Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sophie Mcgivern | -- | 1532 | 2022-06-21 02:58:04 | | | |
| 2 | Dean Liu | -2 word(s) | 1530 | 2022-06-21 03:01:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mcgivern, S.; Boutouil, H.; Al-Kharusi, G.; Little, S.; Dunne, N.J.; Levingstone, T.J. 3D Bioprinting for Cartilage Tissue Engineering. Encyclopedia. Available online: https://encyclopedia.pub/entry/24242 (accessed on 07 February 2026).
Mcgivern S, Boutouil H, Al-Kharusi G, Little S, Dunne NJ, Levingstone TJ. 3D Bioprinting for Cartilage Tissue Engineering. Encyclopedia. Available at: https://encyclopedia.pub/entry/24242. Accessed February 07, 2026.
Mcgivern, Sophie, Halima Boutouil, Ghayadah Al-Kharusi, Suzanne Little, Nicholas J. Dunne, Tanya J. Levingstone. "3D Bioprinting for Cartilage Tissue Engineering" Encyclopedia, https://encyclopedia.pub/entry/24242 (accessed February 07, 2026).
Mcgivern, S., Boutouil, H., Al-Kharusi, G., Little, S., Dunne, N.J., & Levingstone, T.J. (2022, June 21). 3D Bioprinting for Cartilage Tissue Engineering. In Encyclopedia. https://encyclopedia.pub/entry/24242
Mcgivern, Sophie, et al. "3D Bioprinting for Cartilage Tissue Engineering." Encyclopedia. Web. 21 June, 2022.
Copy Citation
Cartilage is an avascular tissue with extremely limited self-regeneration capabilities. Three-dimensional (3D) bioprinted constructs for cartilage repair applications. 3D bioprinting is an evolutionary additive manufacturing technique that enables the precisely controlled deposition of a combination of biomaterials, cells, and bioactive molecules, collectively known as bioink, layer-by-layer to produce constructs that simulate the structure and function of native cartilage tissue.
cartilage
3D bioprinting
tissue engineering
1. Introduction
Articular cartilage is a smooth, wear-resistant, highly specialised hyaline cartilage found at the ends of bones within synovial joints where it reduces friction to allow smooth joint movement [1]. As a result of its avascularity and aneurality, cartilage has extremely limited self-regeneration capabilities, thus damage to the articular cartilage from pathological conditions such as osteoarthritis (OA) and rheumatoid arthritis (RA), and traumatic injury pose a significant challenge to orthopaedic surgeons. OA is the most common joint disorder in the world. Minor symptoms experienced during early-stage disease can be managed through medication and physiotherapy; however, as the disease progresses, severe articular cartilage damage occurs. OA has a significant impact on a patient’s quality of life, causing severe pain, stiffness, and swelling in the affected region. Over 300 million people globally suffer from OA as of 2019 [2], resulting in a significant economic burden [3]. The current treatments for conditions affecting the articular cartilage consist primarily of pain management medication and physiotherapy, with surgical intervention required in more severe cases. Current surgical approaches include microfracture, subchondral drilling, abrasion arthroplasty, autologous chondrocyte implantation (ACI), matrix-assisted ACI (MACI), and osteochondral autograft/allograft transplantation (OAT) [4]. While these techniques are widely applied clinically, there are associated limitations and complications such as donor site mobility, graft hypertrophy, and inconsistent repair tissue associated with them [4]. Ultimately, a total joint replacement is required for end-stage disease. Thus, the development of new approaches capable of effectively regenerating damaged cartilage tissue is imperative.
Tissue engineering, an interdisciplinary field that combines biomaterial scaffolds, cells, and signalling agents to develop biological substitutes capable of restoring, maintaining, or improving tissue function, shows promise for the development of new approaches for the repair of cartilage tissue [5]. Within the tissue engineered construct, the scaffold and signalling agents function to direct cells to produce the required tissue type, thus this approach offers advantages over standard cell-based therapies. An ideal scaffold should replicate the unique mechanical and biological properties of the native ECM of the desired tissue and have a porous structure that allows for cell attachment and nutrients exchange. Three-dimensional bioprinting, an additive manufacturing process, has recently been applied to the fabrication of tissue-engineered constructs for a range of applications including cartilage defect repair. The process involves the layer-by-layer deposition of cell-laden biomaterials, called bioinks. The 3D bioprinting technique can be applied to replicate the complex organisation of cells and ECM within native tissues due to its ability to precisely control material deposition [6]. Additionally, cells, drugs, and bioactive molecules can be incorporated in a spatially controlled manner within the constructs for an enhanced cellular response, and thus, 3D bioprinting boasts major advantages over current scaffold fabrication techniques. The selection of an appropriate bioink is a critical consideration when designing 3D bioprinted constructs. Bioinks must comply with a wide range of stringent requirements, including biocompatibility and biodegradability, while also possessing the necessary rheological properties to ensure good printability. Often, adjusting factors that improve printability such as increased viscosity, induce a harsh environment for the survival and functionality of cells. A delicate compromise between these factors is therefore required to achieve the optimal bioink and construct compositions [7]. Three-dimensional bioprinted constructs require the ideal biochemical composition, architecture, surface properties, and mechanical properties to support cell growth, proliferation, and differentiation, and to withstand the biological environment post-implantation. Researchers focuses on the recent advances in the development of bioinks and 3D bioprinted constructs for cartilage tissue engineering applications and discusses the potential for the translation of these constructs to the clinic for the treatment of damaged articular cartilage.
2. Tissue Engineering Approaches for Cartilage Tissue Engineering
Cartilage has a dense structure comprised of highly specialised cells, known as chondrocytes and chondroblasts, embedded in the cartilaginous extracellular matrix (ECM) which is comprised mainly of proteoglycans, glycoproteins, collagen fibres, elastin fibres, and water. Articular cartilage has a complex layered structure consisting of four zones: (i) a superficial zone, (ii) a transitional zone, (iii) a deep zone, and (iv) a calcified zone, each with different matrix compositions, structural organization, and cell density. The superficial zone contains collagen type II fibers aligned parallel to the cartilage surface, the transition zone contains randomly orientated collage II fibers, while the in the deep zone type II collagen fibers are arranged vertically. This unique anatomy results in gradient physical, mechanical, and biological properties which makes articular cartilage damage increasingly complex to repair and poses challenges for the design of tissue-engineered constructs for cartilage repair.
A wide range of fabrication techniques have been used to fabricate porous scaffolds for cartilage tissue engineering applications including porogen-leaching [8], gel-pressing [9], solvent-casting [10], electrospinning [11], and freeze-drying [12][13]. More recently, approaches that enable the fabrication of layered scaffolds that more closely replicate the graduate nature of cartilage tissue have been developed [13][14]. While these techniques allow control of the material composition in each layer, spatial control over the organisation of cells and growth factors within the constructs cannot be effectively achieved. Thus, 3D bioprinting offers the potential to achieve constructs for cartilage tissue repair that more closely mimic the native tissue environment and thus hold a greater potential to achieve rapid, long-lasting repair of cartilage tissue.
3. Clinical Translation of 3D Bioprinted Constructs for Cartilage Repair Applications
Extrusion-based 3D bioprinting has shown promise for the fabrication of constructs composed of both natural and synthetic biomaterial-based bioinks for cartilage tissue engineering applications. While the ability of these constructs to promote chondrogenesis has been demonstrated in vitro, further pre-clinical studies are required to demonstrate their efficacy in vivo. To date, 3D bioprinted constructs have yet to be successfully translated to the clinic. The technique has been shown to have good reproducibility and potential for mass scalability and it also shows promise for use in personalised medicine. However, limitations remain including high costs and complex regulatory pathways for the approval of tissued engineered constructs. The proposed clinical application of this technique in a personalised medicine approach involves three stages: (i) medical imagery, (ii) construct design, and (iii) construct bioprinting (Figure 1). The medical imaging stage employs imaging techniques such as computed tomography (CT) and magnetic resonance (MRI) to obtain a 3D image of the cartilage defect. This data is stored in the Digital Imaging and Communications in Medicine (DICOM) format, the standard image file format for medical imaging. Following this, the DICOM file is reverse-engineered and imported into computer-aided design (CAD) software. This enables the generation of a surface model that mimics the shape and structure of the defect site. This model is converted into an STL file and then used to create two-dimensional (2D) slices of the construct. A motion programme is then created which contains codes that provide the tool path information for the printer. Patient cells would then be harvested and combined with the desired biomaterial to produce a bioink. The desired construct would then be bioprinted in a layer-by-layer fashion. Finally, any post-processing or crosslinking required would be applied to achieve a final 3D bioprinted construct ready for implantation into the defect site [15].
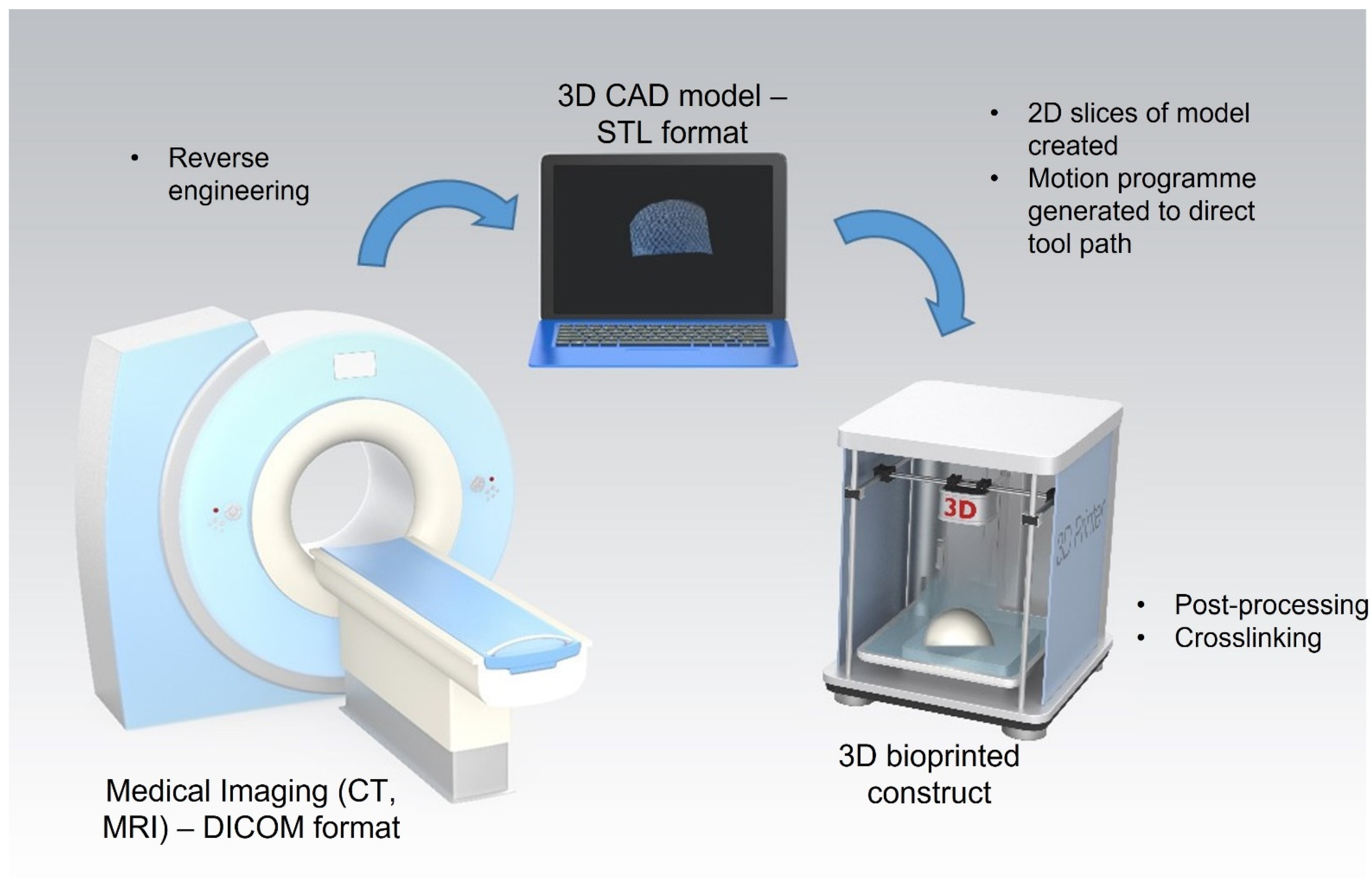
Figure 1 Process for 3D bioprinting of patient specific constructs for cartilage tissue engineering applications. Stage 1: 3D image of cartilage defect captured using computed tomography (CT) or magnetic resonance (MRI) and stored in the Digital Imaging and Communications in Medicine (DICOM) format. Stage 2: DICOM file is reverse-engineered and imported into computer-aided design (CAD) software and then converted into an STL file. Stage 3: A motion programme is created to provide the tool path information for the printer. The desired construct is bioprinted in a layer-by-layer fashion and post-processing/crosslinking applied.
An alternative approach is the use of in situ bioprinting where bioinks are directly printed into the defect site by the surgeon within a clinical setting. This removes the requirement for the bioprinted construct to be handled by the surgeon prior to implantation. This approach may provide particular advantages for the reconstruction of complex geometries, such as curved surfaces [16]. An interesting example of this approach is the BioPen (Australian Research Council Centre of Excellence for Electromaterials Sciences (ACES), University of Wollongong (UOW)), a handheld, 3D bioprinting device dedicated to in situ 3D bioprinting for cartilage tissue repair [17]. This device is a handheld co-axial extrusion device that allows the deposition of cells embedded in a hydrogel material in the surgical setting. The complex regulatory pathway for tissue-engineered constructs presents a major challenge to the successful translation of 3D bioprinting technologies to the clinic. Further research is required to ensure that bioprinted products are reproducible, high quality, safe, and effective at achieving repair of cartilage tissue [18]. Obtaining ethical approval for the harvest and expansion of stem cells in the laboratory and subsequently, their use in surgery presents a challenge to clinical translation. As a relatively new technique, there is a lack of bioprinting-specific standards and this poses further challenges when obtaining regulatory approval for bioprinted constructs. In order to overcome these challenges, close collaboration between academia, industry, and regulators will be essential.
References
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468.
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248.
- Bitton, R. The economic burden of osteoarthritis. Am. J. Manag. Care 2009, 15, S230–S235.
- Hotham, W.E.; Malviya, A. A systematic review of surgical methods to restore articular cartilage in the hip. Bone Jt. Res. 2018, 7, 336–342.
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40.
- Dijkgraaf, L.C.; de Bont, L.G.M.; Boering, G.; Liem, R.S.B. Normal cartilage structure, biochemistry, and metabolism. A review of the literature. J. Oral Maxillofac. Surg. 1995, 53, 924–929.
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055.
- Dudziński, K.; Chwojnowski, A.; Gutowska, M.; Płończak, M.; Czubak, J.; Łukowska, E.; Wojciechowski, C. Three dimensional polyethersulphone scaffold for chondrocytes cultivation—The future supportive material for articular cartilage regeneration. Biocybern. Biomed. Eng. 2010, 30, 65–76.
- Lee, S.; Lee, K.; Kim, S.H.; Jung, Y. Enhanced cartilaginous tissue formation with a cell aggregate-fibrin-polymer scaffold complex. Polymers 2017, 9, 348.
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacant, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993, 14, 323–330.
- Zhou, Y.; Chyu, J.; Zumwalt, M. Recent Progress of Fabrication of Cell Scaffold by Electrospinning Technique for Articular Cartilage Tissue Engineering. Int. J. Biomater. 2018, 2018, 1953636.
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J. Advanced strategies for articular cartilage defect repair. Materials 2013, 6, 637–668.
- Levingstone, T.J.; Matsiko, A.; Dickson, G.R.; O’Brien, F.J.; Gleeson, J.P. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014, 10, 1996–2004.
- Fu, L.; Yang, Z.; Gao, C.; Li, H.; Yuan, Z.; Wang, F.; Sui, X.; Liu, S.; Guo, Q. Advances and prospects in biomimetic multilayered scaffolds for articular cartilage regeneration. Regen. Biomater. 2020, 7, 527–542.
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023.
- Singh, S.; Choudhury, D.; Yu, F.; Mironov, V.; Naing, M.W. In situ bioprintingBioprinting from benchside to bedside? Acta Biomater. 2020, 101, 14–25.
- Onofrillo, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; O’Connor, A.J.; Scott, M.; Wallace, G.G.; Choong, P.F.M.; Di Bella, C. Biofabrication of human articular cartilage: A path towards the development of a clinical treatment. Biofabrication 2018, 10, 045006.
- Stanco, D.; Urbán, P.; Tirendi, S.; Ciardelli, G.; Barrero, J. 3D bioprinting for orthopaedic applications: Current advances, challenges and regulatory considerations. Bioprinting 2020, 20, e00103.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
829
Revisions:
2 times
(View History)
Update Date:
21 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




