
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gohar Shaghayegh | -- | 3100 | 2022-06-20 12:23:03 | | | |
| 2 | Beatrix Zheng | -1 word(s) | 3099 | 2022-06-21 03:27:50 | | |
Video Upload Options
Chronic rhinosinusitis (CRS) is a persistent inflammation of the nasal cavity and paranasal sinuses associated with tissue remodelling, dysfunction of the sinuses’ natural defence mechanisms, and induction of different inflammatory clusters. The etiopathogenesis of CRS remains elusive, and both environmental factors, such as bacterial biofilms and the host’s general condition, are thought to play a role. Bacterial biofilms have significant clinical relevance due to their potential to cause resistance to antimicrobial therapy and host defenses. Despite substantial medical advances, some CRS patients suffer from recalcitrant disease that is unresponsive to medical and surgical treatments. Those patients often have nasal polyps with tissue eosinophilia, S. aureus-dominant mucosal biofilm, comorbid asthma, and a severely compromised quality of life.
1. Chronic Rhinosinusitis
2. Aetiology of CRS
3. CRS and Asthma
4. CRS and Dysbiosis
5. Staphylococcus aureus
6. Staphylococcal Biofilm
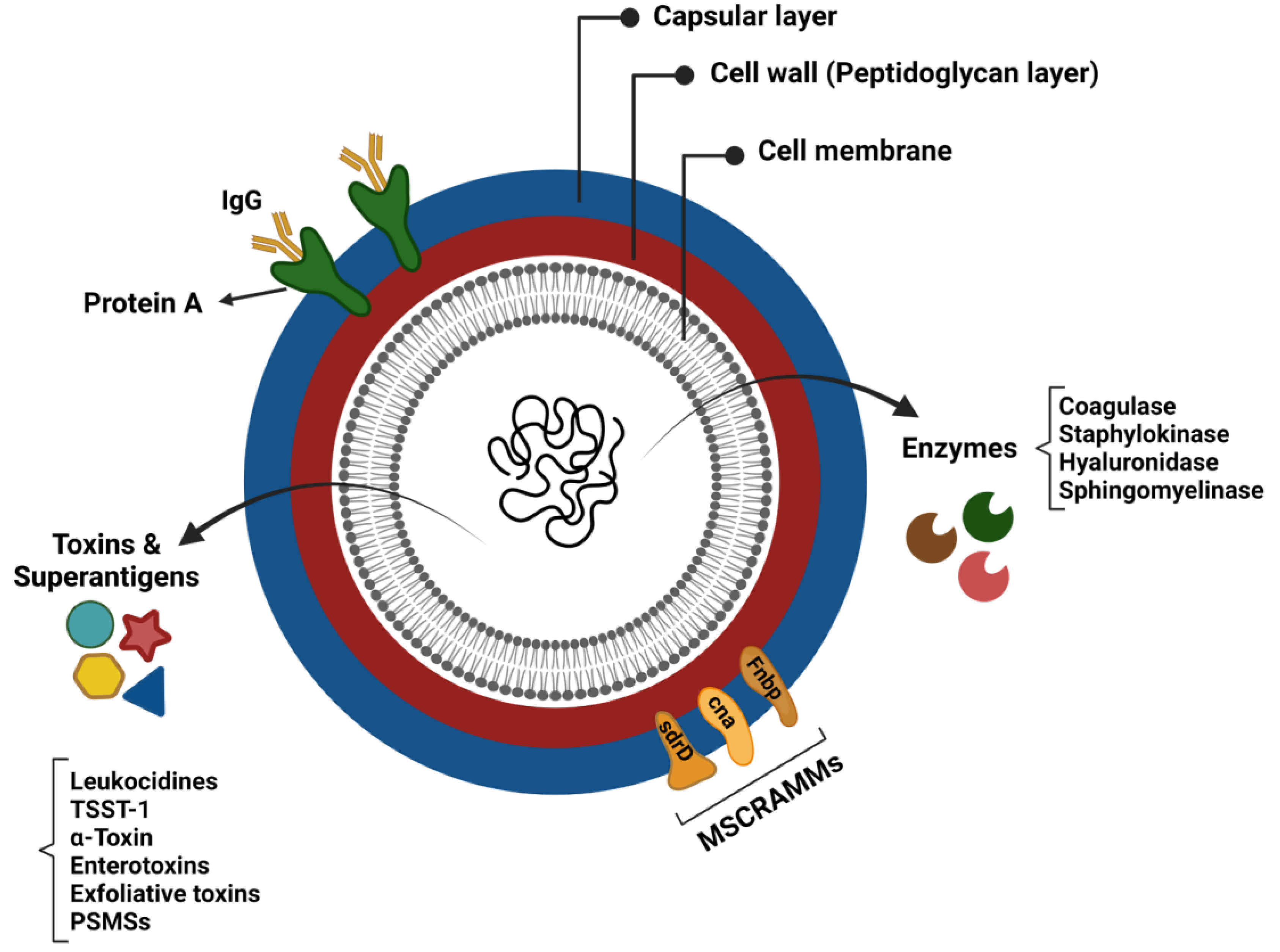
7. Staphylococcal Virulence Factors
8. Immune Response in CRS
9. CRS Inflammatory Endotypes
10. Current Therapeutic Strategies for CRS
References
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12.
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Ashok Kumar, K.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A. Clinical practice guideline (update) adult sinusitis executive summary. Otolaryngol.-Head Neck Surg. 2015, 152, 598–609.
- Brook, I. Microbiology of chronic rhinosinusitis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1059–1068.
- DeConde, A.S.; Soler, Z.M. Chronic rhinosinusitis: Epidemiology and burden of disease. Am. J. Rhinol. Allergy 2016, 30, 134–139.
- Lee, S.; Lane, A.P. Chronic rhinosinusitis as a multifactorial inflammatory disorder. Curr. Infect. Dis. Rep. 2011, 13, 159–168.
- Stevens, W.W.; Schleimer, R.P.; Kern, R.C. Chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. Pract. 2016, 4, 565–572.
- Kucuksezer, U.C.; Ozdemir, C.; Akdis, M.; Akdis, C.A. Chronic rhinosinusitis: Pathogenesis, therapy options, and more. Expert Opin. Pharmacother. 2018, 19, 1805–1815.
- Fastenberg, J.H.; Hsueh, W.D.; Mustafa, A.; Akbar, N.A.; Abuzeid, W.M. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J. Otorhinolaryngol.-Head Neck Surg. 2016, 2, 219–229.
- Chapurin, N.; Pynnonen, M.A.; Roberts, R.; Schulz, K.; Shin, J.J.; Witsell, D.L.; Parham, K.; Langman, A.; Carpenter, D.; Vambutas, A. CHEER national study of chronic rhinosinusitis practice patterns: Disease comorbidities and factors associated with surgery. Otolaryngol.–Head Neck Surg. 2017, 156, 751–756.
- Frendø, M.; Håkansson, K.; Schwer, S.; Rix, I.; Ravn, A.T.; Backer, V.; von Buchwald, C. Asthma in ear, nose, and throat primary care patients with chronic rhinosinusitis with nasal polyps. Am. J. Rhinol. Allergy 2016, 30, e67–e71.
- Benninger, M.S.; Sindwani, R.; Holy, C.E.; Hopkins, C. Impact of medically recalcitrant chronic rhinosinusitis on incidence of asthma. Proc. Int. Forum Allergy Rhinol. 2016, 6, 124–129.
- Chung, S.D.; Chen, P.Y.; Lin, H.C.; Hung, S.H. Comorbidity profile of chronic rhinosinusitis: A population-based study. Laryngoscope 2014, 124, 1536–1541.
- Kariyawasam, H.H.; Rotiroti, G. Allergic rhinitis, chronic rhinosinusitis and asthma: Unravelling a complex relationship. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 79–86.
- Jarvis, D.; Newson, R.; Lotvall, J.; Hastan, D.; Tomassen, P.; Keil, T.; Gjomarkaj, M.; Forsberg, B.; Gunnbjornsdottir, M.; Minov, J. Asthma in adults and its association with chronic rhinosinusitis: The GA2LEN survey in Europe. Allergy 2012, 67, 91–98.
- Yoshimura, K.; Kawata, R.; Haruna, S.; Moriyama, H.; Hirakawa, K.; Fujieda, S.; Masuyama, K.; Takenaka, H. Clinical epidemiological study of 553 patients with chronic rhinosinusitis in Japan. Allergol. Int. 2011, 60, 491–496.
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.-O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4.
- Langdon, C.; Mullol, J. Nasal polyps in patients with asthma: Prevalence, impact, and management challenges. J. Asthma Allergy 2016, 9, 45.
- Ryu, G.; Min, C.; Park, B.; Choi, H.G.; Mo, J.-H. Bidirectional association between asthma and chronic rhinosinusitis: Two longitudinal follow-up studies using a national sample cohort. Sci. Rep. 2020, 10, 9589.
- Zhang, N.; Van Zele, T.; Perez-Novo, C.; Van Bruaene, N.; Holtappels, G.; DeRuyck, N.; Van Cauwenberge, P.; Bachert, C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J. Allergy Clin. Immunol. 2008, 122, 961–968.
- Klossek, J.M.; Neukirch, F.; Pribil, C.; Jankowski, R.; Serrano, E.; Chanal, I.; El Hasnaoui, A. Prevalence of nasal polyposis in France: A cross-sectional, case–control study. Allergy 2005, 60, 233–237.
- Laidlaw, T.M.; Mullol, J.; Woessner, K.M.; Amin, N.; Mannent, L.P. Chronic rhinosinusitis with nasal polyps and asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1133–1141.
- Bachert, C.; Zhang, N.; Holtappels, G.; De Lobel, L.; Van Cauwenberge, P.; Liu, S.; Lin, P.; Bousquet, J.; Van Steen, K. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J. Allergy Clin. Immunol. 2010, 126, 962–968.e966.
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Frank, D.N. The microbiome of the middle meatus in healthy adults. PLoS ONE 2013, 8, e85507.
- Bordin, A.; Sidjabat, H.E.; Cottrell, K.; Cervin, A. Chronic rhinosinusitis: A microbiome in dysbiosis and the search for alternative treatment options. Microbiol. Aust. 2016, 37, 149–152.
- Feazel, L.M.; Robertson, C.E.; Ramakrishnan, V.R.; Frank, D.N. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope 2012, 122, 467–472.
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124.
- Choi, E.B.; Hong, S.W.; Kim, D.K.; Jeon, S.; Kim, K.R.; Cho, S.H.; Gho, Y.; Jee, Y.K.; Kim, Y.K. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy 2014, 69, 517–526.
- Ramakrishnan, V.R.; Feazel, L.M.; Abrass, L.J.; Frank, D.N. Prevalence and abundance of Staphylococcus aureus in the middle meatus of patients with chronic rhinosinusitis, nasal polyps, and asthma. Proc. Int. Forum Allergy Rhinol. 2013, 3, 267–271.
- Ramakrishnan, V.R.; Hauser, L.J.; Feazel, L.M.; Ir, D.; Robertson, C.E.; Frank, D.N. Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J. Allergy Clin. Immunol. 2015, 136, 334–342.e1.
- Koutsourelakis, I.; Halderman, A.; Khalil, S.; Hittle, L.E.; Mongodin, E.F.; Lane, A.P. Temporal instability of the post-surgical maxillary sinus microbiota. BMC Infect. Dis. 2018, 18, 441.
- Paramasivan, S.; Bassiouni, A.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B. The international sinonasal microbiome study: A multicentre, multinational characterization of sinonasal bacterial ecology. Allergy 2020, 75, 2037–2049.
- Frank, D.N.; Zhu, W.; Sartor, R.B.; Li, E. Investigating the biological and clinical significance of human dysbioses. Trends Microbiol. 2011, 19, 427–434.
- Vickery, T.W.; Ramakrishnan, V.R.; Suh, J.D. The role of Staphylococcus aureus in patients with chronic sinusitis and nasal polyposis. Curr. Allergy Asthma Rep. 2019, 19, 1–8.
- Chalermwatanachai, T.; Zhang, N.; Holtappels, G.; Bachert, C. Association of mucosal organisms with patterns of inflammation in chronic rhinosinusitis. PLoS ONE 2015, 10, e0136068.
- Van Zele, T.; Gevaert, P.; Watelet, J.-B.; Claeys, G.; Holtappels, G.; Claeys, C.; van Cauwenberge, P.; Bachert, C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J. Allergy Clin. Immunol. 2004, 114, 981–983.
- Schmidt, F.; Meyer, T.; Sundaramoorthy, N.; Michalik, S.; Surmann, K.; Depke, M.; Dhople, V.; Salazar, M.G.; Holtappels, G.; Zhang, N. Characterization of human and Staphylococcus aureus proteins in respiratory mucosa by in vivo-and immunoproteomics. J. Proteom. 2017, 155, 31–39.
- Schwartz, J.S.; Peres, A.G.; Endam, L.M.; Cousineau, B.; Madrenas, J.; Desrosiers, M. Topical probiotics as a therapeutic alternative for chronic rhinosinusitis: A preclinical proof of concept. Am. J. Rhinol. Allergy 2016, 30, e202–e205.
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323.
- Seiberling, K.A.; Conley, D.B.; Tripathi, A.; Grammer, L.C.; Shuh, L.; Haines, G.K., III; Schleimer, R.; Kern, R.C. Superantigens and chronic rhinosinusitis: Detection of staphylococcal exotoxins in nasal polyps. Laryngoscope 2005, 115, 1580–1585.
- Monaco, M.; de Araujo, F.P.; Cruciani, M.; Coccia, E.M.; Pantosti, A. Worldwide epidemiology and antibiotic resistance of Staphylococcus aureus. In Staphylococcus aureus; Springer: New York, NY, USA, 2016; pp. 21–56.
- Thammavongsa, V.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 2013, 342, 863–866.
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Mongodin, E.F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367, 731–739.
- Drilling, A.; Coombs, G.W.; Tan, H.l.; Pearson, J.C.; Boase, S.; Psaltis, A.; Speck, P.; Vreugde, S.; Wormald, P.J. Cousins, siblings, or copies: The genomics of recurrent Staphylococcus aureus infections in chronic rhinosinusitis. Proc. Int. Forum Allergy Rhinol. 2014, 4, 953–960.
- Grumann, D.; Nübel, U.; Bröker, B.M. Staphylococcus aureus toxins–their functions and genetics. Infect. Genet. Evol. 2014, 21, 583–592.
- Teymournejad, O.; Montgomery, C.P. Evasion of immunological memory by S. aureus infection: Implications for vaccine design. Front. Immunol. 2021, 12, 430.
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells”. J. Bacteriol. 2007, 189, 7945–7947.
- Gil, C.; Solano, C.; Burgui, S.; Latasa, C.; García, B.; Toledo-Arana, A.; Lasa, I.; Valle, J. Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infect. Immun. 2014, 82, 1017–1029.
- Singhal, D.; Psaltis, A.J.; Foreman, A.; Wormald, P.-J. The impact of biofilms on outcomes after endoscopic sinus surgery. Am. J. Rhinol. Allergy 2010, 24, 169–174.
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193.
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745.
- Suh, J.D.; Ramakrishnan, V.; Palmer, J.N. Biofilms. Otolaryngol. Clin. N. Am. 2010, 43, 521–530.
- Donné, J.; Dewilde, S. The challenging world of biofilm physiology. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 67, pp. 235–292.
- Belas, R. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol. 2014, 22, 517–527.
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Boyd, C.D.; O’Toole, G.A. Second messenger regulation of biofilm formation: Breakthroughs in understanding c-di-GMP effector systems. Annu. Rev. Cell Dev. Biol. 2012, 28, 439–462.
- Romeo, T. When the party is over: A signal for dispersal of Pseudomonas aeruginosa biofilms. J. Bacteriol. 2006, 188, 7325–7327.
- Ramakrishnan, Y.; Shields, R.; Elbadawey, M.; Wilson, J. Biofilms in chronic rhinosinusitis: What is new and where next? J. Laryngol. Otol. 2015, 129, 744–751.
- Singh, P.; Mehta, R.; Agarwal, S.; Mishra, P. Bacterial biofilm on the sinus mucosa of healthy subjects and patients with chronic rhinosinusitis (with or without nasal polyposis). J. Laryngol. Otol. 2015, 129, 46–49.
- Sanderson, A.R.; Leid, J.G.; Hunsaker, D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope 2006, 116, 1121–1126.
- Sanclement, J.A.; Webster, P.; Thomas, J.; Ramadan, H.H. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope 2005, 115, 578–582.
- Dlugaszewska, J.; Leszczynska, M.; Lenkowski, M.; Tatarska, A.; Pastusiak, T.; Szyfter, W. The pathophysiological role of bacterial biofilms in chronic sinusitis. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1989–1994.
- Prince, A.A.; Steiger, J.D.; Khalid, A.N.; Dogrhamji, L.; Reger, C.; Claire, S.E.; Chiu, A.G.; Kennedy, D.W.; Palmer, J.N.; Cohen, N.A. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am. J. Rhinol. 2008, 22, 239–245.
- Foreman, A.; Psaltis, A.J.; Tan, L.W.; Wormald, P.-J. Characterization of bacterial and fungal biofilms in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2009, 23, 556–561.
- Psaltis, A.J.; Weitzel, E.K.; Ha, K.R.; Wormald, P.-J. The effect of bacterial biofilms on post-sinus surgical outcomes. Am. J. Rhinol. 2008, 22, 1–6.
- Pratt, E.; Collins, A.M.; Sewell, W.A.; Harvey, R.J. Antigen selection in IgE antibodies from individuals with chronic rhinosinusitis with nasal polyps. Am. J. Rhinol. Allergy 2010, 24, 416–421.
- Cryer, J.; Schipor, I.; Perloff, J.R.; Palmer, J.N. Evidence of bacterial biofilms in human chronic sinusitis. J. Oto-Rhino-Laryngol. 2004, 66, 155–158.
- Bachert, C.; Claeys, S.E.; Tomassen, P.; Van Zele, T.; Zhang, N. Rhinosinusitis and asthma: A link for asthma severity. Curr. Allergy Asthma Rep. 2010, 10, 194–201.
- Cirkovic, I.; Pavlovic, B.; Bozic, D.D.; Jotic, A.; Bakic, L.; Milovanovic, J. Antibiofilm effects of topical corticosteroids and intranasal saline in patients with chronic rhinosinusitis with nasal polyps depend on bacterial species and their biofilm-forming capacity. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1897–1903.
- Mladina, R.; Skitarelić, N.; Musić, S.; Ristić, M. A biofilm exists on healthy mucosa of the paranasal sinuses: A prospectively performed, blinded, scanning electron microscope study. Clin. Otolaryngol. 2010, 35, 104–110.
- Powers, M.E.; Wardenburg, J.B. Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS Pathog. 2014, 10, e1003871.
- Bien, J.; Sokolova, O.; Bozko, P. Characterization of virulence factors of Staphylococcus aureus: Novel function of known virulence factors that are implicated in activation of airway epithelial proinflammatory response. J. Pathog. 2011, 2011, 601905.
- Liu, Y.; Yao, Y.; Wang, Z.C.; Ning, Q.; Liu, Z. Novel innate and adaptive lymphocytes: The new players in the pathogenesis of inflammatory upper airway diseases. Clin. Exp. Allergy 2018, 48, 620–631.
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 2015, 136, 1442–1453.
- Hirschberg, A.; Kiss, M.; Kadocsa, E.; Polyanka, H.; Szabo, K.; Razga, Z.; Bella, Z.; Tiszlavicz, L.; Kemeny, L. Different activations of toll-like receptors and antimicrobial peptides in chronic rhinosinusitis with or without nasal polyposis. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1779–1788.
- Van Zele, T.; Claeys, S.; Gevaert, P.; Van Maele, G.; Holtappels, G.; Van Cauwenberge, P.; Bachert, C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006, 61, 1280–1289.
- Bachert, C.; Zhang, N.; Hellings, P.W.; Bousquet, J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2018, 141, 1543–1551.
- Ahern, S.; Cervin, A. Inflammation and endotyping in chronic rhinosinusitis—A paradigm shift. Medicina 2019, 55, 95.
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635.
- De Greve, G.; Hellings, P.W.; Fokkens, W.J.; Pugin, B.; Steelant, B.; Seys, S.F. Endotype-driven treatment in chronic upper airway diseases. Clin. Transl. Allergy 2017, 7, 22.
- Bachert, C.; Zhang, N. Medical algorithm: Diagnosis and treatment of chronic rhinosinusitis. Allergy 2019, 75, 240–242.
- Fokkens, W.J.; Lund, V.; Luong, A.U.; Orlandi, R.R. A comparison of international guidelines for rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2022, 10, 1418–1422.
- Chong, L.Y.; Head, K.; Hopkins, C.; Philpott, C.; Schilder, A.G.; Burton, M.J. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2016, 26, CD011996.
- Bachert, C.; Pawankar, R.; Zhang, L.; Bunnag, C.; Fokkens, W.J.; Hamilos, D.L.; Jirapongsananuruk, O.; Kern, R.; Meltzer, E.O.; Mullol, J. ICON: Chronic rhinosinusitis. World Allergy Organ. J. 2014, 7, 25.
- Rudmik, L.; Soler, Z.M. Medical therapies for adult chronic sinusitis: A systematic review. J. Am. Med. Assoc. 2015, 314, 926–939.
- Sharma, R.; Lakhani, R.; Rimmer, J.; Hopkins, C. Surgical interventions for chronic rhinosinusitis with nasal polyps. Cochrane Database Syst. Rev. 2014, 12, CD006991.
- Cardona, V.; Luengo, O.; Labrador-Horrillo, M. Immunotherapy in allergic rhinitis and lower airway outcomes. Allergy 2017, 72, 35–42.
- Mann, C.; Schmidtmann, I.; Bopp, T.; Brieger, J.; Fruth, K. Treg activation and their role in different subtypes of chronic rhinosinusitis. Allergy 2020, 75, 2687–2689.
- Hong, H.; Liao, S.; Chen, F.; Yang, Q.; Wang, D.Y. Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy 2020, 75, 2794–2804.
- Staudacher, A.G.; Peters, A.T.; Kato, A.; Stevens, W.W. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann. Allergy Asthma Immunol. 2020, 124, 318–325.
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650.
- Bachert, C.; Sousa, A.R.; Lund, V.J.; Scadding, G.K.; Gevaert, P.; Nasser, S.; Durham, S.R.; Cornet, M.E.; Kariyawasam, H.H.; Gilbert, J. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J. Allergy Clin. Immunol. 2017, 140, 1024–1031.e14.
- Laidlaw, T.M.; Prussin, C.; Panettieri, R.A.; Lee, S.; Ferguson, B.J.; Adappa, N.D.; Lane, A.P.; Palumbo, M.L.; Sullivan, M.; Archibald, D. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope 2019, 129, E61–E66.
- Gevaert, P.; Calus, L.; Van Zele, T.; Blomme, K.; De Ruyck, N.; Bauters, W.; Hellings, P.; Brusselle, G.; De Bacquer, D.; Van Cauwenberge, P. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J. Allergy Clin. Immunol. 2013, 131, 110–116.e1.
- Fokkens, W.J.; Lund, V.; Bachert, C.; Mullol, J.; Bjermer, L.; Bousquet, J.; Canonica, G.W.; Deneyer, L.; Desrosiers, M.; Diamant, Z. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy 2019, 74, 2312–2319.
- Kim, C.; Han, J.; Wu, T.; Bachert, C.; Fokkens, W.; Hellings, P.; Hopkins, C.; Lee, S.; Mullol, J.; Lee, J.T. Role of biologics in chronic rhinosinusitis with nasal polyposis: State of the art review. Otolaryngol.–Head Neck Surg. 2021, 164, 57–66.
- Brown, W.C.; Senior, B. A critical look at the efficacy and costs of biologic therapy for chronic rhinosinusitis with nasal polyposis. Curr. Allergy Asthma Rep. 2020, 20, 16.
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.-P.; Molle, V. Staphylococcus aureus toxins: An update on their pathogenic properties and potential treatments. Toxins 2021, 13, 677.
- Karauzum, H.; Venkatasubramaniam, A.; Adhikari, R.P.; Kort, T.; Holtsberg, F.W.; Mukherjee, I.; Mednikov, M.; Ortines, R.; Nguyen, N.T.; Doan, T. IBT-V02: A multicomponent toxoid vaccine protects against primary and secondary skin infections caused by Staphylococcus aureus. Front. Immunol. 2021, 12, 475.




