Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chin-Wei Huang | -- | 1606 | 2022-06-21 17:11:56 | | | |
| 2 | Catherine Yang | Meta information modification | 1606 | 2022-06-22 04:40:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Huang, C.; Chen, T.; Lai, M.; Huang, H.I.; Wu, S. Immunity, Ion Channels and Epilepsy. Encyclopedia. Available online: https://encyclopedia.pub/entry/24292 (accessed on 07 February 2026).
Huang C, Chen T, Lai M, Huang HI, Wu S. Immunity, Ion Channels and Epilepsy. Encyclopedia. Available at: https://encyclopedia.pub/entry/24292. Accessed February 07, 2026.
Huang, Chin-Wei, Tsang-Shan Chen, Ming-Chi Lai, Huai-Ying Ingrid Huang, Sheng-Nan Wu. "Immunity, Ion Channels and Epilepsy" Encyclopedia, https://encyclopedia.pub/entry/24292 (accessed February 07, 2026).
Huang, C., Chen, T., Lai, M., Huang, H.I., & Wu, S. (2022, June 21). Immunity, Ion Channels and Epilepsy. In Encyclopedia. https://encyclopedia.pub/entry/24292
Huang, Chin-Wei, et al. "Immunity, Ion Channels and Epilepsy." Encyclopedia. Web. 21 June, 2022.
Copy Citation
Epilepsy is a common chronic neurological disorder in modern society. One of the major unmet challenges is that current antiseizure medications are basically not disease-modifying. Among the multifaceted etiologies of epilepsy, the role of the immune system has attracted considerable attention in recent years. It is known that both innate and adaptive immunity can be activated in response to insults to the central nervous system, leading to seizures. Moreover, the interaction between ion channels, which have a well-established role in epileptogenesis and epilepsy, and the immune system is complex and is being actively investigated.
immunity
ion channel
epilepsy
seizure
1. Introduction
Epilepsy is a chronic brain disorder that causes chronic, recurrent seizures as part of its clinical presentation. It is estimated that between 1% and 1.5% of the global population experiences at least one seizure in their lifetime. Although the techniques and technologies used in brain imagery and in neurophysiological research have undergone substantial development in recent years, some of the etiologies of epilepsy have not yet been identified, and the mechanisms of epilepsy are still not fully understood. Consequently, the treatment of epilepsy is not always satisfactory. It is estimated that 30% of patients with epilepsy suffer from pharmacoresistant epilepsy [1][2]. One of the unmet challenges is the difficulty of explaining epileptogenesis. The problem stems from the fact that the antiepileptic drugs (AEDs) currently used to treat epilepsy are basically not disease-modifying drugs; instead, they are antiseizure drugs that are designed to reduce the frequency of seizures but not to alter epileptogenesis [3].
Epilepsy is a multifaceted condition with complex etiologies, including genetic, toxic, and metabolic causes; infection; and structural lesions in the brain. Another possible cause has come to light recently, as the investigation of the role of immune mechanisms in the pathogenesis of seizures has gained momentum over the past two decades. Furthermore, the classification of seizures and epilepsies published in 2017 by the International League Against Epilepsy (ILAE) included a novel immune-mediated origin as one of the six etiologies of epilepsy [4]. It is known that both innate and adaptive immunity can be activated in response to central nervous system (CNS) insults, which, in turn, could lead to seizures [5]. Several neural-specific autoantibodies have been identified, such as the anti-Hu antibody in patients with paraneoplastic encephalomyelitis, the anti-Ma1 antibodies associated with paraneoplastic neurological syndromes, the anti-Ma2 antibodies associated with limbic encephalitis, and the anti-N-methyl-D-aspartate (NMDA) receptor antibodies in patients with limbic encephalitis [6][7][8][9][10][11][12]. Additionally, a retrospective population-based study in the US revealed a fourfold increase in the risk of epilepsy among patients with autoimmune disease [13]. These findings shed light on the role of immunity in the pathogenesis of epilepsy. In addition, some studies have suggested that the mammalian target of the rapamycin (mTOR) pathway plays a key role in the proper development of neural networks and that it is involved in epileptogenesis triggered by both genetic and acquired factors [14][15][16].
The role of ion channels in epilepsy and epileptogenesis is an active focus of current research, and the alteration of the ion channels involved in epileptogenesis has been established in numerous studies [17][18][19][20][21]. It has been further suggested that some ion channels are associated with altered immunological/inflammatory responses involved in the generation of epilepsy [22][23] and in immune-mediated epilepsy.
2. Adaptive Immunity in Seizures and Epilepsy
Unlike innate immune responses, which are triggered by glial activation and inflammation, which, in turn, play a role in epileptogenesis, adaptive immune responses are caused by immune cells infiltrating the brain as a result of infectious or noninfectious encephalitis, trauma, or hypoxia. Autoimmune epilepsy originates with a maladaptive immune response, which results in the formation of autoantibodies reacting to self-antigens. Adaptive immunity is not only related to recurrent seizures but is also involved in the progressive degeneration of the brain. For example, in patients with Rasmussen’s encephalitis, staining for CD8 has demonstrated the large-scale infiltration of cytotoxic T lymphocytes into the cortex [24] (Figure 1).
In the case brain infection by the herpes simplex virus (HSV), proinflammatory cytokines are produced by microglia and locally infiltrating macrophages. HSV sometimes triggers the late onset of antibodies attacking NMDA receptors, leading to the development of anti-NMDA receptor encephalitis [25][26][27][28]. Chemokines are also released during HSV infection and play a role in immunity by modulating leukocyte trafficking to the focus of infection [29][30][31]. As the virus continues replicating, both CD4+ and CD8+ T lymphocytes infiltrate the brain [32][33][34]. Incidentally, HSV can escape from immune response targeting in the brain through the mediating effect of HSV-1 UL13 kinase. This kinase facilitates the evasion of HSV-1-specific CD8+ T cells at infection sites by downregulating the expression of CXCL9, a chemokine that attracts the CD8+ T cells, thereby increasing the severity and fatality of the HSV infection [35].
Another example of adaptive immunity is paraneoplastic neurological syndromes (PNSs), in which the production of antibodies is triggered by cancer cells and attack neurons. This spectrum of diseases is usually caused by antibodies targeting intracellular onconeural antigens. The pathogenesis is most likely mediated by T cells, as conspicuous cytotoxic T-cell infiltration has been found surrounding neurons in patients with anti-Yo, anti-Hu, and anti-Ma2 antibodies [36][37][38]. In the past two decades, autoimmune encephalitis (AE) has become known and has changed the approach to evaluating the etiologies of epilepsy [39][40][41] (Figure 1). AE might occur in conjunction with PNSs or not be related to cancers. The antigens in AE tend to be located on the cell surface or synapse and are targeted by antibodies, such as the antibodies against NMDA receptors, leucine-rich glioma-inactivated protein 1 (LGI1), and myelin oligodendrocyte glycoprotein (MOG). On the other hand, the antigens in PNSs have an intracellular location and are targeted by antibodies such as the anti-Yo and the anti-Hu antibodies [42][43][44].
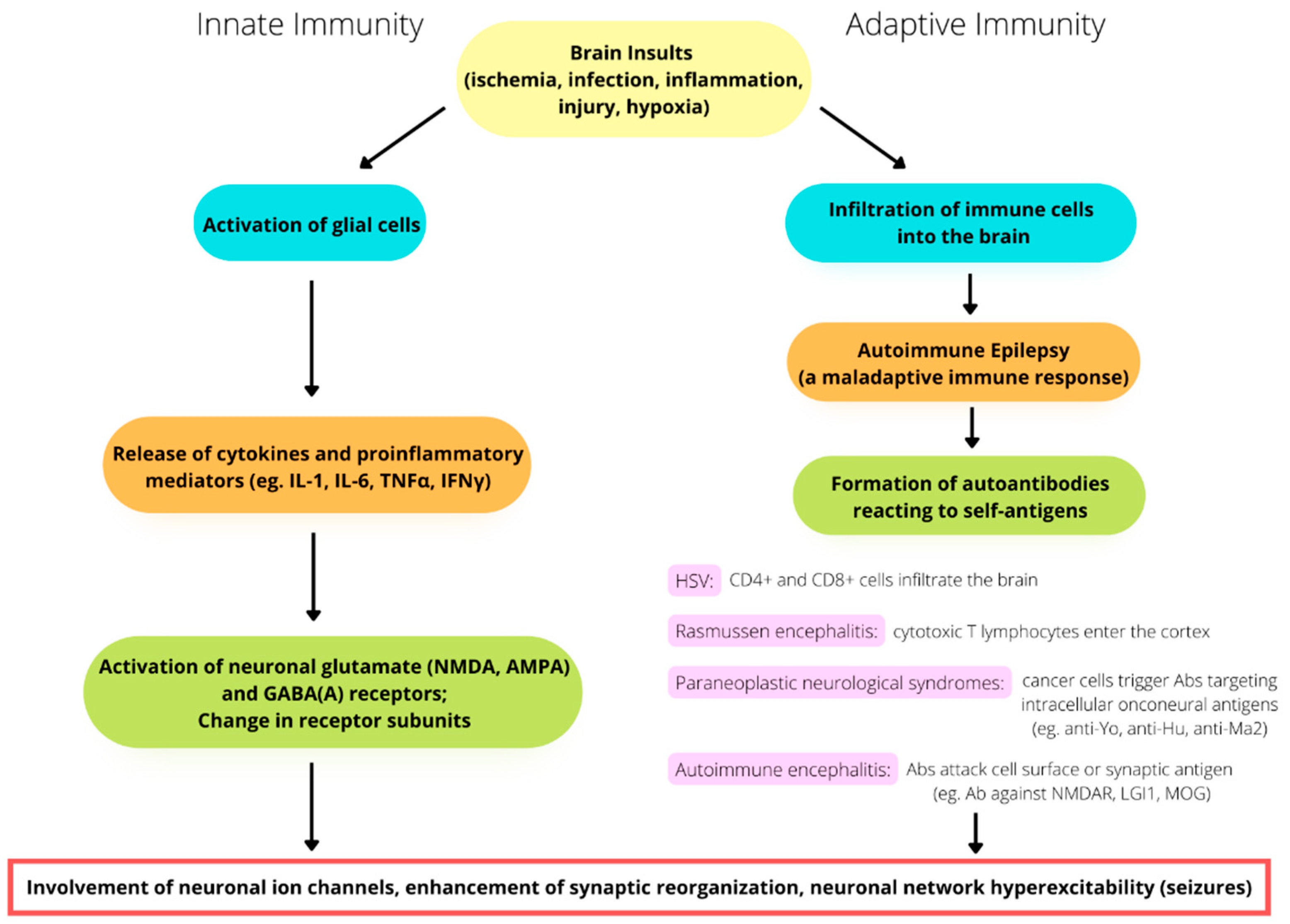
Figure 1. The involvement of immunity in the pathomechanism of seizure generation. Both innate and adaptive immunity alterations play a potential role in seizures and epileptogenesis.
It is also worth mentioning that the incidence of seizure disorders in patients with multiple sclerosis has been reported to exceed their incidence in the general population. Several studies have reported seizures occurring at the onset of multiple sclerosis [45]. The increased risk of seizures for patients with multiple sclerosis may reflect the effects of inflammation, which provides a theoretical basis for the application of immunomodulation to the treatment of seizure disorders.
3. Involvement of Ion Channels and the Immune System in Epileptogenesis and Epilepsy
Molecular studies of epileptogenesis and epilepsy have demonstrated that specific ion channels play an important role in both genetic and acquired forms of epilepsy [46][47][48], especially voltage-gated sodium channels [49][50][51][52][53]. The ionic mechanisms underlying burst-firing behavior in neurons are not fully understood, although sodium channels have been found to be significantly involved [48][54]. Different types of sodium channels expressed in both glutamatergic and GABAergic cell types might play unequal roles in neuronal excitability, the enhancement of synaptic potentials, the generation of subthreshold oscillations, the facilitation of repetitive firing, and the prolongation of depolarized potentials [49][55].
Generalized epilepsy with febrile seizures plus (GEFS+) is caused by missense mutations in NaV1.1 channels. Furthermore, familial febrile seizures are caused by mild loss-of-function mutations in NaV1.1 channels, which are involved in febrile seizures associated with vaccination [22][56][57]. In addition, calmodulin, a small protein acting as a signal transducer that regulates neuronal plasticity, muscle contraction, and immune response [58], modulates the voltage-gated sodium-channel gating process, alters the sodium current density, and regulates the trafficking and expression of sodium channel proteins. Many mutations in the calmodulin-binding IQ domain give rise to diseases, including epilepsy [23].
As mentioned earlier, mTOR is an evolutionarily conserved serine/threonine kinase that plays a central role in integrating environmental cues in the form of growth factors, amino acids, and energy. It is now considered to be a central regulator of immune responses [59]. Mutations along the mTOR signaling pathway can indirectly affect the expression level and activity of ion channels that determine neuronal firing rate, neural activity patterns, and neuronal network activity [60]. The mTOR signaling pathway is considered one of the disease mechanisms that underlie monogenic epilepsies (i.e., mTORopathy) [61]. Both gain- and loss-of-function mutations of ion channels, synaptic proteins, and signaling molecules that are located along the mTOR pathway have been linked to this imbalance of network excitability [62]. Furthermore, in in vitro studies, mTOR was found to regulate intrinsic neuronal excitability by increasing the expression of large-conductance calcium-activated potassium channels (BK channels) [63].
The PI3K/AKT/mTOR pathway is an intracellular signaling pathway that is important in regulating the cell cycle. The PI3K/AKT pathway has a natural inhibitor called phosphatase and tensin homolog (PTEN), the function of which is to limit proliferation in cells. The NMDA receptor recruits PTEN to the postsynaptic terminal and decreases AMPA receptor-mediated responses [64]. In addition, PTEN knockdown has been shown to directly alter the properties of AMPA receptors [65]. On the other hand, mTOR inactivation has been shown to increase the expression of potassium channels (Kv1.1 and Kv1.2) and regulate the activity of NMDA receptors, which control the influx of Ca2+ into the cell and alter mTOR activity [66]. Thus, cellular excitability is maintained because the changes in ion channels and mTOR activity are balanced. A study by Nguyen and Anderson [67] revealed a link between ion channels and the mTOR pathway in PTEN KO mice that was associated with an increase in the expression of hippocampal Kv1.1 protein, which was normalized by the inhibition of mTOR by rapamycin. Therefore, it is possible that mTOR is capable of altering the translation and activity of a variety of ion channels in neurons, thus regulating excitability in neuronal networks [62]. Interestingly, the immunosuppressor everolimus has been shown to inhibit mTOR signaling by reducing the phosphorylation of downstream mTOR effects, which suggests that it has potential antiepileptogenic properties [14][15][16]. Whether the modulation of the mTOR pathway and immune responses, in terms of antiepileptoge
References
- Kalilani, L.; Sun, X.; Pelgrims, B.; Noack-Rink, M.; Villanueva, V. The epidemiology of drug resistant epilepsy: A systemic revies and meta-analysis. Epilepsia 2018, 59, 2179–2193.
- Sultana, B.; Panzini, M.A.; Carpentier, A.V.; Comtois, J.; Rioux, B.; Gore, G.; Bauer, P.R.; Kwon, C.S.; Jetté, N.; Josephson, C.B.; et al. Incidence and prevalence of drug-resistant epilepsy: A systemic review and meta-analysis. Neurology 2021, 96, 805–817.
- French, J.A.; Perucca, E. Time to start calling things by their own names? The case for antiseizure medicines. Epilepsy Curr. 2020, 20, 69–72.
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position papter of the ILAE commission for Classification and Terminology. Epilepsia 2017, 58, 512–521.
- Granata, T.; Cross, H.; Theodore, W.; Avanzini, G. Immune-mediated epilepsies. Epilepsia 2011, 52, 5–11.
- Dalmau, J.; Graus, F.; Rosenblum, M.K.; Posner, J.B. Anti-Hu associated paraneoplastic encephalomyelitis/sensory neuropathy: A clinical study of 71 patients. Medicine 1992, 71, 59–72.
- Dalmau, J.; Gultekin, S.H.; Voltz, R.; Hoard, R.; DesChamps, T.; Balmaceda, C.; Batchelor, T.; Gerstner, E.; Eichen, J.; Frennier, J.; et al. Ma1, a novel neuron- and testis-specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain 1999, 122, 27–39.
- Dalmau, J.; Graus, F.; Villarejo, A.; Posner, J.B.; Blumenthal, D.; Thiessen, B.; Saiz, A.; Meneses, P.; Rosenfeld, M.R. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004, 127, 1831–1844.
- Overeem, S.; Dalmau, J.; Bataller, L.; Nishino, S.; Mignot, E.; Verschuuren, J.; Lammers, G.J. Hypocretin-1 CSF levels in anti-Ma2 associated encephalitis. Neurology 2004, 62, 138–140.
- Dalmau, J. Limbic encephalitis and variants related to neuronal cell membrane autoantigens. Rinsho Shinkeigaku 2008, 48, 871–874.
- Dalmau, J.; Tüzün, E.; Wu, H.Y.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.B.; Shimazaki, H.; Koide, R.; King, D.; et al. Paraneoplastic antiN-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36.
- Graus, F.; Saiz, A. Limbic encephalitis: An expanding concept. Neurology 2008, 70, 500–501.
- Ong, M.S.; Kohane, I.S.; Cai, T.; Gorman, M.P.; Mandl, K.D. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol. 2014, 71, 569–574.
- Krueger, D.A.; Wilfong, A.A.; Mays, M.; Talley, C.M.; Agricola, K.; Tudor, C.; Capal, J.; Holland-Bouley, K.; Franz, D.N. Long-term treatment of epilepsy with everolimus in tuberous sclerosis. Neurology 2016, 87, 2408–2415.
- Vezzani, A. Before epilepsy unfolds: Finding the epileptogenesis switch. Nat. Med. 2012, 18, 1626–1627.
- Curatolo, P.; Moavero, R.; van Scheppingen, J.; Aronica, E. mTOR dysregulation and tuberous sclerosis-related epilepsy. Expert Rev. Neurother. 2018, 18, 185–201.
- Oyrer, J.; Maljevic, S.; Scheffer, I.E.; Berkovic, S.F.; Petrou, S.; Reid, C.A. Ion Channels in Genetic Epilepsy: From Genes and Mechanisms to Disease-Targeted Therapies. Pharmacol. Rev. 2018, 70, 142–173.
- Symonds, J.D.; Zuberi, S.M.; Johnson, M.R. Advances in epilepsy gene discovery and implications for epilepsy diagnosis and treatment. Curr. Opin. Neurol. 2017, 30, 193–199.
- Wei, F.; Yan, L.M.; Su, T.; He, N.; Lin, Z.J.; Wang, J.; Shi, Y.W.; Yi, Y.H.; Liao, W.P. Ion Channel Genes and Epilepsy: Functional Alteration, Pathogenic Potential, and Mechanism of Epilepsy. Neurosci. Bull. 2017, 33, 455–477.
- Catterall, W.A. Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem. Res. 2017, 42, 2495–2504.
- Lai, M.C.; Lin, K.M.; Yeh, P.S.; Wu, S.N.; Huang, C.W. The novel effect of immunomodulatory-glatiramer acetate on epileptogenesis and epileptic seizures. Cell. Physiol. Biochem. 2018, 50, 150–168.
- Catterall, W.A.; Kalume, F.; Oakley, J.C. NaV1.1 channels and epilepsy. J. Physiol. 2010, 588, 1849–1859.
- Wu, X.; Hong, L. Calmodulin Interactions with Voltage-Gated Sodium Channels. Int. J. Mol. Sci. 2021, 22, 9798.
- Varadkar, S.; Bien, C.G.; Kruse, C.A.; Jensen, F.E.; Bauer, J.; Pardo, C.A.; Vincent, A.; Mathern, G.W.; Cross, J.H. Rasmussen’s encephalitis: Clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014, 13, 195–205.
- Pruss, H.; Finke, C.; Holtje, M.; Hofmann, J.; Klingbeil, C.; Probst, C.; Borowski, K.; Ahnert-Hilger, G.; Harms, L.; Schwab, J.M.; et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann. Neurol. 2012, 72, 902–911.
- Hacohen, Y.; Deiva, K.; Pettingill, P.; Waters, P.; Siddiqui, A.; Chretien, P.; Menson, E.; Lin, J.P.; Tardieu, M.; Vincent, A.; et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov. Disord. 2014, 29, 90–96.
- Norsadini, M.; Mohammad, S.S.; Coraza, F.; Ruga, E.M.; Kothur, K.; Perilongo, G.; Frigo, A.C.; Toldo, I.; Dale, R.C.; Sartori, S. Herpes simplex virus-induced anti-N-methyl-D-aspartate receptor encephalitis: A systematic literature review with analysis of 43 cases. Dev. Med. Child Neurol. 2017, 59, 796–805.
- Armangue, T.; Spatola, M.; Vlagea, A.; Mattozzi, S.; Cárceles-Cordon, M.; Martinez-Heras, E.; Llufriu, S.; Muchart, J.; Erro, M.E.; Abraira, L.; et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 2018, 17, 760–772.
- Enquist, L.W.; Husak, P.J.; Banfield, B.W.; Smith, G.A. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus. Res. 1998, 51, 237–347.
- Wickham, S.; Lu, B.; Ash, J.; Daniel, J.J.; Carr, D.J. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J. Neuroimmunol. 2005, 162, 51–59.
- Luster, A.D. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 2002, 14, 129–135.
- Chan, W.L.; Javanovic, T.; Lukic, M.L. Infiltration of immune T cells in the brain of mice with herpes simplex virus-induced encephalitis. J. Neuroimmunol. 1989, 23, 195–201.
- Hudson, S.J.; Streilein, J.W. Functional cytotoxic T cells are associated with focal lesions in the brains of SJL mice with experimental herpes simplex encephalitis. J. Immunol. 1994, 152, 5540–5547.
- Marques, C.P.; Cheeran, M.C.; Palmquist, J.M.; Hu, S.; Urban, S.L.; Lokensgard, J.R. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J. Immunol. 2008, 181, 6417–6426.
- Koyanagi, N.; Imai, T.; Shindo, K.; Sato, A.; Fujii, W.; Ichinohe, T.; Takemura, N.; Kakuta, S.; Uematsu, S.; Kiyono, H.; et al. Herpes simplex virus-1 evasion of CD8+ T cell accumulation contributes to viral encephalitis. J. Clin. Investig. 2017, 127, 3784–3795.
- McKeon, A.; Pittock, S.J. Paraneoplastic encephalomyelopathies: Pathology and mechanisms. Acta Neuropathol. 2011, 122, 381–400.
- Roberts, W.K.; Deluca, I.J.; Thomas, A.; Fak, J.; Williams, T.; Buckley, N.; Dousmanis, A.G.; Posner, J.B.; Darnell, R.B. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J. Clin. Investig. 2009, 119, 2042–2051.
- Bien, C.G.; Vincent, A.; Barnett, M.H.; Becker, A.J.; Blümcke, I.; Graus, F.; Jellinger, K.A.; Reuss, D.E.; Ribalta, T.; Schlegel, J.; et al. Immunopathology of autoantibody-associated encephalitides: Clues for pathogenesis. Brain 2012, 135, 1622–1638.
- Chefdeville, A.; Honnorat, J.; Hampe, C.S.; Desestret, V. Neuronal central nervous system syndromes probably mediated by autoantibodies. Eur. J. Neurosci. 2016, 43, 1535–1552.
- Dalmau, J.; Graus, F. Antibody-mediated encephalitis. N. Engl. J. Med. 2018, 378, 840–851.
- Ramanathan, S.; Al-Diwani, A.; Waters, P.; Irani, S.R. The autoantibody-mediated encephalitides: From clinical observations to molecular pathogenesis. J. Neurol. 2021, 268, 1689–1707.
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol. Rev. 2017, 97, 839–887.
- Armangue, T.; Olivé-Cirera, G.; Martínez-Hernandez, E.; Sepulveda, M.; Ruiz-Garcia, R.; Muñoz-Batista, M.; Ariño, H.; González-Álvarez, V.; Felipe-Rucián, A.; Martínez-González, M.J.; et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: A multicentre observational study. Lancet Neurol. 2020, 19, 234–246.
- Vogrig, A.; Gigli, G.L.; Segatti, S.; Corazza, E.; Marini, A.; Bernardini, A.; Valent, F.; Martina Fabris, M.; Curcio, F.; Brigo, F.; et al. Epidemiology of paraneoplastic neurological syndromes: A population-based study. J. Neurol. 2020, 267, 26–35.
- Marrie, R.A.; Reider, N.; Cohen, J.; Trojano, M.; Sorensen, P.S.; Cutter, G.; Reingold, S.; Stuve, O. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult. Scler. 2015, 21, 342–349.
- Mody, I. Ion channels in epilepsy. Int. Rev. Neurobiol. 1998, 42, 199–226.
- Blumenfeld, H. Cellular and network mechanisms of spike-wave seizures. Epilepsia 2005, 46, 21–23.
- Lerche, H.; Shah, M.; Beck, H.; Noebels, J.; Johnston, D.; Vincent, A. Ion channels in genetic and acquired forms of epilepsy. J. Physiol. 2013, 591, 753–764.
- Waszkielewicz, A.M.; Gunia, A.; Szkaradek, N.; Słoczyńska, K.; Krupińska, S.; Marona, H. Ion channels as drug targets in central nervous system disorders. Curr. Med. Chem. 2013, 20, 1241–1285.
- Hargus, N.J.; Nigam, A.; Bertram, E.H., 3rd; Patel, M.K. Evidence for a role of Nav1.6 in facilitating increases in neuronal hyperexcitability during epileptogenesis. J. Neurophysiol. 2013, 110, 1144–1157.
- Singh, N.A.; Pappas, C.; Dahle, E.J.; Claes, L.R.; Pruess, T.H.; De Jonghe, P.; Thompson, J.; Dixon, M.; Gurnett, C.; Peiffer, A.; et al. A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PLoS Genet. 2009, 5, e1000649.
- Ashraf, M.N.; Gavrilovici, C.; Shah, S.U.; Shaheen, F.; Choudhary, M.I.; Rahman, A.U.; Fahnestock, M.; Simjee, S.U.; Poulter, M.O. A novel anticonvulsant modulates voltage-gated sodium channel inactivation and prevents kindling-induced seizures. J. Neurochem. 2013, 126, 651–661.
- Mulley, J.C.; Hodgson, B.; McMahon, J.M.; Iona, X.; Bellows, S.; Mullen, S.A.; Farrell, K.; Mackay, M.; Sadleir, L.; Bleasel, A.; et al. Role of the sodium channel SCN9A in genetic epilepsy with febrile seizures plus and Dravet syndrome. Epilepsia 2013, 54, e122–e126.
- Stafstrom, C.E. The role of the subiculum in epilepsy and epileptogenesis. Epilepsy Curr. 2005, 4, 121–129.
- Yan, B.; Li, P. An integrative view of mechanisms underlying generalized spike-and-wave epileptic seizures and its implication on optimal therapeutic treatments. PLoS ONE 2011, 6, e22440.
- Gataullina, S.; Dulac, O. From genotype to phenotype in Dravet disease. Seizure 2017, 44, 58–64.
- Fang, Z.X.; Hong, S.Q.; Li, T.S.; Wang, J.; Xie, L.L.; Han, W.; Jiang, L. Genetic and phenotypic characteristics of SCN1A-related epilepsy in Chinese children. Neuroreport 2019, 30, 671–680.
- Zoidl, G.R.; Spray, D.C. The Roles of Calmodulin and CaMKII in Cx36 Plasticity. Int. J. Mol. Sci. 2021, 22, 4473.
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of Immune Responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68.
- Cho, C.H. Frontier of epilepsy research—mTOR signaling pathway. Exp. Mol. Med. 2011, 43, 231–274.
- Moloney, P.B.; Cavalleri, G.L.; Delanty, N. Epilepsy in the mTORopathies: Opportunities for precision medicine. Brain Commun. 2021, 3, fcab222.
- Weng, O.Y.; Li, Y.; Wang, L.Y. Modeling Epilepsy Using Human Induced Pluripotent Stem Cells-Derived Neuronal Cultures Carrying Mutations in Ion Channels and the Mechanistic Target of Rapamycin Pathway. Front. Mol. Neurosci. 2022, 15, 810081.
- Wang, Y.; Tao, J.; Wang, M.; Yang, L.; Ning, F.; Xin, H.; Xu, X.; Cai, H.; Zhang, W.; Yu, K.; et al. Mechanism of regulation of big-conductance Ca2+-activated K+ channels by mTOR complex 2 in podocytes. Front. Physiol. 2019, 10, 167.
- Jurado, S.; Benoist, M.; Lario, A.; Knafo, S.; Petrok, C.N.; Esteban, J.A. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 2010, 29, 2827–2840.
- Yang, D.-J.; Wang, X.-L.; Ismail, A.; Ashman, C.J.; Valori, C.F.; Wang, G.; Gao, S.; Higginbottom, A.; Ince, P.G.; Azzouz, M.; et al. PTEN regulates AMPA receptor-mediated cell viability in iPS-derived motor neurons. Cell Death Dis. 2014, 5, e1096.
- Niere, F.; Raab-Graham, K.F. mTORC1 is a local, postsynaptic voltage sensor regulated by positive and negative feedback pathways. Front. Cell. Neurosci. 2017, 11, 152.
- Nguyen, L.H.; Anderson, A.E. mTOR-dependent alterations of Kv1.1 subunit expression in the neuronal subset-specific Pten knockout mouse model of cortical dysplasia with epilepsy. Sci. Rep. 2018, 8, 3568.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
754
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
22 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




