Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chin-Wei Huang and Version 2 by Catherine Yang.
Epilepsy is a common chronic neurological disorder in modern society. One of the major unmet challenges is that current antiseizure medications are basically not disease-modifying. Among the multifaceted etiologies of epilepsy, the role of the immune system has attracted considerable attention in recent years. It is known that both innate and adaptive immunity can be activated in response to insults to the central nervous system, leading to seizures. Moreover, the interaction between ion channels, which have a well-established role in epileptogenesis and epilepsy, and the immune system is complex and is being actively investigated.
- immunity
- ion channel
- epilepsy
- seizure
1. Introduction
Epilepsy is a chronic brain disorder that causes chronic, recurrent seizures as part of its clinical presentation. It is estimated that between 1% and 1.5% of the global population experiences at least one seizure in their lifetime. Although the techniques and technologies used in brain imagery and in neurophysiological research have undergone substantial development in recent years, some of the etiologies of epilepsy have not yet been identified, and the mechanisms of epilepsy are still not fully understood. Consequently, the treatment of epilepsy is not always satisfactory. It is estimated that 30% of patients with epilepsy suffer from pharmacoresistant epilepsy [1][2][1,2]. One of the unmet challenges is the difficulty of explaining epileptogenesis. The problem stems from the fact that the antiepileptic drugs (AEDs) currently used to treat epilepsy are basically not disease-modifying drugs; instead, they are antiseizure drugs that are designed to reduce the frequency of seizures but not to alter epileptogenesis [3].
Epilepsy is a multifaceted condition with complex etiologies, including genetic, toxic, and metabolic causes; infection; and structural lesions in the brain. Another possible cause has come to light recently, as the investigation of the role of immune mechanisms in the pathogenesis of seizures has gained momentum over the past two decades. Furthermore, the classification of seizures and epilepsies published in 2017 by the International League Against Epilepsy (ILAE) included a novel immune-mediated origin as one of the six etiologies of epilepsy [4]. It is known that both innate and adaptive immunity can be activated in response to central nervous system (CNS) insults, which, in turn, could lead to seizures [5]. Several neural-specific autoantibodies have been identified, such as the anti-Hu antibody in patients with paraneoplastic encephalomyelitis, the anti-Ma1 antibodies associated with paraneoplastic neurological syndromes, the anti-Ma2 antibodies associated with limbic encephalitis, and the anti-N-methyl-D-aspartate (NMDA) receptor antibodies in patients with limbic encephalitis [6][7][8][9][10][11][12][6,7,8,9,10,11,12]. Additionally, a retrospective population-based study in the US revealed a fourfold increase in the risk of epilepsy among patients with autoimmune disease [13]. These findings shed light on the role of immunity in the pathogenesis of epilepsy. In addition, some studies have suggested that the mammalian target of the rapamycin (mTOR) pathway plays a key role in the proper development of neural networks and that it is involved in epileptogenesis triggered by both genetic and acquired factors [14][15][16][14,15,16].
The role of ion channels in epilepsy and epileptogenesis is an active focus of current research, and the alteration of the ion channels involved in epileptogenesis has been established in numerous studies [17][18][19][20][21][17,18,19,20,21]. It has been further suggested that some ion channels are associated with altered immunological/inflammatory responses involved in the generation of epilepsy [22][23][22,23] and in immune-mediated epilepsy.
24. Adaptive Immunity in Seizures and Epilepsy
Unlike innate immune responses, which are triggered by glial activation and inflammation, which, in turn, play a role in epileptogenesis, adaptive immune responses are caused by immune cells infiltrating the brain as a result of infectious or noninfectious encephalitis, trauma, or hypoxia. Autoimmune epilepsy originates with a maladaptive immune response, which results in the formation of autoantibodies reacting to self-antigens. Adaptive immunity is not only related to recurrent seizures but is also involved in the progressive degeneration of the brain. For example, in patients with Rasmussen’s encephalitis, staining for CD8 has demonstrated the large-scale infiltration of cytotoxic T lymphocytes into the cortex [24][48] (Figure 1). In the case brain infection by the herpes simplex virus (HSV), proinflammatory cytokines are produced by microglia and locally infiltrating macrophages. HSV sometimes triggers the late onset of antibodies attacking NMDA receptors, leading to the development of anti-NMDA receptor encephalitis [25][26][27][28][49,50,51,52]. Chemokines are also released during HSV infection and play a role in immunity by modulating leukocyte trafficking to the focus of infection [29][30][31][53,54,55]. As the virus continues replicating, both CD4+ and CD8+ T lymphocytes infiltrate the brain [32][33][34][56,57,58]. Incidentally, HSV can escape from immune response targeting in the brain through the mediating effect of HSV-1 UL13 kinase. This kinase facilitates the evasion of HSV-1-specific CD8+ T cells at infection sites by downregulating the expression of CXCL9, a chemokine that attracts the CD8+ T cells, thereby increasing the severity and fatality of the HSV infection [35][59]. Another example of adaptive immunity is paraneoplastic neurological syndromes (PNSs), in which the production of antibodies is triggered by cancer cells and attack neurons. This spectrum of diseases is usually caused by antibodies targeting intracellular onconeural antigens. The pathogenesis is most likely mediated by T cells, as conspicuous cytotoxic T-cell infiltration has been found surrounding neurons in patients with anti-Yo, anti-Hu, and anti-Ma2 antibodies [36][37][38][60,61,62]. In the past two decades, autoimmune encephalitis (AE) has become known and has changed theour approach to evaluating the etiologies of epilepsy [39][40][41][63,64,65] (Figure 1). AE might occur in conjunction with PNSs or not be related to cancers. The antigens in AE tend to be located on the cell surface or synapse and are targeted by antibodies, such as the antibodies against NMDA receptors, leucine-rich glioma-inactivated protein 1 (LGI1), and myelin oligodendrocyte glycoprotein (MOG). On the other hand, the antigens in PNSs have an intracellular location and are targeted by antibodies such as the anti-Yo and the anti-Hu antibodies [42][43][44][66,67,68].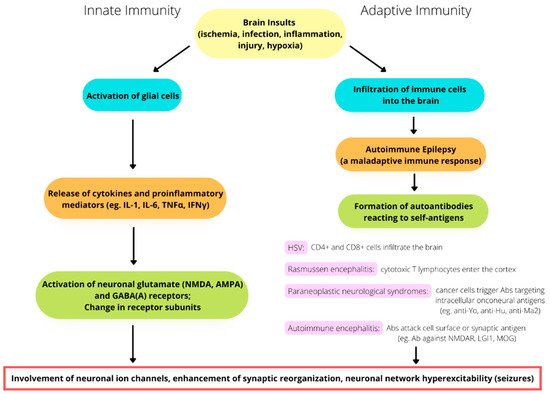
Figure 1. The involvement of immunity in the pathomechanism of seizure generation. Both innate and adaptive immunity alterations play a potential role in seizures and epileptogenesis.
It is also worth mentioning that the incidence of seizure disorders in patients with multiple sclerosis has been reported to exceed their incidence in the general population. Several studies have reported seizures occurring at the onset of multiple sclerosis [45][69]. The increased risk of seizures for patients with multiple sclerosis may reflect the effects of inflammation, which provides a theoretical basis for the application of immunomodulation to the treatment of seizure disorders.
