorption Spectra is at 260–284 nanometers (nm).
KA is a weak acid with multidimensional uses. It reacts at every position on the ring, thus forming several products, such as ethers, pyridines, metal chelates, azodyes, mannich base, pyridines, and cyanoethylation products [10][12]. Several chemical reactions of KA have been studied over the years since its isolation. At the carbon 5 position of this compound, the hydroxy becomes a weak acid, therefore forming salts when reacted with metals such as cadmium, nickel, copper, zinc, and sodium due to its weakly acidic properties [10]. Introducing new functional groups on the KA skeleton via the hydroxy ketone or hydroxyalkyl allows for the improvement in the solubility of subsequent complexes [13].
3. Safety Assessment of Kojic Acid
Several studies have been conducted to evaluate the safety and efficiency of tyrosinase inhibitors in the cosmeceutical and medicinal industries [2][14]. These inhibitors are important due to their ability to prevent pigmentation disorders.
The safety studies performed recommend the use of KA in topical preparations at a concentration of 1% or less because, in these ranges, it shows efficient and safe properties [2].
KA is listed as an ‘additive’ in the Inventory of Cosmetic Ingredients database of Europe, and in countries such as Switzerland, there is a ban on the use of KA as a cosmetic ingredient [15]. Other skin sensitization data have reinforced the safety of KA at a dosage of 2% in leave-on products [1][14][16]
KA depigmented black guinea pig skin at a dosage of 4%, but these results were not observed at 1%. The CIR Expert Panel also concluded that at concentrations below 1%, dermal sensitization and skin lightening would not be seen, thus recommending the usage at 1% [9][17].
KA was also not found to be toxic in chronic, reproductive, genotoxicity, and acute studies [14] Another study on acute, chronic, reproductive, and genotoxic aspects by Aytemir and Karakay (2012), revealed that KA was not toxic, as it is slowly released into the human skin; it would not reach the limit of tumor promotion and low carcinogenicity [1].
KA produced from bearberry leaves is safe and efficient for topical use, although it is not satisfactorily effective and not stable for use in cosmeceuticals [1][7][18].
A survey conducted by the cosmetics industry also indicated that it is safe for use at a concentration ranging from (0.1 to 2.0)% [14][16]
A determination by the European Commission’s Scientific Committee on Consumer Products (SCCP) indicated that KA is safe for use at a concentration limit of 1% (Burnett et al., 2010; Mann et al., 2018).
Available data to date indicate that KA is safe for application as a skin lightening agent at a concentration of 1% in leave-on creams [2][9].
Various investigations have shown that when used at 1 and 2%, KA does not show any ocular or allergenic sensitivity. It was also declared a group 3 carcinogen by the International Agency For Research On Cancer (IARC) [2]. In addition, the Food and Drug Administration (FDA) does not permit the use of KA in pharmaceutical products without a prescription; however, the SCCP reported that the dose of KA should be 1.0% in skincare products and that it is not a toxicant in generative, chronic, acute, and genotoxicity form [2].
Despite the extensive benefits of using KA in topical products, there are some disadvantages, including contact dermatitis and possible photo-damaging of the skin [2]. These are outlined in Table 1 below.
Table 1. Positive and negative effects of using KA for skin lightening.
| Benefits | Disadvantages |
|---|---|
|
|
4. Kojic Acid Derivatives
KA causes skin irritation, has inadequate inhibitory activity, and is not stable during storage, thus reducing its use in cosmetic products [1][15][19][20][21]. To overcome these disadvantages, many derivatives of KA have been produced [22]. These derivatives were produced to improve stability and solubility.
By modifying the alcoholic hydroxyl group of KA, it can be converted into an ester, glycoside, amino acid derivatives, hydroxyphenyl ether, or tripeptide derivatives [1].
The KA derivatized through an ethylene linkage of the phosphonate with aldehyde using intermediates derived from KA is about eight times more effective in tyrosinase inhibitory activity than KA [1][10].
Recently, methods for the synthesis of a variety of KA derivatives, such as KA di-palmitate, KA ester, and KA laureate, have been reported [10][23]. KA peptides have also been investigated as potent tyrosinase inhibitors [24].
5. Cosmetic Applications of Kojic Acid
KA is a popular ingredient and is used by various industries globally [25][26]. In the cosmetic industry, it is used as a topical treatment for skin conditions such as spots, melasma, and patches of light brown color resulting from post-inflammatory hyperpigmentation [25][27][28][29][30].
KA has skin-lightening properties and can act as a UV protector, whereby it prevents the development of hyperpigmentation in human skin by inhibiting the formation of melanin through the prevention of tyrosinase formation [10][26][28].
KA also enhances the shelf life of cosmetic products through its preservative properties [31]. It is normally combined with alpha-hydroxy acid in the formulation of skin-lightening products to manage age spots and lightened freckles. Due to its manganese and zinc complexes, it can be used as a radioprotective agent against γ-ray [10]. Figure 1 below summarizes the above-discussed applications of KA.
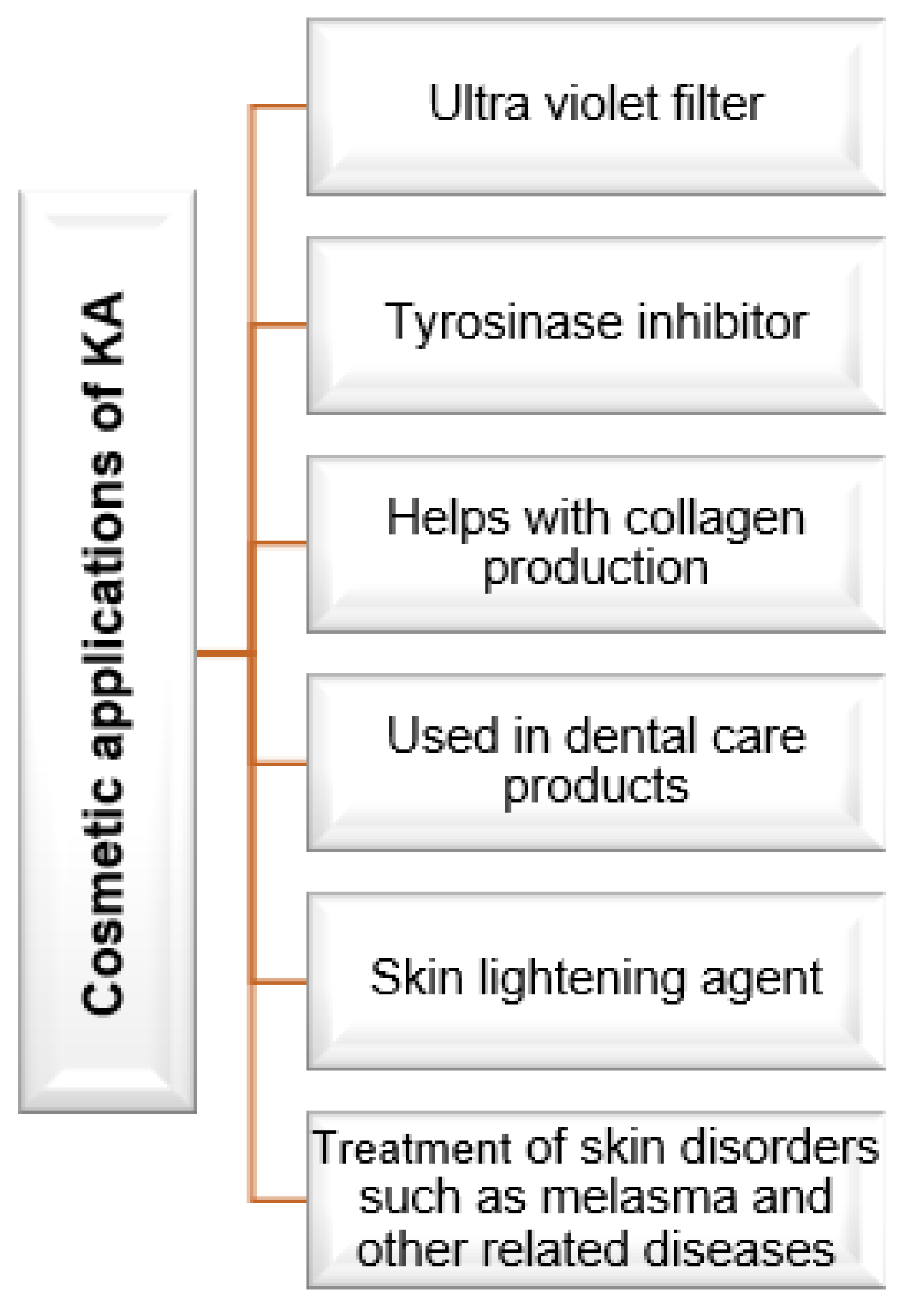
Figure 1. Cosmetic applications of kojic acid [2].
6. Biological Activities of Kojic Acid
The available literature indicates that this ingredient has various biological activities, and they are listed below.
6.1. Antibacterial and Antimicrobial Activity
KA has antifungal and antibacterial properties [32]. Preceding antimicrobial activity assays showed that KA was more active against Gram-negative bacteria than against Gram-positive bacteria [1]. However, some of its derivatives have shown conflicting effects distinct from KA’s antibacterial activity [1].
When used in cosmetic products, KA can prevent the growth of microorganisms and can be used as a preservative [33].
The antimicrobial activity of the ethyl acetate (EtOAc) extract of Colletotrichum gloeosporioides and its major compound KA were evaluated, and the results showed considerable antimicrobial activity against all tested strains [29]. When tested against various microorganisms, KA was most active against Micrococcus luteus and least active against Pseudomonas aeruginosa [29].
Due to its antifungal properties, KA is incorporated into some antifungal products to improve their effectiveness [29]. Furthermore, it could be useful in treating various fungal infections of the skin as well as yeast infections, ringworm, athlete’s foot, and candidiasis [29].
KA and its derivatives have potent activity against bacteria such as Staphylococcus aureus [10]. The KA derivatives were also validated for antifungal activities against Fusarium oxysporum, Rhizoctonia solani, and Pythium graminicola, which cause fungal infections such as fusarium wilt, sheath blight, and seedling blight. Besides its antibiotic properties, KA also shows some insecticidal activity against Spodoptera frugiperda and Heliothis zea insects [10].
6.2. Antioxidant Activity
KA has anti-oxidant properties [7] and is used as a substitute for hydroquinone (HQ) for skin lightening by the cosmeceutical industry [1][34].
Studies by Zhang et al., (2017) showed that KA improved oxidative stress response in fungi, thus showing the anti-oxidant ability of this metabolite [34]. Other preceding bioactivity studies on KA revealed that it has anti-oxidant properties [29].
The correlation between anti-melanogenic activity with oxidative effects of KA and KA esters was investigated by Lajis et al.,2012. The results of the study showed that both KA and its esters had mild free radical scavenging activities at concentrations ranging from 1.95 to 1000 μg/mL [35].
6.3. Anti-Inflammatory Activity
KA may exert slight anti-inflammatory effects that may favorably improve by subsequent derivation of chosen KA derivatives [36]. In a recent study to develop a safe anti-inflammatory compound, a derivative of KA and p-coumaric acid were synthesized, as they are known to have anti-inflammatory properties. The study suggested that the anti-inflammatory action of KA was enhanced by the addition of cinnamate moiety in p-coumaric acid as an hydrophobic part [37]. A study assessed the anti-inflammatory activity of KA and p-coumaric acid and revealed that both possessed anti-inflammatory properties [34].
In another study, KA and its two novel derivatives were isolated from the fungus Aspergillus versicolor and evaluated for their anti-inflammatory effects [38], showing that KA has a moderate anti-inflammatory effect, while the derivatives 1 and 2 were found to have improved effects [38].
6.4. Tyrosinase Inhibition Activity
KA is regarded as one of the best skin-lightening agents in the beauty industry [1]. It exerts a slow and effective reversible inhibition of tyrosinase, thus preventing melanin formation, and also plays an important role in cellular melanin formation [1]. According to available data from various studies, it can be used as a monotherapy or combined with other agents [27]. In Japan, this ingredient is known as a quasi-drug [14][39]. Due to its ability to inhibit tyrosinase activity, KA has been used in several studies as a standard [1][24][40][41][42][43][44][45]. Another study revealed that KA inhibits melanosis by interfering with the uptake of oxygen required for enzymatic browning [1].
References
- Aytemir, D.M.; Karakay, G. Kojic Acid Derivatives. Med. Chem. Drug Des. 2012, 1–27.
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593.
- Desai, S. Effect of a Tranexamic Acid, Kojic Acid, and Niacinamide Containing Serum on Facial Dyschromia: A Clinical Evaluation. J. Drugs Dermatol. 2019, 18, 454–459.
- Lourith, N.; Kanlayavattanakul, M.; Ruktanonchai, U. Formulation and stability of Moringa oleifera Oil Microemulsion. Soft Mater. 2016, 14, 64–71.
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58.
- Hasil, A.; Mehmood, A.; Noureen, S.; Ahmed, M. Experimental and theoretical charge density analysis of skin whitening agent kojic acid. J. Mol. Struct. 2020, 1216, 128295.
- Van Tran, V.; Loi Nguyen, T.; Moon, J.Y.; Lee, Y.C. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: Challenges and development strategies. Chem. Eng. J. 2018, 368, 88–114.
- Bashir, F.; Sultana, K.; Khalid, M.; Rabia, H.; Khan, H. Kojic Acid: A Comprehensive Review Abstract: Keywords: The Applications of Kojic Acid Kojic acid. AJAHAS 2021, 6, 13–17.
- Chambers, C. Opinion on kojic acid. Sci. Committees Consum. Prod. 2008, 1–79.
- Rosfarizan, M.; Mohamed, M.S.; Suhaili, N.; Salleh, M.M.; Ariff, A.B. Kojic acid: Applications and development of fermentation process for production. Biotechnol. Mol. Biol. Rev. 2010, 5, 24–37.
- Zborowski, K.; Gryboś, R.; Proniewicz, L.M. Determination of the most stable structures of selected hydroxypyrones and their cations and anions. J. Mol. Struct. THEOCHEM 2003, 639, 87–100.
- Chaudhary, J. Production Technology and Applications of Kojic Acid. Annu. Res. Rev. Biol. 2014, 4, 3165–3196.
- Annan, N.A.; Butler, I.S.; Titi, H.M.; El-Lazeik, Y.; Jean-Claude, B.J.; Mostafa, S.I. DNA interaction and anticancer evaluation of new zinc(II), ruthenium(II), rhodium(III), palladium(II), silver(I) and platinum(II) complexes based on kojic acid; X-ray crystal structure of ·H2O. Inorganica Chim. Acta 2019, 487, 433–447.
- Burnett, C.L. Final report of the safety assessment of kojic acid as used in cosmetics. Int. J. Toxicol. 2010, 29.
- Masse, M.O.; Duvallet, V.; Borremans, M.; Goeyens, L. Identification and quantitative analysis of kojic acid and arbutine in skin-whitening cosmetics. Int. J. Cosmet. Sci. 2001, 23, 219–232.
- Mann, T. Inhibition of Human Tyrosinase Requires Molecular Motifs Distinctively Different from Mushroom Tyrosinase. J. Invest. Dermatol. 2018, 138, 1601–1608.
- Bergfeld, W.F. Final Report of the Cosmetic Ingredient Review Expert Panel Safety Assessment of Simmondsia Chinensis (Jojoba) Seed Oil , Simmondsia Chinensis (Jojoba) Seed Wax, Hydrogenated Jojoba Oil , Hydrolyzed Jojoba Esters , Isomerized Jojoba Oil, Jojoba Este. Cosmet. Ingred. Rev. 2008, 1–32. Available online: https://online.personalcarecouncil.org/ctfa-static/online/lists/cir-pdfs/FR525.pdf (accessed on 10 May 2022).
- Velliou, E.G. In vitro Studies. Model. Optim. Control Biomed. Syst. 2017, 233–264.
- Buschmann, H.J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191.
- Hashemi, H. Climate change and the future of water management in Iran. Middle East Critique 2015, 24, 307–323.
- Kwak, S.Y.; Choi, H.R.; Park, K.C.; Lee, Y.S. Kojic acid-amino acid amide metal complexes and their melanogenesis inhibitory activities. J. Pept. Sci. 2011, 17, 791–797.
- Seyedeh Mahdieh Hashemi, S.E. Kojic acid-derived tyrosinase inhibitors: Synthesis and bioactivity. Pharm. Biomed. Res. 2015, 1, 1–17. Available online: http://pbr.mazums.ac.ir (accessed on 10 May 2022).
- Hariri, R.; Saeedi, M.; Akbarzadeh, T. Naturally occurring and synthetic peptides: Efficient tyrosinase inhibitors. J. Pept. Sci. 2021, 27, 1–10.
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S. H Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418.
- Gomes, C.; Silva, A.C.; Marques, A.C.; Lobo, S.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33.
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27.
- De, A. Hyperpigmentation Case Kojic Acid in the Management of Melasma : An Effective Therapeutic Weapon. Indian J. Dermatol. 2019, 1–4.
- Davis, E.C.; Calender, V.D. A Review of the Epidemiology, Clinical Features, and Treatment Options in Skin of Color Year study population prevalence rank. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31.
- Nurunnabi, T. Antimicrobial activity of kojic acid from endophytic fungus Colletotrichum gloeosporioides isolated from Sonneratia apetala, a mangrove plant of the Sundarbans. Asian Pac. J. Trop. Med. 2018, 11, 350–354.
- Tetali, B.; Fahs, F.M.; Mehregan, D. Popular over-the-counter cosmeceutical ingredients and their clinical efficacy. Int. J. Dermatol. 2020, 59, 393–405.
- El-Kady, I.A.; Zohri, A.N.A.; Hamed, S.R. Kojic Acid Production from Agro-Industrial By-Products Using Fungi. Biotechnol. Res. Int. 2014, 2014, 642385.
- Owolabi, J.O.; Fabiyi, O.S.; Adelakin, L.A.; Ekwerike, M.C. Effects of skin lightening cream agents - hydroquinone and kojic acid, on the skin of adult female experimental rats. Clin. Cosmet. Investig. Dermatol. 2020, 13, 283–289.
- Wang, X.R. Intercalation assembly of kojic acid into Zn-Ti layered double hydroxide with antibacterial and whitening performances. Chinese Chem. Lett. 2019, 30, 919–923.
- Zhang, J. Kojic acid-mediated damage responses induce mycelial regeneration in the basidiomycete Hypsizygus marmoreus. PLoS ONE 2017, 12, e0187351.
- Lajis, A.F.B.; Hamid, M.; Ariff, A.B. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012, 2012, 952452.
- Brtko, J.; Rondahl, L.; Ficková, M.; Hudecová, D.; Eybl, V.; Uher, M. Kojic acid and its derivatives: History and present state of art. Cent. Eur. J. Public Health 2004, 12, S16–S17.
- Lee, M.; Rho, H.S.; Choi, K. Anti-inflammatory Effects of a P-coumaric Acid and Kojic Acid Derivative in LPS-stimulated RAW264.7 Macrophage Cells. Biotechnol. Bioprocess Eng. 2019, 24, 653–657.
- Li, T.; Liang, J.; Liu, L.; Shi, F.; Jia, X.; Li, M. Fitoterapia Novel kojic acid derivatives with anti-inflammatory effects from Aspergillus versicolor. Fitoterapia 2021, 154, 105027.
- Ando, H.; Matsui, M.S.; Ichihashi, M. Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders. Int. J. Mol. Sci. 2010, 11, 2566–2575.
- Li, J. Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem. 2021, 224, 113744.
- Aygun, R.B.; Zengin, G.; Yıldıztugay, E.; Jugreet, S.; Yılmaz, M.A.; Mahomoodally, F.M. Chemical characterization, anti-oxidant and anti-enzymatic properties of extracts from two Silene species: A focus on different plant parts and extraction methods. Process Biochem. 2022, 116, 206–213.
- Bang, E.J. In vitro and in vivo evidence of tyrosinase inhibitory activity of a synthesized (Z)-5-(3-hydroxy-4-methoxybenzylidene)-2-thioxothiazolidin-4-one (5-HMT). Exp. Dermatol. 2019, 28, 734–737.
- Pavic, A.; Ilic-Tomic, T.; Glamočlija, J. Unravelling anti-melanogenic potency of edible mushrooms laetiporus sulphureus and agaricus silvaticus in vivo using the zebrafish model. J. Fungi 2021, 7, 834.
- Meziant, L.; Bachir-bey, M.; Bensouici, C.; Saci, F.; Boutiche, M.; Louaileche, H. Assessment of inhibitory properties of flavonoid-rich fig (Ficus carica L.) peel extracts against tyrosinase, α-glucosidase, urease and cholinesterases enzymes, and relationship with antioxidant activity. Eur. J. Integr. Med. 2021, 43, 101272.
- Dulić, M.; Ciganović, P.; Vujić, L.; Končić, M.Z. Antidiabetic and Cosmeceutical Potential of Common Barbery (Berberis vulgaris L.) Root Bark Extracts Obtained by Optimization of ‘Green’ Ultrasound-Assisted Extraction. Molecules 2019, 24, 3613.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
23 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





