Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jörg Männer | -- | 3179 | 2022-06-17 12:06:57 | | | |
| 2 | Rita Xu | -13 word(s) | 3166 | 2022-06-20 04:17:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Männer, J. Human Embryonic Heart Start Beating. Encyclopedia. Available online: https://encyclopedia.pub/entry/24161 (accessed on 07 February 2026).
Männer J. Human Embryonic Heart Start Beating. Encyclopedia. Available at: https://encyclopedia.pub/entry/24161. Accessed February 07, 2026.
Männer, Jörg. "Human Embryonic Heart Start Beating" Encyclopedia, https://encyclopedia.pub/entry/24161 (accessed February 07, 2026).
Männer, J. (2022, June 17). Human Embryonic Heart Start Beating. In Encyclopedia. https://encyclopedia.pub/entry/24161
Männer, Jörg. "Human Embryonic Heart Start Beating." Encyclopedia. Web. 17 June, 2022.
Copy Citation
The onset of embryonic heart beating may be regarded as the defining feature for the beginning of personal human life. Clarifying the timing of the first human heartbeat, therefore, has religious, philosophical, ethical, and medicolegal implications.
first heartbeat
human embryonic heart
1. Introduction
From all the organs of human's body, the heart and the brain have received an eminent position in the diverse philosophical, theological and biomedical concepts of human life that evolved in the Near East and Europe during the past 5000 years. Each of the two organs were proposed as the location of the soul or the source of the “vital principle” that defined personal human life [1]. Therefore, if researchers neglect the existence of concepts that attribute the soul or the vital principle to the whole body, researchers can roughly distinguish between heart-centered and brain-centered concepts of human life. Seeing either the heart or the brain as the source of the vital principle has great impact on the practical work of physicians. This is because the presence of heart or brain activity can be regarded as the indicator of life. The irreversible cessation of heart or brain activity then can be regarded as the defining sign of death of an individual human being. Up to the 1960s, the traditional legal definition of death, in Western countries, was the cessation of cardio-respiratory function. During the 1960s and 1970s, the medicolegal definition of death shifted from a heart-centered to a brain-centered definition of death, which had great impact on the development of transplantation medicine [2].
In analogy to the medicolegal definitions of death, the beginning of heart or brain activity may be regarded as defining features for the beginning of personal life [3]. These ideas also have consequences for the practical work of physicians since they have influence on the development of laws regulating abortion [4]. In the United States, for example, there is currently a tendency to define the beginning of personal human life by the onset of embryonic heart beating with the consequence of banning abortions after the time point of the first detection of the embryonic heart beating [5].
In view of the eminent status of the heart in researchers' thinking about personal human life, it is no wonder that, in my lectures of medical embryology, the students frequently ask me when the human embryonic heart starts beating. Up until last year, my answer to this question has corresponded to the current textbook knowledge, which says that the cardiovascular system is the first organ system to function and that the human embryonic heart starts beating at 21 to 23 days after fertilization [6][7][8]. During this short time period, human embryos are said to pass through stage 10 of the so-called Carnegie stages (CS) of human embryonic development, and their length is said to vary between 1.5 and 3 mm [9]. The heart of CS-10 embryos has a relatively simple form design, which resembles a tubular blood vessel. At the beginning of CS-10, the heart is a relatively short and straight tube aligned along the ventral midline of the foregut (Figure 1A). Up to the end of CS-10, the tubular heart undergoes a considerable lengthening and changes its 3-dimensional configuration from a straight to a C-shaped heart loop (Figure 1B–D).
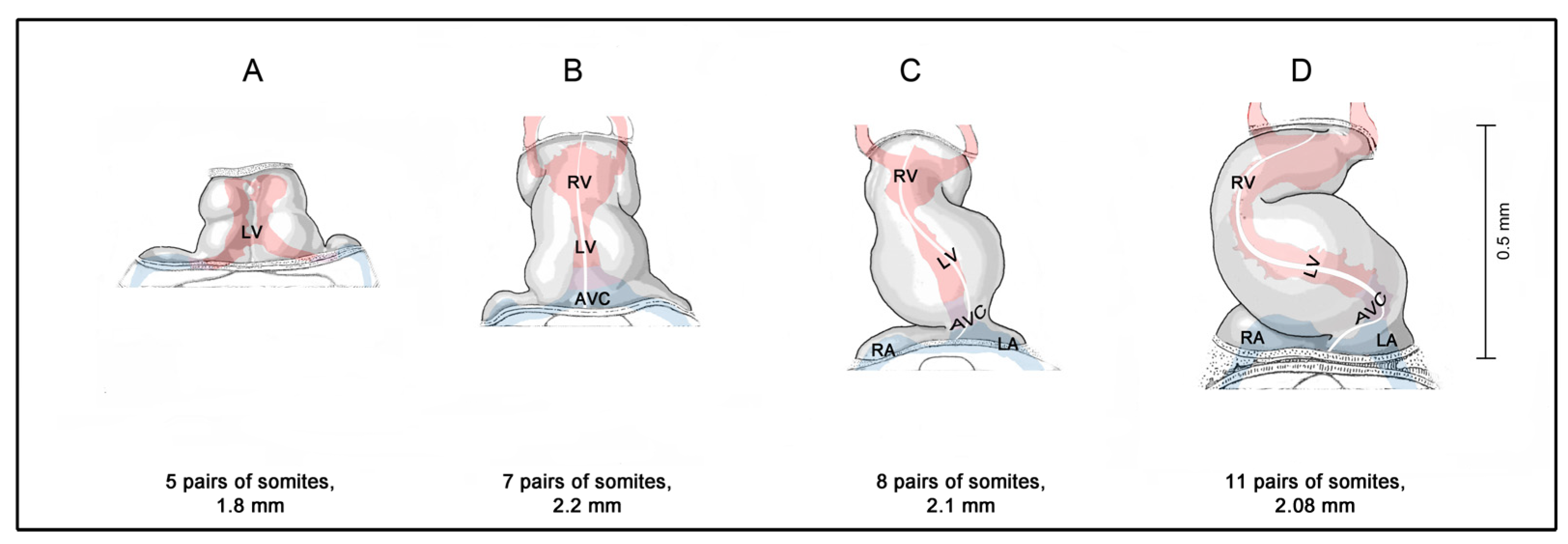
Figure 1. Morphogenesis of the human embryonic heart tube during Carnegie-stage 10. (A,B) depict early and late linear heart tube stages; (C) depicts a heart tube at the onset of cardiac looping; and (D) depicts a C-shaped heart tube. Hearts are shown in ventral views. The longitudinal white line indicates the original ventral midline of the heart tube. The shape of the endocardial lumen can be seen through the semitransparent wall of the hearts. It is indicated by red (ventricles, outflow tract, arteries) and blue (veins, atria, atrioventricular canal) colors. Drawings are based on Figures 18–24 shown in reference [10]. Abbreviations: AVC = atrioventricular canal; LA = embryonic left atrium; LV = embryonic left ventricle; RA = embryonic right atrium; RV = embryonic right ventricle.
It is obvious that a tubular embryonic heart mechanically cannot work in the same way as the mature four-chambered heart of human beings. Thus, if researchers use, in the context of the early embryonic heart activity, the term “heartbeat”, which is used to describe “the regular movement that the heart makes as it sends blood around your body” [11], researchers should be aware of the fact that researchers deal with a kind of heart movement that differs considerably from the movement of the mature four-chambered heart. Observations on animal embryos have shown that the pumping action of embryonic heart tubes is characterized by the cyclic generation of traveling mechanical waves sweeping from its venous to its arterial end. These waves were traditionally interpreted as peristaltic movements of the myocardial wall of the heart tube (for review see [12]). I should, furthermore, note that, in the embryological literature, the term heartbeat sometimes was used already for the first weak contractions of early embryonic cardiomyocytes [13][14][15][16]. These contractions appear as small, irregular twitches within circumscribed areas of the developing myocardium and do not generate coordinated movements of the developing heart that cause fluid flow. Calling these contractions heartbeats does not match with the above-mentioned everyday usage of the term heartbeat and, therefore, should be avoided.
For the illustration of the general anatomy of CS-10 embryos, I usually show pictures of three-dimensional models of a well-known historical human embryo that has been described, for the first time, by the Swiss anatomist Auguste-Francois-Charles de’Eternod in 1896 [17] and 1899 [18] (Figure 2). The Eternod embryo had a greatest length of 2.11 mm and is best suited for illustrating an abridged and simplified version of the current textbook knowledge of the beginning of human heart activity, which says that the human embryonic heart starts beating around post-fertilization day 21, when the embryo passes through CS-10 and is about 2.1 mm long (21:10 = 2.1).
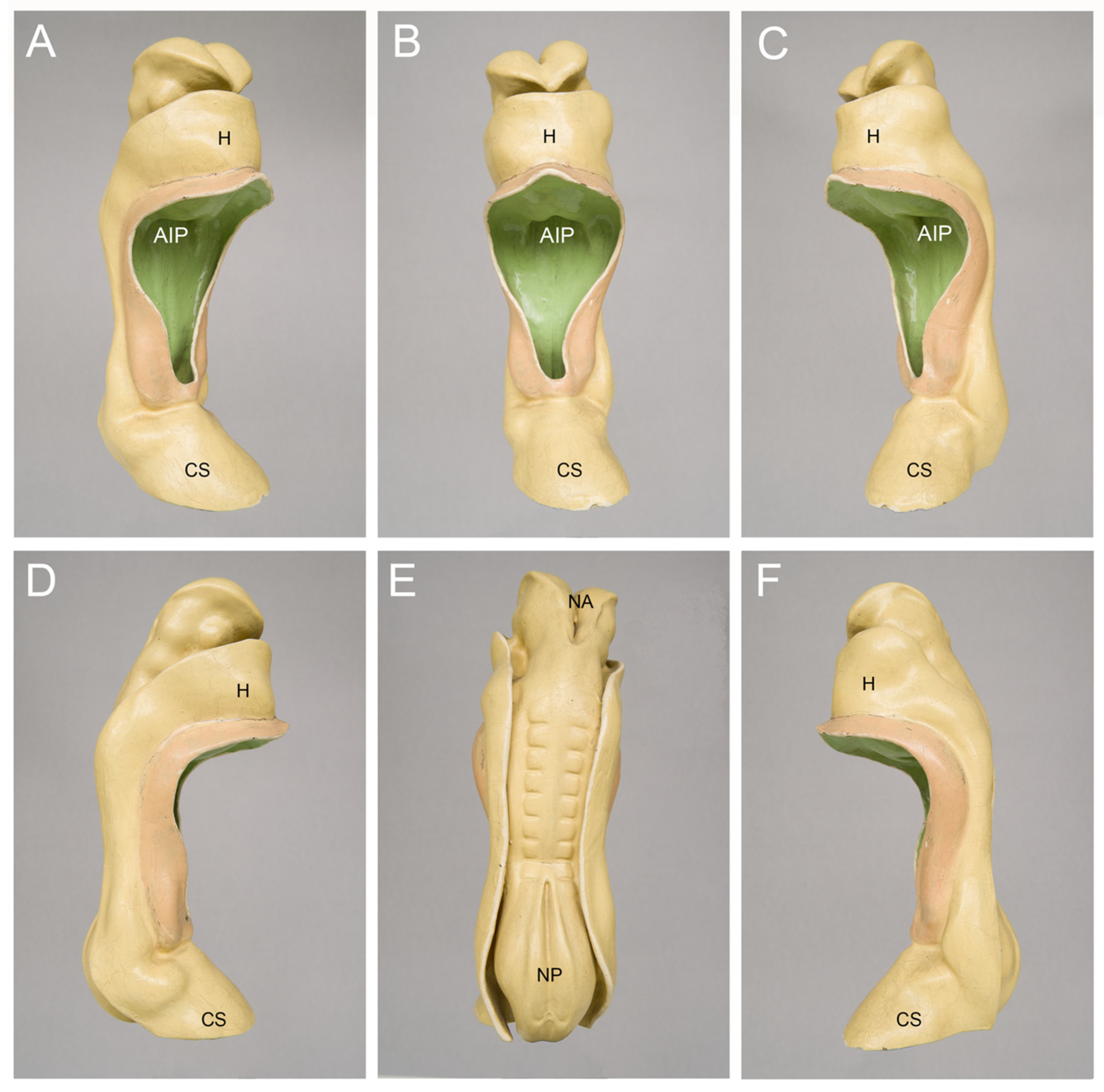
Figure 2. Outer morphology of a Carnegie-stage 10 human embryo as depicted by a historical three-dimensional model manufactured in the studio of Friedrich Ziegler in Freiburg/Breisgau, Germany. This embryo was described, for the first time, by the Swiss anatomist Auguste-Francois-Charles de’Eternod in 1896 and 1899 [17][18]. Pictures of this embryo or its heart were published in many textbooks, atlases and articles on human embryology during the 20th century. The embryo had a greatest length of 2.11 mm and seven pairs of complete somites + one pair of incomplete somites. The model is shown in right ventral (A), ventral (B), left ventral (C), right lateral (D), dorsal (E), and left lateral (F) views. Abbreviations: AIP = anterior intestinal portal; CS = connecting stalk; H = bulge of the body wall produced by the heart and pericardial cavity; NA = neuroporus anterior; NP = neuroporus posterior.
It should be noted here that the usage of the post-fertilization age is mainly confined to the community of embryologists and developmental biologists, which are especially interested in knowledge of the true age of human embryos. The date of fertilization of the human oocyte normally cannot be determined with high accuracy, since fertilization normally occurs within the body of the mother and is normally not associated with any physiological phenomenon that comes regularly to the attention of the mother. Physicians, therefore, determine the age of a pregnancy—the so-called “gestational age”—on the basis of a regular and visible phenomenon that normally does not escape the attention of a woman. This is the beginning of the last menstrual period [19]. The gestational age is approximately 14 days older than the post-fertilization age.
2. How Can We Clarify the Timing of the First Heartbeats in Human Embryos?
The ideal approach to clarify the timing of the first human heartbeats would be the direct observation of living human embryos during the critical phase of the fourth post-fertilization week (sixth gestational week). However, such an approach is not a trivial approach since fertilization as well as embryonic and fetal development of human beings normally takes place inside of the mother’s body, where the oocyte, the sperm, and the embryo are protected from direct visual observations. In other words, researchers normally do not know the exact fertilization date, nor can researchers see the embryo. If researchers want to obtain information on the beginning of human heart activity, by direct observations on living human embryos, researchers principally have to solve two practical tasks: (1) to make possible the exact determination of the post-fertilization age of the embryos to be examined; and (2) to make possible the visualization of their heart activity during the early stages of embryonic development.
3. Contemporary Sources of Knowledge
Today’s medical students may not see any problem in solving the two above-mentioned tasks. This is because modern medicine uses a wide spectrum of different diagnostic and therapeutic procedures, and two of these procedures seem to be ideally suited for solving the two tasks. These are: (1) assisted reproductive procedures, such as in vitro fertilization (IVF), which facilitate precise timing of fertilization in a very small population of human embryos since the late 1970s [20]; and (2) modern ultrasonographic imaging techniques, which make it possible to visualize, noninvasively and at relatively high spatial and temporal resolutions, human embryos and fetuses within the uterus from the beginning of the fourth week after fertilization (beginning of the sixth gestational week) up to the birth of the baby [21].
It appears that the currently most elegant design for a study intended to document the beginning of human embryonic heart activity seems to be an ultrasonographic examination of the embryonic heart activity in pregnancies resulting from assisted reproductive procedures. During the past 30 years, such studies were indeed conducted by several groups. The main purpose of these studies, however, was not to clarify the timing of the first heartbeat but was to provide reference curves for the changes in the embryonic heart rate in relation to the age and length of human embryos. Thus, in some of these studies, examinations have not started at the beginning of the fourth post-fertilization week, when the heart is said to start beating in some embryos but have started at the end of the fourth post-fertilization week (end of the sixth gestational week), when ultrasonographically detectable heart activity normally is present in every embryo [22][23]. It is obvious that such studies cannot provide information on the onset of embryonic heart activity. Only a relatively small number of studies, all published in the 1990s, started their examinations at the beginning of the fourth post-fertilization week and, therefore, could provide information on the onset of human embryonic heart activity [24][25][26][27][28][29][30][31][32][33]. Table 1 presents the age, greatest length, and heart rate of human embryos at the onset of ultrasonographically detectable heart activity as documented by these studies.
Table 1. Age, greatest length and heart rate of human embryos at the onset of ultrasonographically detectable heart activity as observed in pregnancies resulting from IVF.
Gestational ages were estimated by adding 14 days to the known post-fertilization age. References are listed according to publication date. Note that the data from two studies were used as the basis for more than one paper. Abbreviations: bpm = beats per minute, Post-fertil. = post-fertilization, gest. = gestational, info = information.
The analysis of the published data disclosed the following facts:
(1) Embryos of identical age can differ in the timing of the onset of ultrasonographically detectable heart activity. This means that human embryonic heart activity normally does not appear at a certain day of development but appears during a certain time slot. The length of this time slot varied between the studies listed in Table 1. The shortest time slot was 4 days [31][32] and the longest was 8 days [29]. Information on the length of the time slot was missed in three studies [27][30][33]. In these studies, the embryonic age was documented only for the earliest time point at which heart activity could be detected by transvaginal ultrasound. If the time slot for the onset of heart beating was defined by the data from normal continuing pregnancies, it was found that the appearance of the heartbeat after this time slot was associated with a higher chance of miscarriage [24][25]. The existence of time slots for the onset of embryonic heart activity corresponds to embryological data, which show that, among human embryos of the same coital age (=age determined after an isolated fruitful coitus), there is a great variability in the stage of development [34].
(2) In most studies, the reported time slot for the appearance of human embryonic heart activity was located at the end of the fourth and beginning of the fifth post-fertilization week [24][25][26][27][28][29] and, therefore, did not match with the time slot reported in contemporary textbooks of human embryology (21 to 23 days after fertilization). Only two studies documented the onset of heart beating at 20 to 23 days after fertilization and, thereby, provided data that matched with the textbook statements [30][31][32]. A third study documented the onset of heart beating on the 24th post-fertilization day, which was close to the textbook statements [33]. It should be noted here that the data from the last three studies, which were in accord with the textbook statements, were published in 1994, 1996, and 1998. The data from the other studies, which did not match with the textbook statements, all were published before 1994. In view of this fact, it is tempting to speculate that the mismatch between the data from the latter studies and the textbook statements may be explained by the usage of older ultrasound equipment, the resolutions of which did not facilitate the recognition of the embryonic heart activity before the end of the fourth post-fertilization week.
(3) In most of the studies listed in Table 1, the greatest length of human embryos, measured on the first day of ultrasonographically detectable heart activity, corresponded to the values obtained from post-mortem measurements on aborted CS-10 embryos (1.6 to 3 mm vs. 1.5 to 3 mm) [25][28][30][31][32] and, therefore, was in accord with the textbook statement that the human embryonic heart started beating at CS-10. Higher values (4 to 6 mm) were measured in three studies [26][27][29], while information on the embryonic length was missed in only a single study [33]. The higher values for the embryonic length were associated with heart rates that were markedly higher than those found in the studies in which the embryonic lengths were in the range of CS-10 embryos. This suggests that the deviations from the expected values of CS-10 embryos may be explained by the usage of ultrasound equipment, the resolutions of which permitted only a delayed detection of early embryonic heart movements. The textbook statement that the human embryonic heart starts beating during CS-10 is supported not only by the above-mentioned data from IVF pregnancies. It is also supported by data from ultrasound measurements on pregnancies resulting from physiological fertilization, which have shown that the smallest embryos in which heart activity could be detected had a greatest length of 2 mm [35][36]. It should be noted here, however, that the interpretations of the above-reported length values have some limitations: (1) To the best of the author’s knowledge, up to now, no study has addressed the question as to whether the values obtained from ultrasonographical in utero measurements of the greatest length of early human embryos correspond to the values that would be obtained by visual length measurements made on the same embryos after interruption of pregnancy. (2) The ultrasonographically determined length values may not be free of measurement errors.
With regard to ultrasonographic examinations of the activity of human embryonic hearts, I should finally note that the information on the age and length of human embryos, at the time of the first detectable heartbeats, was only a by-product of such studies. As stated above, the main purpose of such studies was to provide reference curves for the changes in the embryonic heart rate in relation to the age and greatest length of human embryos during the first trimester of pregnancy. This information is of great interest not only to physicians caring for pregnant women and their unborn children. It is also of interest to embryologists and developmental biologists working on the developing heart. This is because such data can be compared with historical and current data from non-human vertebrates that are frequently used as animal models for human embryonic development, such as the chick and mouse embryo. For unknown reasons, however, the data on the prenatal development of the heart rate did not find their way into contemporary textbooks of human embryology.
First trimester ultrasound examinations have consistently shown that the changes in embryonic heart rate follow a characteristic pattern [22][29][30][35][36][37]. This pattern is characterized by a first phase of a rapid and almost linear increase in the heart rate and a subsequent second phase of a moderate decrease in the heart rate (Figure 3). The first phase starts with the onset of heart beating and lasts up to the end of the seventh post-fertilization week. It coincides with the morphogenetic processes that transform the tubular embryonic heart into a four-chambered heart. Furthermore, during this phase, a significant correlation was found between the heart rate and the greatest length of the embryo [22][23][25][29][36][38]. The second phase starts at the beginning of the eighth post-fertilization week and lasts up to the end of the first/beginning of the second trimester. It coincides temporally with the structural maturation of the atrio-ventricular valves [39] and the maturation of the diastolic function of the ventricles [40].
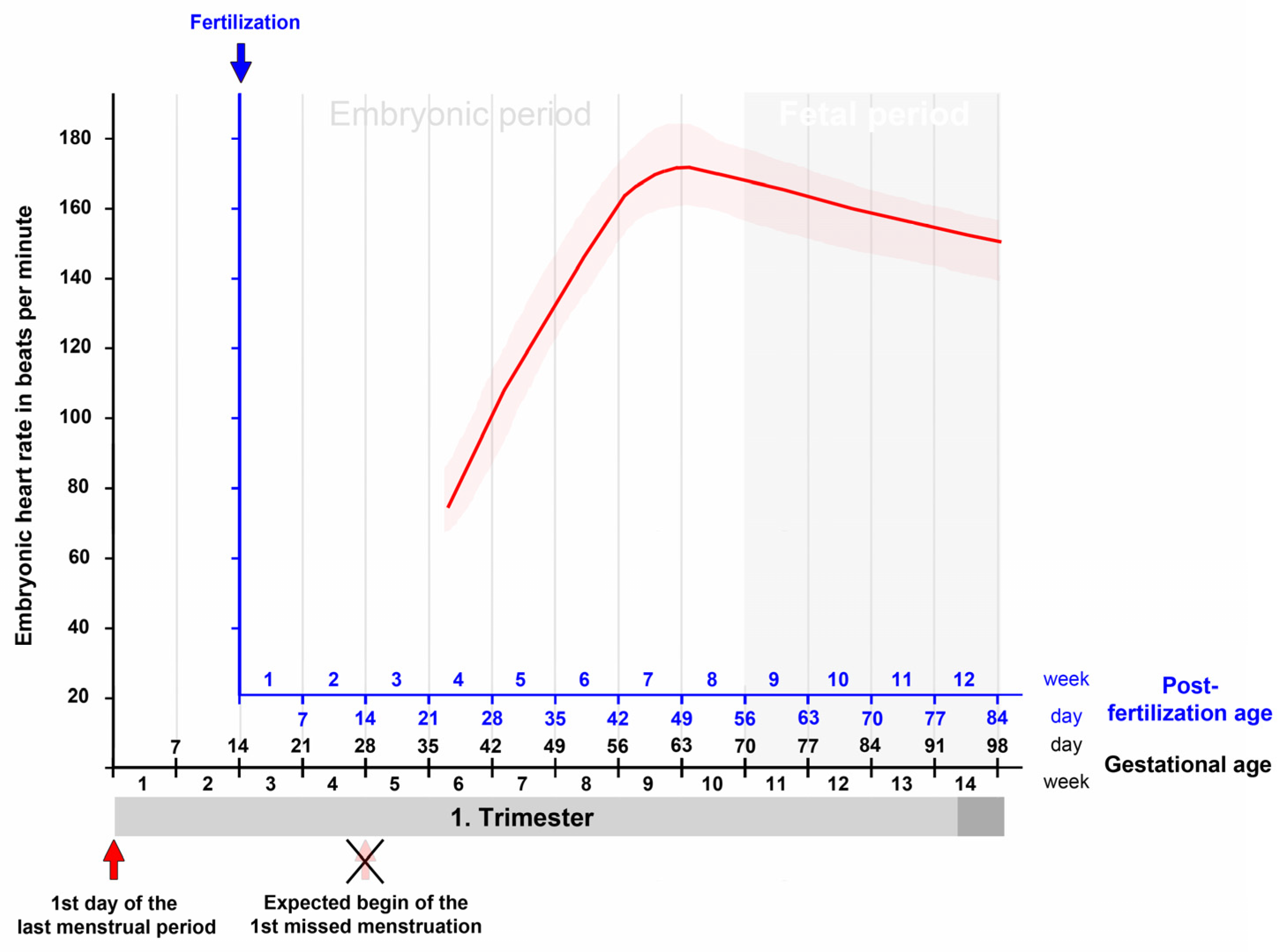
Figure 3. This graph depicts the typical changes in the embryonic/fetal heart rate in relation to the gestational as well as post-fertilization age as recorded by transvaginal ultrasonographic measurements during the first trimester. There is a rapid and almost linear increase in the embryonic heart rate from the onset of heart beating up to the end of the seventh post-fertilization week (ninth gestational week) followed by a moderate decrease up to the end of the first/beginning of the second trimester. Based on data from [30].
If the heart rate data from the first trimester are complemented by measurements from the second and third trimester, it is found that the phase of moderate decrease in the heart rate is followed by a phase of a slow decrease that lasts up to the birth of the baby [41][42]. A curve depicting the changes in the embryonic and fetal heart rate during all periods of prenatal development is shown in Figure 4.
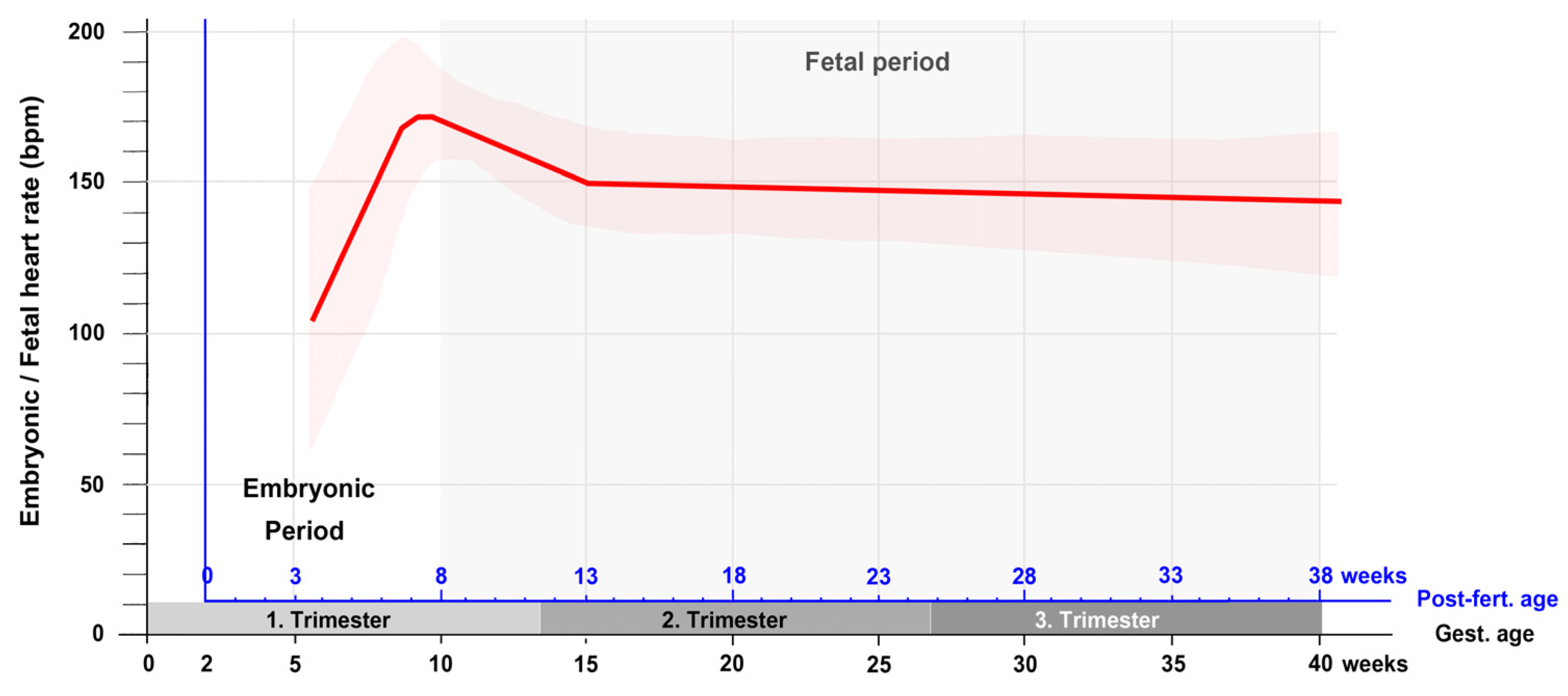
Figure 4. This graph depicts the typical changes in the embryonic/fetal heart rate in relation to the gestational as well as post-fertilization age as recorded by ultrasonographic measurements during all periods of pregnancy. Note that after the first trimester, there is only a slow linear decrease in the heart rate up to the birth of the baby. Based on data from [41][42]. Abbreviations: bpm = beats per minute; Gest. = Gestational; Post-fert. = Post-fertilization.
If this figure is compared with corresponding figures from chick (Figure 1 in [43]) and mouse (Figure 1 in [16]) embryos, it becomes apparent that the patterns of prenatal changes in the heart rate are very similar between humans and chicks. Mice, on the other hand, do not show a decrease in the heart rate during the fetal period of development. In these animals the prenatal heart rate does not only increase during the embryonic period (mouse post-fertilization days 1 to 14.5) but also increase during the fetal period (mouse post-fertilization days 14.5 to 21) as well as during postnatal heart development until the animals reach adulthood.
References
- Santoro, G.; Wood, M.D.; Merlo, L.; Anastasi, G.P.; Tomasello, F.; Germano, A. The anatomic location of the soul from the heart, through the brain, to the whole body, and beyond: A journey through western history, science, and philosophy. Neurosurgery 2009, 65, 633–643.
- Powner, D.J.; Ackerman, B.M.; Grenvil, A. Medical diagnosis of death in adults: Historical contributions to current controversies. Lancet 1996, 348, 1219–1223.
- Smolensky, K. Defining life from the perspective of death: An introduction to the forced symmetry approach. Univ. Chic. Leg. Forum 2006, 41–86.
- English, J. Abortion evolution: How Roe v. Wade has come to support a pro-life & pro-choice position. Creighton Law Rev. 2019, 53, 157–210.
- Evans, D.P.; Narasimhan, S. A narrative analysis of anti-abortion testimony and legislative debate related to Georgia’s fetal “heartbeat” abortion ban. Sex. Reprod. Health Matters 2020, 28, 1686201.
- Carlson, B.M. Human Embryology and Developmental Biology, 6th ed.; Elsevier: St. Louis, MI, USA, 2019.
- Schoenwolf, G.C.; Bleyl, S.B.; Brauer, P.R.; Francis-West, P.H. Larsen’s Human Embryology, 5th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015.
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. The Developing Human. Clinically Oriented Embryology, 11th ed.; Elsevier: Edinburgh, UK; London, UK; New York, NY, USA; Oxford, UK; Philidelphia, PA, USA; St. Louis, MI, USA; Sidney, Australia, 2020.
- O’Rahilly, R.; Müller, F. Developmental Stages in Human Embryos; Carnegie Institution Washington Publication 673: Washington DC, USA, 1987.
- Davis, C.L. The development of the human heart from its first appearance to the stage found in embryos of twenty paired somites. Contr. Embryol. Carnegie Inst. 1927, 19, 245–284.
- Cambridge Advanced Learner’s Dictionary, 4th ed.; Cambridge University Press: Cambridge, UK, 2013.
- Männer, J.; Wessel, A.; Yelbuz, T.M. How does the tubular embryonic heart work? Looking for the physical mechanism driving unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. 2010, 239, 1035–1046.
- Minot, C.S. Human Embryology; William Wood and Company: New York, NY, USA, 1892.
- Heuser, C.H.; Corner, G.W. Developmental horizons in human embryos. Description of age group X, 4 to 12 somites. Contr. Embryol. Carnegie Inst. 1957, 36, 29–39.
- Tyser, R.C.V.; Miranda, A.M.A.; Chen, C.; Davidson, S.M.; Srinivas, S.; Riley, P.R. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLIFE 2016, 5, e17113.
- Tyser, R.C.V.; Srinivas, S. The first heartbeat—Origin of cardiac contractile activity. Cold Spring Harb. Perspect. Biol. 2020, 12, a037135.
- Eternod, A.C.F. Sur un œuf humain de 16.3 mm avec embryon de 2.1 mm (uterus et adnexes). Soc Helvet Sci Nat. 1896, 79, 164–169.
- Eternod, A.C.F. Il y a un canal notochordal dans l’embryon humain. Anat. Anz. 1899, 16, 131–143.
- Naegele, F.C. Erfahrungen und Abhandlungen aus dem Gebiethe der Krankheiten des Weiblichen Geschlechtes. Nebst Grundzügen einer Methodenlehre der Geburtshülfe; Löffler: Mannheim, Germany, 1812.
- Johnson, M.H. A short history of in vitro fertilization. Int. J. Dev. Biol. 2019, 63, 82–92.
- Doubilet, P.M. Ultrasound evaluation of the first trimester. Radiol. Clin. N. Am. 2014, 52, 1191–1199.
- van Heeswijk, M.; Nijhuis, J.G.; Hollander, H.M.G. Fetal heart rate in early pregnancy. Early Hum. Dev. 1990, 22, 151–156.
- Ouyang, Y.; Qin, J.; Lin, G.; Xiang, S.; Li, X. Reference intervals of gestational sac, yolk sac, embryonic length, embryonic heart rate at 6–10 weeks after in vitro fertilization-embryo transfer. BMC Pregnancy Childbirth 2020, 20, 533.
- Schats, R.; Jansen, C.A.; Wladimiroff, J.W. Embryonic heart activity: Appearance and development in early human pregnancy. Br. J. Obs. Gynaecol. 1990, 97, 989–994.
- van Os, H.C.; Hout, J.I.T.; Hermans, J.; Jansen, C.A.M. Embryonic length, crown-rump length and fetal heart activity in early human pregnancy determination by transvaginal ultrasound. BMUS Bull. 1993, 1, 18–23.
- Howe, R.S.; Isaacson, K.J.; Albert, J.L.; Coutifaris, C.B. Embryonic heart rate in human pregnancy. J. Ultrasound Med. 1991, 10, 367–371.
- Elnekheli, M.; Kahles, G.; Khoshyomn, S.; Kirisits, R.; Troger, P.; Boldizsar, A.; Feichtinger, W. Transvaginale Doppleruntersuchung der embryonalen Herzaktion in der Frühschwangerschaft. (Transvaginal doppler sonography of embryonic cardiac activity in early human pregnancy). Ultraschall Med. 1992, 13, 12–14.
- Dickey, R.P.; Gasser, R.F. Ultrasound evidence for variability in the size and development of normal human embryos before the tenth post-insemination week after assisted reproductive technologies. Hum. Reprod. 1993, 8, 331–337.
- Rotsztejn, D.; Rana, N.; Dmowski, W.P. Correlation between fetal heart rate, crown-rump length, and ß-human chorionic gonadotropin levels during the first trimester of well-timed conceptions resulting from infertility treatment. Fertil. Steril. 1993, 59, 1169–1173.
- Wisser, J.; Dirschedl, P. Embryonic heart rate in dated human embryos. Early Hum. Dev. 1994, 37, 107–115.
- Britten, S.; Soenksen, D.M.; Bustillo, M.; Coulam, C.B. Very early (24–56 days from the last menstrual period) embryonic heart rate in normal pregnancies. Hum. Reprod. 1994, 9, 2424–2426.
- Coulam, C.B.; Britten, S.; Soenksen, D.M. Early (34–56 days from last menstrual period) ultrasonographic measurements in normal pregnancies. Hum. Reprod. 1996, 11, 1771–1774.
- Tezuka, N.; Sato, S.; Banzai, M.; Saito, H.; Hiroi, M. Development and sexual differences in embryonic heart rate in pregnancies resulting from in vitro fertilization. Gynaecol. Obstet. Investig. 1998, 46, 217–219.
- Shiota, K. Variability in human embryonic development and its implications for the susceptibility to environmental agents. Birth Defects Res. 2009, 85, 661–666.
- Rempen, A. Diagnosis of viability in early pregnancy with vaginal sonography. J. Ultrasound Med. 1990, 9, 711–716.
- Yapar, E.G.; Ekici, E.; Gökmen, O. First trimester fetal heart rate measurements by transvaginal ultrasound combined with pulsed Doppler: An evaluation of 1331 cases. Europ. J. Obstet. Gynec. Reprod. Biol. 1995, 60, 133–137.
- Robinson, H.P.; Shaw-Dunn, J. Fetal heart rate as determined by sonor in early pregnancy. J. Obstet. Gynecol. 1973, 80, 805–809.
- Papaioannou, G.I.; Syngelaki, A.; Poon, L.C.Y.; Ross, J.A.; Nicolaides, K.H. Normal ranges of embryonic length, embryonic heart rate, gestational sac diameter and yolk sac diameter at 6–10 weeks. Fetal. Diagn. Ther. 2010, 28, 207–219.
- Oosthoek, P.W.; Wenink, A.C.G.; Vrolijk, B.C.M.; Wisse, L.J.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Development of the atrioventricular valve tension apparatus in the human heart. Anat. Embryol. 1998, 198, 317–329.
- Acharya, G.; Gui, Y.; Cnota, W.; Huhta, J.; Wloch, A. Human embryonic cardiovascular function. Acta Obstet. Gynecol. Scand. 2016, 95, 621–628.
- DuBose, T.J.; Cunyus, J.A.; Johnson, L.F. Embryonic heart rate and age. JDMS 1990, 6, 151–157.
- DuBose, T.J. Embryonic heart rates. Fertil. Steril. 2009, 92, e57.
- Romanoff, A.L. The heart beat of avian embryos. Anat. Rec. 1944, 89, 313–316.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
20 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




