Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Indrajit ROY | -- | 3232 | 2022-06-20 19:45:49 | | | |
| 2 | Conner Chen | -26 word(s) | 3206 | 2022-06-21 02:26:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Roy, I.; Chakraborty, N.; Gandhi, S.; Verma, R. Nanozyme Platforms for Antibacterial Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/24234 (accessed on 08 February 2026).
Roy I, Chakraborty N, Gandhi S, Verma R. Nanozyme Platforms for Antibacterial Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/24234. Accessed February 08, 2026.
Roy, Indrajit, Nayanika Chakraborty, Sona Gandhi, Rajni Verma. "Nanozyme Platforms for Antibacterial Applications" Encyclopedia, https://encyclopedia.pub/entry/24234 (accessed February 08, 2026).
Roy, I., Chakraborty, N., Gandhi, S., & Verma, R. (2022, June 20). Nanozyme Platforms for Antibacterial Applications. In Encyclopedia. https://encyclopedia.pub/entry/24234
Roy, Indrajit, et al. "Nanozyme Platforms for Antibacterial Applications." Encyclopedia. Web. 20 June, 2022.
Copy Citation
Some key enzymes or classes of enzymes, such as superoxide dismutase (SOD), catalase (CAT), oxidase (OXD), peroxidase (POD), deoxyribonuclease (DNase), etc., have been implicated in antibacterial therapy.
enzyme mimics
nanozyme
reactive oxygen species
1. Nanozyme Platforms for Antibacterial Applications
Bacterial diseases are a major public health concern worldwide [1][2]. Although antibiotic treatment is the most widely recognized paradigm for treating such diseases, the continuous use, overuse, and misuse of antibiotic-based drugs over a long time have prompted the evolution of multi-drug-resistant super-bacteria [3][4]. Drug resistance is further promoted since bacteria thrive predominantly in surface attached extracellular polymeric substance (EPS) matrix-enclosed-multicellular communities or as biofilms. Drug-resistant bacterial diseases currently account for the loss of 700,000 lives annually. Unless novel antibiotic-free interventional strategies are developed urgently, the mortality rate is anticipated to skyrocket to 10 million lives annually by 2050 [5].
To overcome this challenge, enormous efforts have been focused on the discovery and development of alternative broad-spectrum antibacterial agents or strategies [6][7]. In this respect, antibacterial peptides, bacteriophage therapy, and antibacterial enzymes have gained popularity over recent years. In addition, nanomaterial-based antibacterial platforms such as photothermal therapy (PTT) [8], photodynamic therapy (PDT) [9], and photocatalytic therapy (PCT) [10] are also becoming prevalent. Recent studies have confirmed that the excellent activity and high substrate selectivity of certain endogenous enzymes, such as natural POD and OXD, can restrain the escalation of bacterial growth and disrupt biofilms. These enzymes catalyse critical biochemical reactions to locally generate toxic reactive oxygen species (ROS) that oxidize key bacterial components such as cell membrane/wall or intracellular compartments. However, owing to the various shortcomings of natural enzymes, engineered nanozymes are being extensively used as promising alternatives in antibiotic-free antibacterial therapy [11]. Nanozymes are also less likely to develop bacterial resistance owing to good membrane permeability and biocompatibility [12]. More importantly, nanozymes can be equipped with catalytic activities to eradicate bacterial biofilms [13][14][15].
2. Metal-Based Nanozymes
Several noble-metal- (gold, silver, platinum, etc.) based nanozymes display strong catalytic activity. Zheng et al. developed mercaptopyrimidine conjugated Au nanoclusters (Au NCs) targeting intractable superbugs in vitro and in vivo. The positive charge of the nanozyme facilitated their easy adherence on the bacterial surface and subsequent cell membrane damage. The induction of intracellular ROS production in bacterial cells was mainly accredited to the intrinsic oxidase-like and peroxidase-like activity, leading to killing about 99% of bacteria and promoting the wound healing process [16]. Zhang et al. evaluated bimetallic platinum–copper (PtCu) alloy nanoparticles for both peroxidase-like and ferroxidase-like in a weakly acidic medium and detection of Fe2+, in addition to their antibacterial potency [17]. Similarly, Cai et al. formulated core–shell Pd@Ir bimetallic nanostructures using a seed-mediated-growth method with morphology-dependent bactericidal activity. This study established that the Pd@Ir octahedron showed better antibacterial activity when compared to Pd@Ir cubes due to higher OXD-like activity. The oxidation of 3,3′,5,5′-tetramethylbenzidine using these Pd@Ir nano cubes had a 1.7 times higher Vmax value and a 4.4 times higher Vmax value for Pd@Ir nano octahedron than when catalyzed by Pd cubes. Moreover, the study also revealed that the OXD-like activity of Pd@Ir became elevated in the presence of natural organic matter. Upon interaction with humic acid (HA), the nanozyme generated high levels of ROS and also promoted cellular internalization of the nanostructure [18].
The high coordination capability of the Cu ion with amino acids prompted the development of Cu-based nanozymes with intrinsic POD-like activity. Cu-embedded hydrogel-based nanozymes can provide effective coverage of wounds and accelerate the wound-healing process with the assistance of H2O2 by stimulating angiogenesis and collagen deposition [19]. As mentioned previously, the nanozyme activity can be enhanced using external stimuli, such as light, magnetic field, and electricity. Karim et al. demonstrated that upon photoactivation, the POD-like activity shown by CuO nanorods are significantly enhanced owing to their favorable band structure. In the presence of H2O2, about 20 times enhanced production of the ROS was observed upon light illumination of the nanorods, leading to potent physical damage to bacterial cells treated with the nanorods [20]. Bimetallic CuCo2S4 nanoparticles have demonstrated exceptional POD-like activity and high antibacterial capability at neutral pH, when compared to monometallic CuS and CoS NPs. These bimetallic nanozymes could accelerate the healing of burn injuries infected with methicillin-resistant S. aureus (MRSA) in vivo [21].
3. Metal Oxide/Sulfide-Based Nanozymes
Cerium oxide nanoparticles (CeO2) are a classic example of biological catalyst with high POD-like activity attributed to a reversible redox switch between Ce4+ and Ce3+ ions. A CeO–H2O2 system is more conducive to promote ROS due to very high and efficient POD-like activity. Different shapes and sizes of nanoceria lead to multiple enzymatic activities, such as that of SOD, CAT, POD, and OXD, because of surface-rich oxygen vacancies, smooth oxygen diffusion, and high redox potential [22]. Luo et al. developed an imidazolium-type poly (ionic liquid) (PIL)/cerium(IV) ion-based electrospun nanofibrous membrane (PIL-Ce) exhibiting DNase mimetic catalytic activity and rapid wound healing in an MRSA-infected mice model. The high antibacterial potential of PIL-Ce was evaluated, and the disintegration of resistant genes was also investigated to block the spread of drug resistance [23].
S. Enteritidis is a notorious zoonotic food-borne pathogen that evades antibiotic treatment and thrives within host cells in animals. An iron oxide nanozyme (IONzyme) with intrinsic POD-like activity was formulated, which can catalyze H2O2 to generate ·OH radicals under acidic conditions for suppressing the survival of intracellular S. Enteritidis [24]. Following the co-localization of IONzymes with S. Enteritidis in acid autophagic vacuoles of leg-horn male hepatoma-derived cells, ROS-regulated antibacterial action against intracellular S. Enteritidis was observed via autophagic elimination (Figure 1). Furthermore, transcriptomic profiling displayed that IONzymes changed the hepatic oxidation reduction along with autophagy-related gene expression in chicken livers infected with S. Enteritidis.
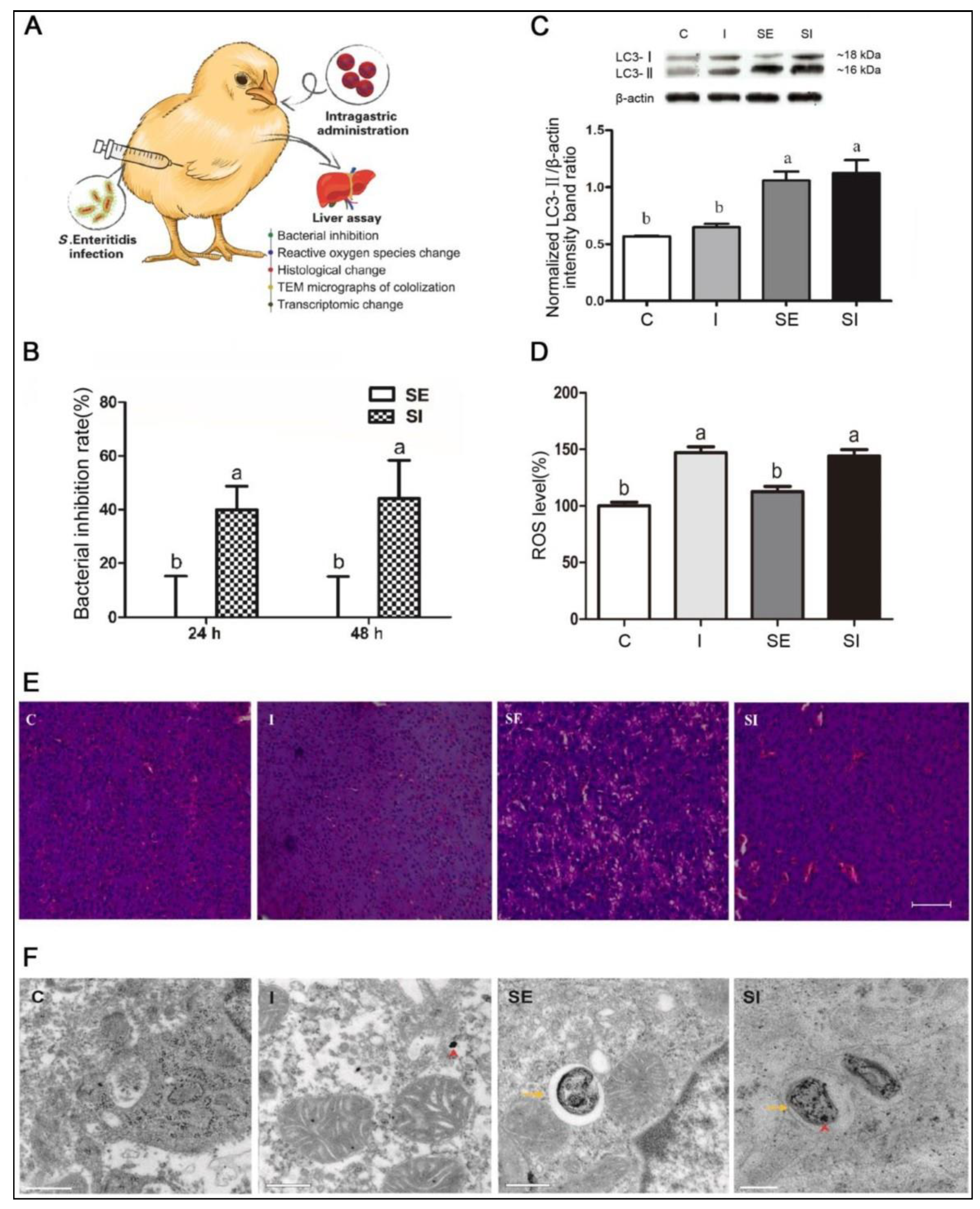
Figure 1. Iron-oxide nanozyme (IONzyme) with peroxidase-like activity in the treatment of chickens infected with S. Enteritidis. Study groups: vehicle control (C); IONzyme alone (I); S. Enteritidis without (SE) or with (SI) IONzyme: (A) schematic illustration of the experiment in chickens, which were subcutaneously administered with the bacteria and orally administered with IONzymes; (B) bacterial inhibition in infected livers, without and with IONzyme treatment, at 24 and 48 h post-treatment; values represent the mean ± SEM (n = 9). Different letters indicate statistically significant difference (p < 0.05).; (C) Western blot analysis showing normalized hepatic LC3 protein expression levels for the various study groups; the values represent the mean ± SEM (n = 3). Different letters indicate statistically significant difference (p < 0.05); (D) the relative levels of ROS in various study groups; values represent the mean ± SEM (n = 6). Different letters indicate statistically significant difference (p < 0.05); (E) photomicrographs of the liver histological sections for the various study groups; scale bar: 50 µm; (F) TEM micrographs showing bacterial co-localization with IONzymes within autophagosomes of chicken liver. IONzyme: red short triangle arrow; S. Enteritidis: yellow arrows. Scale bar: 0.5 µm (in C, I, SE) and 0.2 µm (SI).
Biofilms are a complex consortium of bacterial communities embedded within a self-produced exopolysaccharide extracellular matrix that create a localized and protected microenvironment adhered to the surface. In a recent study by Gao et al., nano-iron sulphide particles were synthesized by the conversion of a natural organo-sulphur compound derived from garlic with broad-spectrum antibacterial activity against resistant bacterial infections. Nano-iron sulphide is a nanozyme exhibiting POD-like and CAT-like activities, which catalyzes the oxidation of H2O2 to accelerate the production of highly toxic hydrogen polysulphide and expedite 500-fold antibacterial efficacy against drug-resistant bacteria. This nanozyme also has the potential to fight biofilms on human dental caries and accelerate wound healing [25]. The bacterial strain S. mutans accumulates on the tooth surface utilizing sugar-rich acidic conditions and demineralizes the enamel-apatite causing caries biofilms. Catalytic Fe3O4 nanoparticles are shown to target and degrade the caries-causing biofilms with high specificity under acidic conditions without impacting surrounding oral tissues in vivo. These nanoparticles generate ·OH radicals at low concentrations of H2O2 (1%), disrupting the embedded S. mutans in the protective biofilm matrix. Furthermore, the dissolution of hydroxyapatite in the acidic conditions was reduced when the caries was treated with the nanoparticles, providing a mechanistic insight in the prevention of dental caries [26].
In another example, it was shown that dextran-coated iron oxide nanoparticles (Dex-NZM) exhibited 49% co-localization with bacteria and 51% with the exopolysaccharides while avoiding binding to gingival cells. Dextran coating concurrently increased the stability, the selectivity catalytic activity, and the treatment efficiency of Dex-NZM nanoparticles against the biofilm matrix due to the ease of incorporation into the extracellular matrix of the biofilm, thus enhancing the matrix breakdown [27]. A ferritin nanozyme (Fenozyme) was developed by the integration of recombinant human ferritin protein shells (HFn) specifically targeting the blood–brain barrier endothelial cells with an inner Fe3O4 nanozyme core exhibiting CAT-like activity. Fenozyme possesses the capability to protect the integrity of the blood–brain barrier and prevent cerebral malaria as was observed in an experimental cerebral malaria mouse model [28]. Fe-based nanozymes possess POD, CAT, OXD, glucose oxidase (GOx), sulphite oxidase (SOx), and SOD-like activity, with the potential to treat biofilm-associated diseases and combat bacteria.
4. Carbon-Based Nanozymes
Carbon-based nanomaterials, such as carbon nanotubes (CNTs), Carbon dots (CDs), graphene and its derivatives, carbon nitride, and fullerene have been widely applied in the field of biomedicine owing to their physiochemical properties, biocompatibility, and multi-enzyme-mimicking activities. Their superior mechanical properties also facilitate their use as dressing materials for the healing of infected wounds. Wang et al. prepared a series of carbon nanotubes with rich oxidized groups (o-CNTs), exhibiting superior POD-like activity over a wide pH range [29]. The carbonyl group on the surface of o-CNTs acted as active catalytic centers, whereas the carboxyl and hydroxyl groups presented competitive sites. Due to an inherent negative charge and the tendency to form hydrogen bonding, the carboxyl group has a higher inhibitory tendency on the catalytic propensity than the hydroxyl group. Therefore, 2-bromo-1-phenylethanone-modified o-CNTs (o-CNTs-BrPE) were prepared to reduce the inhibitory effect of the carboxyl group in the nanozyme. As the number of competing sites decreased, o-CNTs-BrPE exhibited good POD-like activity, thus enabling catalysis of H2O2 to ·OH and leading to the eradication of bacteria and the protection of tissues from bacteria-induced edema and purulent inflammation.
Graphene quantum dots (GQDs) display superior enzyme mimetic activity owing to rapid electron transfer when compared to graphene oxide. Chen et al. developed a GQD-based antibacterial nanozyme system capable of generating highly toxic ROS that showed enhanced and broad-spectrum antibacterial activity. Moreover, GQD-based band-aids exhibited exceptional in vivo wound healing at low concentrations of H2O2 without conceding cell proliferation [11]. In another example, hybrid nanozymes were constructed by incorporating Au NPs within graphitic carbon nitride nanosheets (g-C3N4 NSs). The incorporation of AuNPs increased the POD-like activity and robust antibacterial potency against multidrug resistant S. aureus due to synergism between the nanosheets and the AuNPs [30].
Carbon nanosheet-based stimuli-responsive biomaterials have been developed recently to treat bacterial infections. Ren’s group developed copper ion-doped carbon nanosheets (Cu-SA) to behave as POD and glutathione (GSH) peroxidase mimics. Glutathione is part of the defense mechanism of bacteria that protects it from oxidation by scavenging excess ROS from the bacterial cells. This hybrid nanozyme was incorporated within a biocompatible and thermo-responsive hybrid nanogel made up of bacterial cellulose nanowhiskers (BCNWs) and poly(N-isopropylacrylamide). The resulting Cu-SA@BCNW/PNI hybrid nanogel showed high antibacterial action via the generation of excess ROS at the cost of intracellular GSH (see Figure 2). This intelligent hybrid nanogel has a remarkable sol–gel transition response at human physiological temperature. This ability allows the hybrid nanogel to form a gel over infected regions in situ for faster disinfection of wound sites [31].
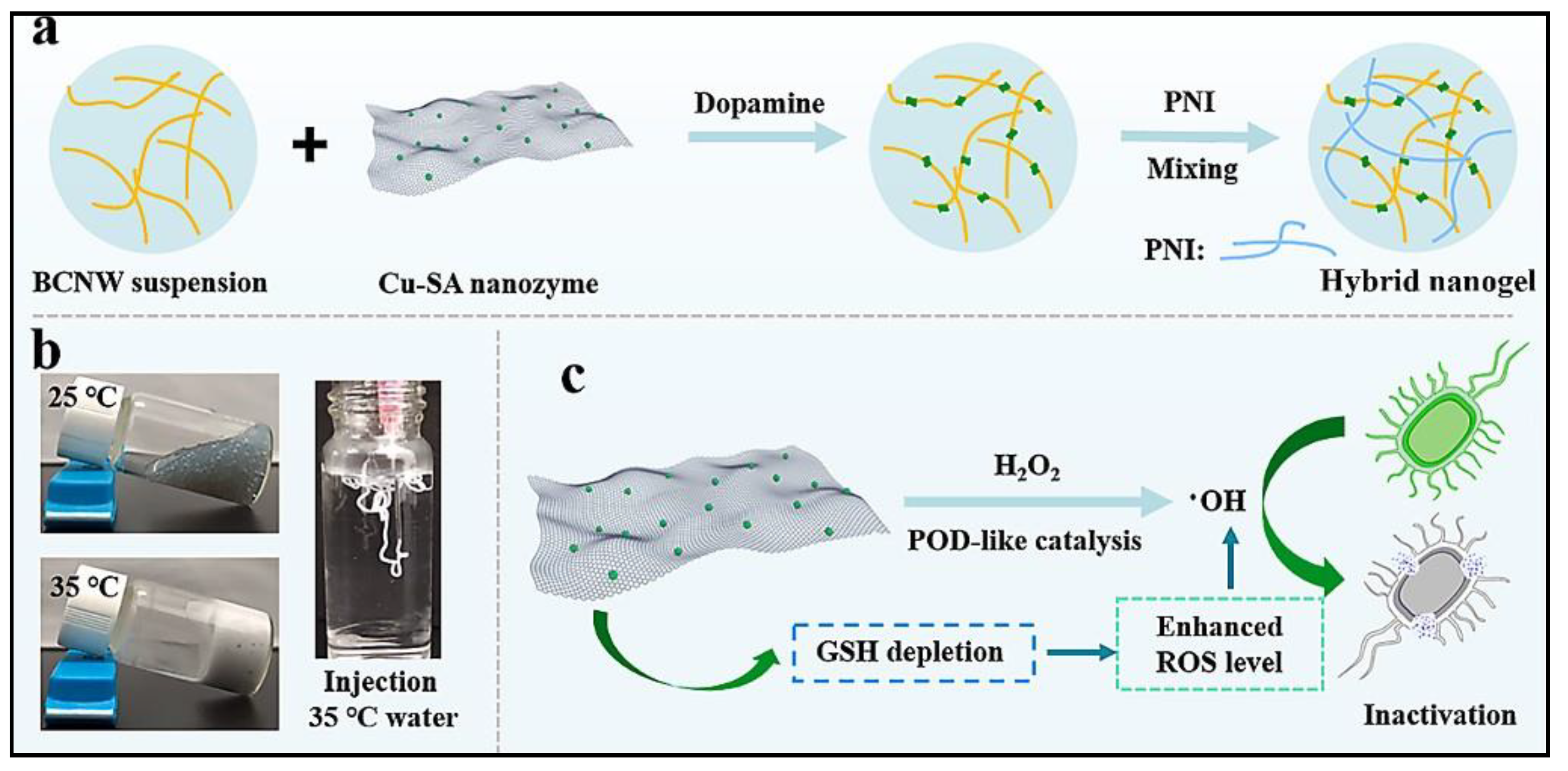
Figure 2. Schematic representation showing: (a) fabricating of Cu-SA@BCNW/PNI hybrid nanogels from BCNW (bacterial cellulose nanowhiskers), Cu-SA nanozyme, dopamine, and PNI (poly-N-isopropylacrylamide); (b) thermo-responsiveness of Cu-SA@BCNW/PNI hybrid nanogels; and (c) mechanism of antibacterial activity of the nanozyme.
5. Transition Metal Dichalcogenide (TMDC)-Based Nanozymes
A number of 2D transition metal dichalcogenides (TMDCs) have also emerged as promising antibacterial agents owing to their large 2D surface area and intrinsic enzyme-like properties. Recently, a defect-rich adhesive MoS2/rGO vertical heterostructure (VHS) was synthesized, with abundant elemental vacancies and a rough surface that facilitates better bacterial capture and ROS production through local topological interactions. The nanozyme exhibited exceptional antibacterial activity because of triple enzymatic (POD-like, CAT-like, and OXD-like) activity and surface defects [32]. Another group has reported that in comparison to MoS2 nanozymes, defect-rich MoS2 flower-shaped nanozymes have superior antibacterial efficacy owing to two reasons. First, the rough surface of the latter promotes higher adhesion to bacterial cells. Second, their defect-rich active edges facilitate improved POD-like activity owing to thermodynamically favorable lower adsorption energy of substrate H2O2 and desorption energy of ·OH radicals [33].
The light-absorbing capability of TMDC-based nanoparticles can be exploited for photoactivated enhancement of enzymatic activity. Shan et al. reported that Cu2MoS4 nanozymes can be activated by irradiation with near infrared light (1064 nm, 1 W cm−1). This photoactivation could enhance the OXD and POD-like activity of the nanozymes, leading to remarkable anti-bacterial activity against 8 log MDR E. coli and 6 log S. aureus [34]. The activity of the Cu2MoS4 nanozymes can be further tuned with topographic modifications. On the other hand, Niu et al. developed an intelligent photoregulated strain-selective bactericidal strategy based on a charge tunable MoS2 nanozyme and a photoactive molecule. The nanozyme was synthesized by citraconic anhydride-modified PEI-MoS2 and the charge tuning was achieved by adjusting the pH responsive citric anhydride. This system has the capability of being modulated by light for charge reversal on the surface and concurrently leading to the enzymatic activation of MoS2 upon varying the pH of the system [35].
Silver nanoparticles (Ag NPs) display a wide-spectrum antibacterial propensity attributed to the release of Ag+ ions that causes structural deformity and protein inactivation of bacterial cells. Recent reports have substantiated that Ag NPs can mimic enzymes such as SOD, CAT, OXD, and POD. The activation of molecular oxygen on the surface of Ag NPs leads to the enzymatic activity. A hybrid Fe3O4@MoS2-Ag nanozyme was constructed by a hydrothermal method and in situ photo deposition of Ag NPs on a defect-rich rough surface of MoS2 to capture the bacteria. The nanozyme resulted in the inactivation of ~69.4% E. coli due to the synergistic disinfection caused by the POD-like activity, the leakage of Ag+ ions, and the NIR (808 nm) light-activated photothermal effect resulting in the production of ROS (see Figure 3). In addition, the magnetic property of Fe3O4 was exploited to recycle the nanozyme [36].
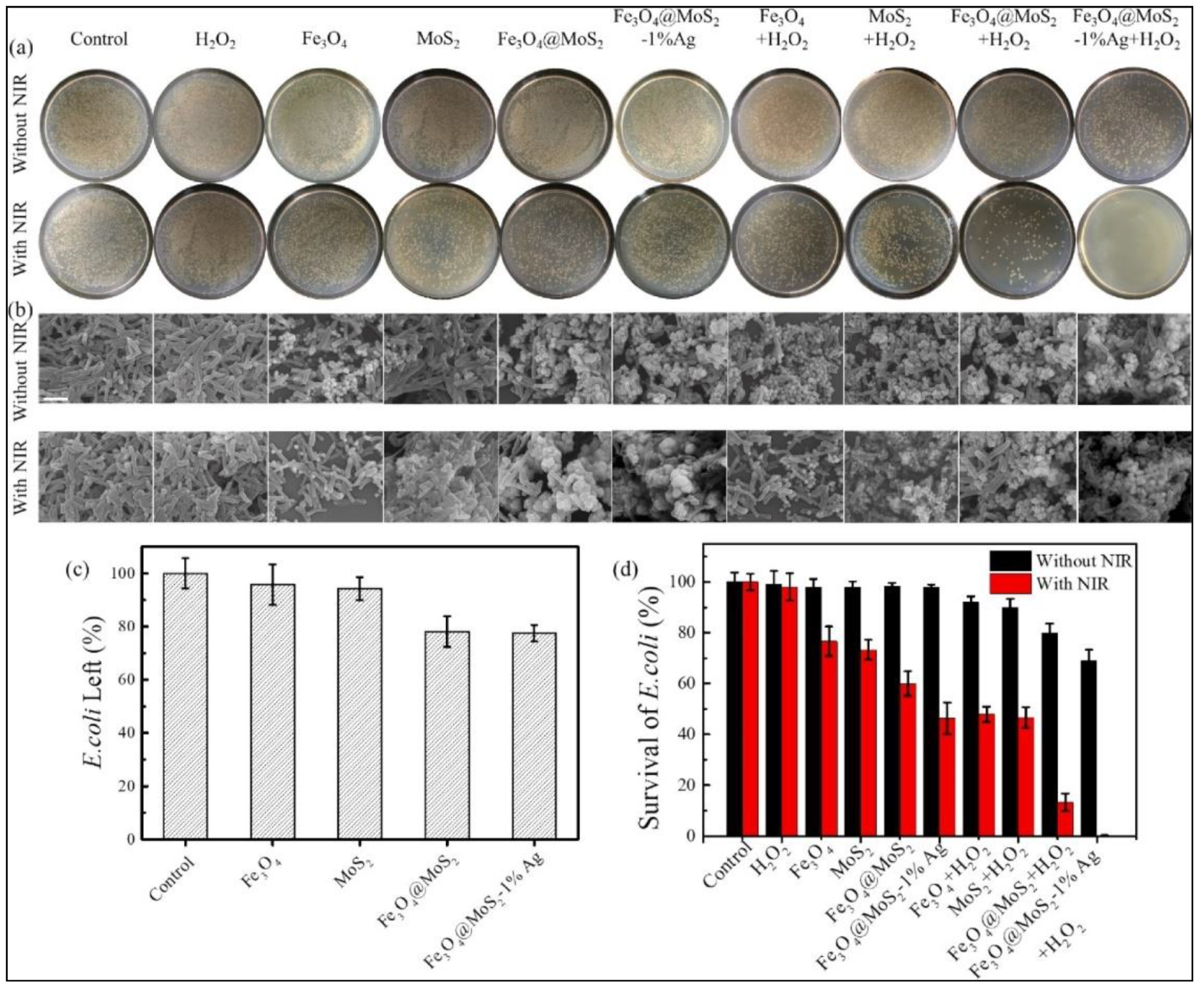
Figure 3. (a) Photographs of standard agar plates showing viable bacterial colonies for various controls and samples, without and with NIR light irradiation; (b) SEM images of bacteria treated with corresponding samples; (c) bacteria percentage left in the suspension after removing the catalysts; (d) percentage survival of bacteria treated with different controls and samples.
6. Prussian Blue (PB) and Metal–Organic Framework (MOF)-Based Nanozymes
Biocompatible Prussian blue (PB) nanocrystals have shown moderate levels of antibacterial activity through POD and OXD-mimetic generation of ROS in bacterial cells. However, as PB nanocrystals generate heating upon exposure to visible light, their antibacterial activity can be significantly enhanced upon combined photothermal therapy. It has been demonstrated that the antibacterial action of the PB nanocrystal platform can be further enhanced upon coating with a layer of chitosan that facilitates high affinity electrostatic interaction with both Gram-positive and Gram-negative bacterial cells [37]. In a separate work, it has been shown that upon doping silver ions in the PB network, very high broad-spectrum antibacterial action could be observed due to the combined effects of ROS generation and silver-ion-mediated toxicity, along with a photothermal effect [38].
Metal organic frameworks (MOFs) are hybrid nanomaterials comprised of organic bridging links and metal nodes, with unique 3D structures having distinctive features, such as specific pore size, diversity in porous structure, and large surface area. Biocompatible MOFs not only serve as hosts for stabilizing natural enzymes, but also serve as catalytic sites with high enzyme-like activities. The high degree of order in the framework provides proper arrangement of active catalytic sites, leading to better interactions with the substrates [39][40]. Ren et al. demonstrated a nature-inspired strategy for the construction of an MOF-based functional enzyme mimic with active sites engineered in a pore microenvironment and pseudopodia-like surface to enhance the bacteria-trapping capability [41]. This nanozyme serves as a faithful POD mimic, with its metal nodes acting as the active centers and nanosized cavities as binding pockets. This system is engineered in such a way that the microenvironment around the active site enriches and activates the substrate molecule for bacterial inhibition. The MOF-nanozyme displayed enhanced antibacterial efficacy as its pseudopodia-like surface promoted effective trapping of bacteria. In another study, an Au-doped MOF/Ce-based nanozyme (MOF-2.5Au-Ce) was designed with DNase- and POD-mimicking activities to disrupt biofilms [42]. The cerium(IV) complexes act as DNase mimics, which hydrolyze the extracellular DNA (eDNA) and components of already manifested biofilms. In addition, the MOF displays strong POD-like catalytic activity against the bacteria rendered in the biofilm, thus controlling the recurrence of biofilm or recolonization of bacteria (Figure 4).
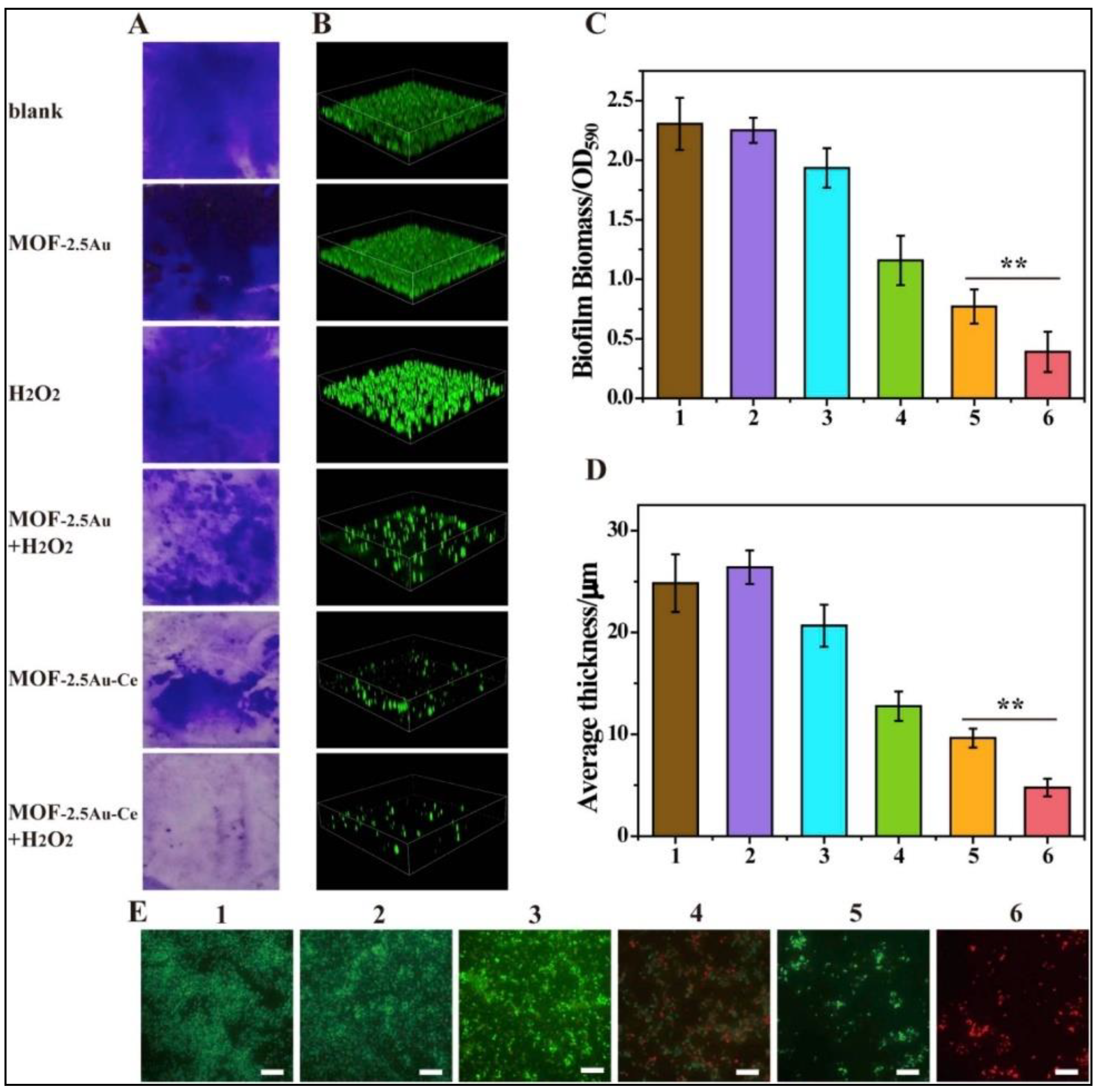
Figure 4. MOF-2.5Au-Ce nanozymes for inhibiting bacterial biofilm formation. (A,C) Crystal violet staining, and (B,D) 3D Confocal Laser Scanning Microscopic (CLSM) imaging of biofilms treated with various samples for 24 h. Optical density at 590 nm (OD590) was measured to quantify the biomass of biofilms. Error bars were calculated on the basis of three independent experiments; ** represents p ≤ 0.01. Image sizes of CLSM: 315 μm × 315 μm. Biofilm thickness was quantified with Comstat 2 software. (E) LIVE/DEAD stain images of residual biofilms using fluorescence microscopy. Green and red stains indicated live and dead bacteria, respectively. Scale bar = 10 μm. Various treatment samples: (1): control (culture medium only); (2): MOF-2.5Au; (3): H2O2; (4): MOF-2.5Au + H2O2; (5): MOF-2.5Au-Ce; and (6): MOF-2.5Au-Ce + H2O2.
7. Single Atom Nanozymes (SANs)
Recently, single atom nanozymes (SANs) have emerged as unique catalytic agents for a variety of applications. As compared to ‘conventional’ nanozymes, where irregular active site distribution or truncated surface densities lead to reduced catalytic activity and specificity, in SANs, the active sites are maximized due to then even distribution of metal centers [43]. Various SANs, such as Pt–Cu, Pt/CeO2, M–N5 and M–N4 (M = Fe, Co, Zn, etc.), have been developed [44][45][46]. These SANs display various enzyme-mimicking properties, such as POD, SOD, CAT, and glutathione peroxidase (GPx-like) activities, and have great potential in anti-inflammation, anti-bacteria, therapeutic diagnosis and degradation of organic pollutants [47][48][49]. Shi et al. synthesized single iron atom nano-catalysts by anchoring single iron atoms in N-doped amorphous carbon (SAF NCs) which induces POD-like activity and is highly effective in bacterial peroxidation. These SAF NCs have shown excellent antibacterial activity against both Gram-positive S. aureus and Gram-negative E. coli bacterial cells, with a low MIC of 62.5 μgmL−1 in vitro. Furthermore, the antibacterial activity can be significantly enhanced by 808 nm NIR laser irradiation. Combined with the good photothermal property of SAF NCs, in vivo bacterial infections can be effectively treated, resulting in better wound healing [50].
Liu et al. synthesized a zinc-based zeolitic-imidazolate framework (ZIF-8)-derived carbon nanomaterial containing atomically dispersed zinc atoms with an efficient POD-like activity. This high activity of this SAN is attributed to the presence of coordinatively unsaturated Zn–N4 active sites which has been confirmed by density functional theory (DFT) calculations. In the presence of H2O2, this SAN inhibited the growth of P. aeruginosa by up to 99.87% and also promoted in vivo bacteria-infected wound disinfection and healing [51]. Recently, SANs with carbon nanoframe-confined FeN5 active centers, which catalytically behaved like the axial ligand-coordinated scheme of cytochrome P450, were reported by Huang et al. Synergistic effects furnish FeN5 SANs with a clear electron push-effect mechanism and also the highest OXD-like activity among other nanozymes (the rate constant 70 times higher than commercial Pt/C) and versatile antibacterial applications [52].
References
- Cattoir, V.; Felden, B. Future Antibacterial Strategies: From Basic Concepts to Clinical Challenges. J. Infect. Dis. 2019, 220, 350–360.
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993.
- Andersson, D.I. Improving predictions of the risk of resistance development against new and old antibiotics. Clin. Microbiol. Infect. 2015, 21, 894–898.
- Li, Y. China’s misuse of antibiotics should be curbed. Br. Med. J. 2014, 348, 1083–1084.
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016; pp. 1–84.
- Mei, L.; Zhu, S.; Yin, W.; Chen, C.; Nie, G.; Gu, Z.; Zhao, Y. Two-dimensional nanomaterials beyond graphene for antibacterial applications: Current progress and future perspectives. Theranostics 2020, 10, 757–781.
- Miao, H.; Teng, Z.; Wang, C.; Chong, H.; Wang, G. Recent progress in two dimensional antimicrobial nanomaterials. Chem. Asian J. 2019, 25, 929–944.
- Ray, P.C.; Khan, S.A.; Singh, A.K.; Senapati, D.; Fan, Z. Nanomaterials for targeted detection and photothermal killing of bacteria. Chem. Soc. Rev. 2012, 41, 3193–3209.
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73.
- Xi, J.; Wei, G.; Wu, Q.; Xu, Z.; Liu, Y.; Han, J.; Fan, L.; Gao, L. Light-enhanced spongelike carbon nanozyme used for synergetic antibacterial therapy. Biomater. Sci. 2019, 7, 4131–4141.
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc. Chem. Res. 2018, 51, 789–799.
- Zhang, R.; Fan, K.; Yan, X. Nanozymes: Created by learning from nature. Sci. China Life Sci. 2020, 63, 1183–1200.
- Liang, M.; Wang, Y.; Ma, K.; Yu, S.; Chen, Y.; Deng, Z.; Liu, Y.; Wang, F. Engineering Inorganic Nanoflares with Elaborate Enzymatic Specificity and Efficiency for Versatile Biofilm Eradication. Small 2020, 16, 2002348.
- Yang, D.; Chen, Z.; Gao, Z.; Tammina, S.K.; Yang, Y. Nanozymes used for antimicrobials and their applications. Colloids Surf. B 2020, 195, 111252.
- Meng, Y.; Li, W.; Pan, X.; Gadd, G.M. Applications of nanozymes in the environment. Environ. Sci. Nano 2020, 7, 1305–1318.
- Zheng, Y.; Liu, W.; Qin, Z.; Chen, Y.; Jiang, H.; Wang, X. Mercaptopyrimidine conjugated gold nanoclusters as nanoantibiotics for combating multidrug resistant superbugs. Bioconjug. Chem. 2018, 29, 3094–3103.
- Zhang, X.; Jiang, X.; Croley, T.R.; Boudreau, M.D.; He, W.; Cai, J.; Li, P.; Yin, J.-J. Ferroxidase-like and antibacterial activity of PtCu alloy nanoparticles. J. Environ. Sci. Health C 2019, 37, 99–115.
- Cai, T.; Fang, G.; Tian, X.; Yin, J.-J.; Chen, C.; Ge, C. Optimization of antibacterial efficacy of noble-metal-based core-shell nanostructures and effect of natural organic matter. ACS Nano 2019, 13, 12694–12702.
- Qiu, H.; Pu, F.; Liu, Z.; Liu, X.; Dong, K.; Liu, C.; Ren, J.; Qu, X. Hydrogel-based artificial enzyme for combating bacteria and accelerating wound healing. Nano Res. 2020, 13, 496–502.
- Karim, M.N.; Singh, M.; Weerathunge, P.; Bian, P.; Zheng, R.; Dekiwadia, C.; Ahmed, T.; Walia, S.; Gaspera, E.D.; Singh, S.; et al. Visible light-triggered reactive oxygen species mediated antibacterial activity of peroxidase-mimic CuO nanorods. ACS Appl. Nano Mater. 2018, 1, 1694–1704.
- Li, D.; Guo, Q.; Ding, L.; Zhang, W.; Cheng, L.; Wang, Y.; Xu, Z.; Wang, H.; Gao, L. Bimetallic CuCo2S4 nanozymes with enhanced peroxidase activity at neutral pH for combating burn infections. Chem. Biochem. 2020, 21, 2620–2627.
- Han, S.I.; Lee, S.-W.; Cho, M.G.; Yoo, J.M.; Oh, M.H.; Jeong, B.; Kim, D.; Park, O.K.; Kim, J.; Namkoong, E.; et al. Epitaxially strained CeO2/Mn3O4 nanocrystals as an enhanced antioxidant for radioprotection. Adv. Mater. 2020, 32, 2001566.
- Luo, Z.; Cui, H.; Guo, J.; Yao, J.; Fang, X.; Yan, F.; Wang, B.; Mao, H. Poly(ionic liquid)/ce-based antimicrobial nanofibrous membrane for blocking drug-resistance dissemination from mrsa-infected wounds. Adv. Funct. Mater. 2021, 31, 2100336.
- Shi, S.; Wu, S.; Shen, Y.; Zhang, S.; Xiao, Y.; He, X.; Gong, J.; Farnell, Y.; Tang, Y.; Huang, Y.; et al. Iron oxide nanozyme suppresses intracellular Salmonella Enteritidis growth and alleviates infection in vivo. Theranostics 2018, 8, 6149–6162.
- Xu, Z.; Qiu, Z.; Liu, Q.; Huang, Y.; Li, D.; Shen, X.; Fan, K.; Xi, J.; Gu, Y.; Tang, Y.; et al. Converting organosulfur compounds to inorganic polysulfides against resistant bacterial infections. Nat. Commun. 2018, 9, 3713.
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284.
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano 2019, 13, 4960–4971.
- Zhao, S.; Duan, H.; Yang, Y.; Yan, X.; Fan, K. Fenozyme protects the integrity of the blood-brain barrier against experimental cerebral malaria. Nano Lett. 2019, 19, 8887–8895.
- Wang, H.; Li, P.; Yu, D.; Zhang, Y.; Wang, Z.; Liu, C.; Qiu, H.; Liu, Z.; Ren, J.; Qu, X. Unraveling the enzymatic activity of oxygenated carbon nanotubes and their application in the treatment of bacterial infections. Nano Lett. 2018, 18, 3344–3351.
- Wang, Z.; Dong, K.; Liu, Z.; Zhang, Y.; Chen, Z.; Sun, H.; Ren, J.; Qu, X. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 2017, 113, 145–157.
- Zhang, S.; Hao, J.; Ding, F.; Ren, X. Nanocatalyst doped bacterial cellulose-based thermosensitive nanogel with biocatalytic function for antibacterial application. Int. J. Biol. Macromol. 2022, 195, 294–301.
- Wang, L.; Gao, F.; Wang, A.; Chen, X.; Li, H.; Zhang, X.; Zheng, H.; Ji, R.; Li, B.; Yu, X.; et al. Defect-rich adhesive molybdenum disulfide/rGO vertical heterostructures with enhanced nanozyme activity for smart bacterial killing application. Adv. Mater. 2020, 32, 2005423.
- Cao, F.; Zhang, L.; Wang, H.; You, Y.; Wang, Y.; Gao, N.; Ren, J.; Qu, X. Defect-rich adhesive nanozymes as efficient antibiotics for enhanced bacterial inhibition. Angew. Chem. 2019, 58, 16236–16242.
- Shan, J.; Yang, K.; Xiu, W.; Qiu, Q.; Dai, S.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. Cu2MoS4 nanozyme with NIR-II light enhanced catalytic activity for efficient eradication of multidrug-resistant bacteria. Small 2020, 16, 2001099.
- Niu, J.; Sun, Y.; Wang, F.; Zhao, C.; Ren, J.; Qu, X. Photomodualted nanozyme used for a gram-selective antimicrobial. Chem. Mater. 2018, 30, 7027–7033.
- Wei, F.; Cui, X.; Wang, Z.; Dong, C.; Li, J.; Han, X. Recoverable peroxidase-like Fe3O4@MoS2-Ag nanozyme with enhanced antibacterial ability. Chem. Eng. J. 2021, 408, 127240.
- Chakraborty, N.; Jha, D.; Gautam, H.K.; Roy, I. Peroxidase-like behavior and photothermal effect of chitosan coated Prussian-blue nanoparticles: Dual-modality antibacterial action with enhanced bioaffinity. Mater. Adv. 2020, 1, 774–784.
- Sharma, S.; Chakraborty, N.; Jha, D.; Gautam, H.K.; Roy, I. Robust Dual Modality Antibacterial Action using Silver-Prussian Blue Nanoscale Coordination Polymer. Mater. Sci. Eng. C 2020, 113, 110982.
- Zhang, Y.; Sun, P.; Zhang, L.; Wang, Z.; Wang, F.; Dong, K.; Liu, Z.; Ren, J.; Qu, X. Silver-infused porphyrinic metal-organic framework: Surface-adaptive, on demand nanoplatform for synergistic bacteria killing and wound disinfection. Adv. Funct. Mater. 2019, 29, 1808594.
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano 2018, 12, 651–661.
- Ren, J.; Zhang, L.; Liu, Z.; Deng, Q.; Sang, Y.; Dong, K.; Qu, X. Nature-inspired construction of nanozyme with active sites in tailored microenvironment and pseudopodia-like surface for enhanced bacterial inhibition. Angew. Chem. 2020, 59, 3469–3474.
- Liu, Z.; Wang, F.; Ren, J.; Qu, X. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials 2019, 208, 21–31.
- Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. When Nanozymes Meet Single-Atom Catalysis. Angew. Chem. 2020, 59, 2565–2576.
- Yan, R.; Sun, S.; Yang, J.; Long, W.; Wang, J.; Mu, X.; Li, Q.; Hao, W.; Zhang, S.; Liu, H.; et al. Nanozyme-based bandage with single-atom catalysis for brain trauma. ACS Nano 2019, 13, 11552–11560.
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-atom nanozymes. Sci. Adv. 2019, 5, eaav5490.
- Liu, P.; Zhao, Y.; Ruixuan, Q.; Shiguang, M.; Guangxu, C.; Lin, G.; Chevrier, D.M.; Peng, Z.; Qing, G.; Dandan, Z.; et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–801.
- Xiang, H.; Feng, W.; Chen, Y. Single-atom catalysts in catalytic biomedicine. Adv. Mater. 2020, 32, 1905994.
- Cheng, N.; Zhang, L.; Doyle-Davis, K.; Sun, X. Single-atom catalysts: From design to application. Electrochem. Energy Rev. 2019, 2, 539–573.
- Su, F.; Li, Y.; Ma, J.; Wang, L.; Zhong, Z.; Wang, L.; Tian, S.; Li, Z.; Liu, H.; Li, J.; et al. Single-atom Sn-Zn pairs in CuO catalyst promote dimethyldichlorosilane synthesis. Natl. Sci. Rev. 2020, 7, 600–608.
- Huo, M.; Wang, L.; Zhang, H.; Zhang, L.; Chen, Y.; Shi, J. Construction of single-iron atom nanocatalysts for highly efficient catalytic antibiotics. Small 2019, 15, 1901834.
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-atom nanozyme for wound disinfection applications. Angew. Chem. 2019, 58, 4911–4916.
- Ouyang, H.; Zhang, L.; Jiang, S.; Wang, W.; Zhu, C.; Fu, Z. Co Single-Atom Catalysts Boost Chemiluminescence. Chem. Eur. J. 2020, 26, 7583–7588.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
21 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




