
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tetsuya Okuda | + 1318 word(s) | 1318 | 2020-01-09 07:35:06 | | | |
| 2 | Dean Liu | Meta information modification | 1318 | 2021-10-13 08:12:07 | | |
Video Upload Options
Gangliosides are series of glycosphingolipids containing sialic acids in the oligosaccharide portion in mammalian cells. Gangliosides are a component of cellular membranes and play roles in modulating membrane function and the activity of membrane proteins. Abnormal expression and metabolism of gangliosides lead to the onset of several conditions in humans, such as neurologic diseases, diabetes, and cancer. A number of studies have been carried out to date to investigate the role of gangliosides in these diseases, and the effect of diet on tissue expression of gangliosides has recently become a topic of interest in this field. As gangliosides are degraded in the intestinal tract, ingested food-derived gangliosides are not directly absorbed into tissues in vivo, but the degradation products can be absorbed and affect ganglioside expression in the tissues. Recent studies have also shown that the expression of gangliosides in tissue cells can be indirectly induced by controlling the expression of ganglioside metabolism-related genes via the diet. These results indicate that dietary control can regulate the expression levels of gangliosides in tissues, which is expected to play a role in preventing and treating ganglioside-related diseases.
1. Introduction
A number of studies are currently underway examining the effect of diet on the expression level of tissue gangliosides. Although the details remain to be fully elucidated, recent progress has revealed new information regarding the effects of diet on ganglioside dynamics, metabolism, and induction. Cellular responses to nutrients and stress have also been identified as factors involved in the regulation of tissue ganglioside expression. This section describes these findings.
2. Gangliosides in Foods
Gangliosides are found in animal-derived foods such as meat, fish, egg yolk, and dairy products. The composition of molecular species of gangliosides in these foods are listed in Table 1. Dairy products primarily contain GD3 [1], but meat and fish contain GM3 [2]. These foods also contain complex gangliosides as minor components. Complex gangliosides are major lipid components in animal brains used as food in certain cultures.
Table 1. Ganglioside components in foods.
|
Food |
Major (Minor) Ganglioside Component |
Reference |
|
|
Meat |
Beef |
GM3 (GD3, GD1a) |
[2] |
|
Pork |
GM3 (GD3, GD1a, GD1b) |
[2] |
|
|
Chicken breast |
GM3 (GD3) |
[2] |
|
|
Chicken thigh |
GM3 (GD3, GD1a, GD1b) |
[2] |
|
|
Fish |
King salmon |
GM3 (GD3) |
[2] |
|
Snapper |
GM3 (GD3, GD1a, GD1b) |
[2] |
|
|
Island mackerel |
GM3 (GD3, GD1a, GD1b) |
[2] |
|
|
Turbot |
GM3 (GD3, GD1a, GD1b, GT1b) |
[2] |
|
|
Tuna |
n.d. |
[3] |
|
|
Egg yolk |
n.d. |
[3] |
|
|
Human breast milk |
Early stage |
GD3 |
|
|
Late stage |
GM3 |
||
|
All stages |
(GM1 and others) |
||
|
Dairy products |
Bovine milk |
GD3 (GM3) |
[8] |
|
Bovine butter |
GD3 (GM3) |
[1] |
|
|
Yogurt |
n.d. |
[3] |
|
|
Infant formula |
GD3 (GM3) |
||
|
Cheddar cheese |
n.d. |
[3] |
|
|
Whey |
GD3 (GM3) |
[9] |
|
|
LCKD (Bio-Serv F3666) |
GD3 (GM3) |
[10] |
|
n.d., not determined; LCKD, low-carbohydrate ketogenic diet.
GD3 is the major ganglioside in human breast milk during the early lactation stage, whereas GM3 becomes the major ganglioside in later stages [4][5]. Some complex gangliosides are also found in breast milk [5][6][7][8]. Bovine milk contains GD3 as the major ganglioside, and therefore, formula prepared using bovine milk also contains this ganglioside [8]. Gangliosides can remain stable in breast milk for long periods at several storage temperatures [11]. Pasteurization has almost no effect on the stability of gangliosides in breast milk.
Plants and fungi contain almost no sialic acid and therefore do not contain gangliosides. However, edible plants such as soybean, corn, rice, wheat, and konjac (Amorphophallus konjac, K. Koch) are rich in glucosylceramide [12][13], a ganglioside precursor. Although not used as a food source, marine sponge (Agelas mauritianus) contains a neutral glycosphingolipid, αGalCer, which activates innate immune responses in mammals [14].
3. Digestion and Absorption of Gangliosides in Foods
Gangliosides contained in foods are degraded in the intestinal tract (Figure 1), and the remaining intact structures are not taken up into the blood and lymph fluid. However, the products of ganglioside degradation, such as saccharides and lipids, are supplied to the tissues, where they promote the biosynthesis of gangliosides. In mammals, gangliosides are first degraded in the small intestine by sialidase/neuraminidase, which removes the sialic acid residues [15], and then sequentially degraded into saccharides and ceramides by glycosylceramidase present in the small intestinal mucosa [16]. Ceramides are further degraded into sphingoid bases and free fatty acids by neutral ceramidase [17][18]. These degradation products (saccharides and lipids) are absorbed by small intestinal epithelial cells and then partially re-synthesized into glycosylceramides [16][19]. Gangliosides are thought to be re-synthesized only in tissue cells that express enzymes involved in ganglioside synthesis. The results of these studies indicate that saccharides and lipids derived from gangliosides induce ganglioside expression in tissues. The sialic acid N-glycolylneuraminic acid (Neu5Gc), which is found in edible animals but not in humans, is abundant in red meat and dairy products [20]. Consumption of these foods supplies tissue cells with Neu5Gc and results in the synthesis of ganglioside species containing Neu5Gc, which are known to be highly expressed in cancer cells [21].
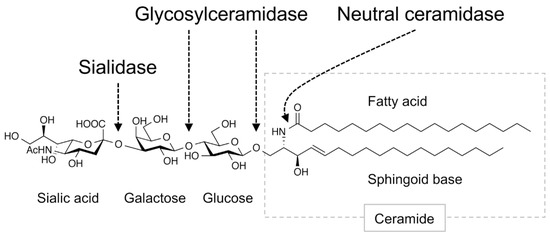
Figure 1. Ganglioside-degradation enzymes in the intestinal tract. Digestive enzymes that degrade gangliosides in the mammalian intestinal tract are shown. Arrows indicate linkage regions in the ganglioside degraded by these enzymes. Figure shows GM3 as an example ganglioside.
Breast milk is a rich source of oligosaccharides containing sialic acid, and the primary oligosaccharide, sialyl lactose, has the same structure as the oligosaccharide of GM3 [22][23]. Infants raised on breast milk have a significantly higher sialic acid content in the gangliosides and glycoproteins in the frontal cortex of the brain than infants fed formula [22]. Sialyl lactose has isomers that exhibit different sialic acid binding patterns, and a model experiment in which pigs were fed these isomers showed that each isomer increases the sialic acid content of gangliosides in a similar manner in several brain regions [23]. These results indicate that the degradation products of sialyl lactose promote ganglioside synthesis in brain tissues. Several animal experiments have shown that a high sialic acid content in gangliosides in the brain is associated with neuronal development, based on the formation and stabilization of functional synapses and neural circuits, leading to improved cognitive function, memory formation, and learning ability [24].
4. Indirect Effects of Dietary Components on Ganglioside Expression
Several studies have found that ganglioside expression in tissues is affected by various nutrient deficiencies or foods that do not provide components for ganglioside synthesis. A study investigating the effects of undernutrition in neonatal rats induced by feeding the mothers a low-protein diet during lactation showed a marked decrease in brain ganglioside content, along with decreases in body and brain weight [25]. Ganglioside expression in neonatal rat brain is also affected by thiamine and vitamin A deficiency [26]. Chronic consumption of ethanol also reportedly affects the expression of gangliosides in the rat brain [27]. Vitamin K deficiency is known to be associated with cognitive and behavioral perturbations in rats, as well as changes in the expression of gangliosides in the hippocampus, striatal striatum, and prefrontal cortex hippocampus in the rat brain [28]. Another study reported that a high-fat diet decreases glycosphingolipid levels in mouse liver [29], whereas our previous study revealed that ganglioside content in the liver increases in mice fed a high-fat, very-low-carbohydrate ketogenic diet (LCKD) [30][31].
5. Dietary Induction of Tissue Ganglioside Expression Via Transcriptional Regulation of Ganglioside Metabolism-Related Genes
Indirect changes in the expression of gangliosides induced by the diet are suggestive of specific changes in the metabolic pathways of ganglioside synthesis and degradation. A number of genes related to ganglioside biosynthesis and metabolism have been identified in recent years, and it has become clear that the expression of gangliosides in tissue cells is regulated by the expression of specific genes, suggesting that transcriptional regulation of ganglioside metabolism-related genes could be targeted by dietary factors that affect tissue ganglioside expression. The molecular species of ganglioside expressed in each type of tissue cell is controlled primarily by the corresponding glycosyltransferase gene. Analyses of the properties of these genes using cultured cells have revealed several targets for modulating ganglioside metabolism, such as transcription factors, signal transduction, and stress responses; controlling these targets thus regulates the expression of gangliosides in cells [32][33].
References
- K Takamizawa; M Iwamori; M Mutai; Y Nagai; Gangliosides of bovine buttermilk. Isolation and characterization of a novel monosialoganglioside with a new branching structure.. Journal of Biological Chemistry 1986, 261, 5625-5630.
- Bertram Y. Fong; Lin Ma; Geok Lin Khor; Yvonne Van Der Does; Angela Rowan; Paul McJarrow; Alastair K. H. MacGibbon; Ganglioside Composition in Beef, Chicken, Pork, and Fish Determined Using Liquid Chromatography–High-Resolution Mass Spectrometry. Journal of Agricultural and Food Chemistry 2016, 64, 6295-6305, 10.1021/acs.jafc.6b02200.
- Pierrette H. Pham; Terri-Lynn Duffy; Andrea L. Dmytrash; Vanessa W. Lien; Alan B. Thomson; M.T. Clandinin; Estimate of dietary ganglioside intake in a group of healthy Edmontonians based on selected foods. Journal of Food Composition and Analysis 2011, 24, 1032-1037, 10.1016/j.jfca.2011.01.011.
- K Takamizawa; M Iwamori; M Mutai; Y Nagai; Selective changes in gangliosides of human milk during lactation: a molecular indicator for the period of lactation.. Biochimica et Biophysica Acta 1986, 879, 73-77.
- Samuel Martín-Sosa; María-Jesús Martín; María-Dolores Castro; José A. Cabezas; Pablo Hueso; Lactational changes in the fatty acid composition of human milk gangliosides. Lipids 2004, 39, 111-116, 10.1007/s11745-004-1208-4.
- A Laegreid; A B Kolstø Otnaess; Trace amounts of ganglioside GM1 in human milk inhibit enterotoxins from Vibrio cholerae and Escherichia coli.. Life Sciences 1987, 40, 55-62.
- R Rueda; R Puente; P Hueso; J Maldonado; A Gil; New data on content and distribution of gangliosides in human milk.. Biological Chemistry Hoppe-Seyler 1995, 376, 723-727.
- Xiao Li Pan; Tatsuro Izumi; Variation of the ganglioside compositions of human milk, cow's milk and infant formulas.. Early Human Development 2000, 57, 25-31, 10.1016/s0378-3782(99)00051-1.
- Zhang, J.; Ren, Y.; Huang, B.; Tao, B.; Pedersen, M.R.; Li, D.; Determination of disialoganglioside GD3 and monosialoganglioside GM3 in infant formulas and whey protein concentrates by ultra-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Journal of Separation Science 2012, 35, 937–946.
- Tetsuya Okuda; Dietary Control of Ganglioside Expression in Mammalian Tissues. International Journal of Molecular Sciences 2019, 21, 177, 10.3390/ijms21010177.
- Jaime Salcedo; Sercan Karav; Annabelle Le Parc; Joshua L. Cohen; Juliana M. L. N. De Moura Bell; Adam Sun; Matthew C. Lange; Daniela Barile; Application of industrial treatments to donor human milk: influence of pasteurization treatments, storage temperature, and time on human milk gangliosides.. npj Science of Food 2018, 2, 5, 10.1038/s41538-018-0013-9.
- Tatsuya Sugawara; Teruo Miyazawa; Separation and determination of glycolipids from edible plant sources by high-performance liquid chromatography and evaporative light-scattering detection.. Lipids 1999, 34, 1231-1237, 10.1007/s11745-999-0476-3.
- Taro Uchiyama; Yusuke Nakano; Osamu Ueda; Hiroshi Mori; Masaya Nakashima; Akira Noda; Chiaki Ishizaki; Masako Mizoguchi; Oral Intake of Glucosylceramide Improves Relatively Higher Level of Transepidermal Water Loss in Mice and Healthy Human Subjects. JOURNAL OF HEALTH SCIENCE 2008, 54, 559-566, 10.1248/jhs.54.559.
- Rossjohn, J.; Pellicci, D.G.; Patel, O.; Gapin, L.; Godfrey, D.; Recognition of CD1d-restricted antigens by natural killer T cells. Nature Reviews Immunology 2012, 12, 845–857, 10.1038/nri3328.
- J J Dickson; M Messer; Intestinal neuraminidase activity of suckling rats and other mammals. Relationship to the sialic acid content of milk.. Biochemical Journal 1978, 170, 407-413.
- A Nilsson; Metabolism of cerebroside in the intestinal tract of the rat.. Biochimica et Biophysica Acta 1969, 187, 113-121.
- A Nilsson; The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract.. Biochimica et Biophysica Acta 1969, 176, 339-347.
- Maria Olsson; Rui-Dong Duan; Lena Ohlsson; Åke Nilsson; Rat intestinal ceramidase: purification, properties, and physiological relevance. American Journal of Physiology-Gastrointestinal and Liver Physiology 2004, 287, G929-G937, 10.1152/ajpgi.00155.2004.
- Tatsuya Sugawara; Digestion and Absorption of Sphingolipids as Functional Food Components. Nippon Eiyo Shokuryo Gakkaishi 2013, 66, 177-183, 10.4327/jsnfs.66.177.
- Pam Tangvoranuntakul; Pascal Gagneux; Sandra Diaz; Muriel Bardor; Nissi Varki; Ajit Varki; Elaine Muchmore; Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proceedings of the National Academy of Sciences 2003, 100, 12045-12050, 10.1073/pnas.2131556100.
- Ichiro Miyoshi; Hideyoshi Higashi; Yoshio Hirabayashi; Shiro Kato; Masaharu Naiki; Detection of 4-O-acetyl-N-glycolylneuraminyl lactosylceramide as one of tumor-associated antigens in human colon cancer tissues by specific antibody. Molecular Immunology 1986, 23, 631-638, 10.1016/0161-5890(86)90100-8.
- Bing Wang; Patricia McVeagh; Peter Petocz; Jennie Brand-Miller; Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. The American Journal of Clinical Nutrition 2003, 78, 1024-1029, 10.1093/ajcn/78.5.1024.
- Sheila K Jacobi; Tanya Yatsunenko; Dongpei Li; Somsankar Dasgupta; Robert K Yu; Brian M Berg; Maciej Chichlowski; Jack Odle; Dietary Isomers of Sialyllactose Increase Ganglioside Sialic Acid Concentrations in the Corpus Callosum and Cerebellum and Modulate the Colonic Microbiota of Formula-Fed Piglets. The Journal of Nutrition 2015, 146, 200-208, 10.3945/jn.115.220152.
- Kate Palmano; Angela Rowan; Rozey Guillermo; Jian Guan; Paul Mc Jarrow; The Role of Gangliosides in Neurodevelopment. Nutrients 2015, 7, 3891-3913, 10.3390/nu7053891.
- K K Vaswani; M Sharma; Effect of neonatal undernutrition on rat brain gangliosides.. International Journal for Vitamin and Nutrition Research 1985, 55, 323-329.
- K K Vaswani; Effect of neonatal thiamine and vitamin A deficiency on rat brain gangliosides.. Life Sciences 1985, 37, 1107-1115.
- Snežana R. Vrbaški; M. Ristić; Bojana Gruji-Injac; Phospholipid and Ganglioside Composition in Rat Brain After Chronic Intake of Ethanol. Journal of Neurochemistry 1984, 42, 1235-1239, 10.1111/j.1471-4159.1984.tb02777.x.
- Sahar Tamadon-Nejad; Bouchra Ouliass; Joseph Rochford; Guylaine Ferland; Vitamin K Deficiency Induced by Warfarin Is Associated With Cognitive and Behavioral Perturbations, and Alterations in Brain Sphingolipids in Rats. Frontiers in Aging Neuroscience 2018, 10, 213, 10.3389/fnagi.2018.00213.
- Kristina Eisinger; Sabrina Krautbauer; Tobias Hebel; Gerd Schmitz; Charalampos Aslanidis; Gerhard Liebisch; Christa Buechler; Lipidomic analysis of the liver from high-fat diet induced obese mice identifies changes in multiple lipid classes. Experimental and Molecular Pathology 2014, 97, 37-43, 10.1016/j.yexmp.2014.05.002.
- Tetsuya Okuda; A low-carbohydrate ketogenic diet promotes ganglioside synthesis via the transcriptional regulation of ganglioside metabolism-related genes.. Scientific Reports 2019, 9, 7627, 10.1038/s41598-019-43952-7.
- Tetsuya Okuda; Naoki Morita; A very low carbohydrate ketogenic diet increases hepatic glycosphingolipids related to regulation of insulin signalling. Journal of Functional Foods 2016, 21, 70-74, 10.1016/j.jff.2015.11.040.
- Thomas Kolter; Richard L. Proia; Konrad Sandhoff; Combinatorial Ganglioside Biosynthesis. Journal of Biological Chemistry 2002, 277, 25859-25862, 10.1074/jbc.r200001200.
- K Furukawa; N Tokuda; O Tajima; Glycosphingolipids in engineered mice: insights into function. Seminars in Cell & Developmental Biology 2004, 15, 389-396, 10.1016/j.semcdb.2004.03.006.




