| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Monika Topa | + 3934 word(s) | 3934 | 2020-09-23 03:38:03 |
Video Upload Options
The photoinduced polymerization of monomers is currently an essential tool in various industries. The photopolymerization process plays an increasingly important role in biomedical applications. It is especially used in the production of dental composites. It also exhibits unique properties, such as a short time of polymerization of composites (up to a few seconds), low e tes with their limitations and disadvantages. nergy consumption, and spatial resolution (polymerization only in irradiated areas). This entry describes a short overview of the history and classification of different typical monomers and photoinitiating systems such as bimolecular photoinitiator system containing camphorquinone and aromatic amine, 1-phenyl-1,2-propanedione, phosphine derivatives, germanium derivatives, hexaarylbiimidazole derivatives, silane-based derivatives and thioxanthone derivatives used in the production of dental composites with their limitations and disadvantages.
1. Introduction
Nowadays, the most modern technologies for the production of polymeric materials are based on photochemically initiated processes. The synthesis of polymeric materials carried out by photopolymerization is one of the most efficient methods, thanks to which it is currently a very widespread and dynamically developing technique[1][2][3][4][5]. Compared to other methods, photopolymerization is considered environmentally friendly due to its low energy consumption, no use of solvents, and high speed at ambient temperature. In industrial practice, two types of photochemically initiated polymerization are most commonly used, namely radical and cationic polymerization[6][7][8][9]. Due to the presence of oxygen inhibition in the case of free radical photopolymerization, much attention is currently paid to the cationic, thiol-ene, and hybrid photopolymerization processes[10][11].
Polymerization using light, mainly ultraviolet (UV) light, was initially used in the coating industry, especially in varnishing for solvent-free paints and varnishes for the furniture and automotive industries[11]. Achieving high polymerization rates in fractions of seconds, resulting from the rapid formation of radicals or initiating ions, allows for high throughput of the production line[12]. Besides, the possibility of conducting photopolymerization processes at ambient temperature prepares polymeric materials carried out by photoinduced polymerization process one of the most efficient photochemical technologies. Currently, this type of polymerization is also used in many other industries, namely in photolithography for the production of printed circuits, in micro-replication for the production of spherical lenses, for photocuring polymeric adhesives, and in microelectronics for encapsulating integrated circuits[13]. The dynamically developing printing industry is a different direction of the application of photopolymerization, which enables printing on plastic or metal materials. Moreover, in recent years, a particular emphasis has been put on the use of photopolymerization processes for 3D-printing technology[14][15][16][17][18][19][20][21][22][23], including stereolithography in the design and formation of three-dimensional models[24].
All this means that not only has recently been an astonishingly rapid growth in the applications of technologies based on photopolymerization processes, but also the development of new materials determining the pace of this development[25]. Dynamic progress in the field of chemistry and technology of photoinitiated processes leads to the emergence of more and more sophisticated solutions in this field, an example of which may be successively developed new generation monomers [26][27], new, more effective photoinitiation systems[28][29][30] or new light sources[31] and methods of monitoring the online polymerization processes[32][33][34][35].
Photopolymerization processes play an increasingly important role in biomedical applications, for instance, in obtaining hydrogel polymer materials[36][37][38][39] or in vivo photocurable dental composites[40][41][42]. Applying photochemically initiated polymerization for obtaining dental polymer composites enables the use of unique and innovative features. The most important are:
- Short time of monomer/filler compositions curing (up to a few seconds);
- Conducting the reaction at room temperature;
- Low energy consumption;
- Spatial resolution (polymerization only in irradiated areas).
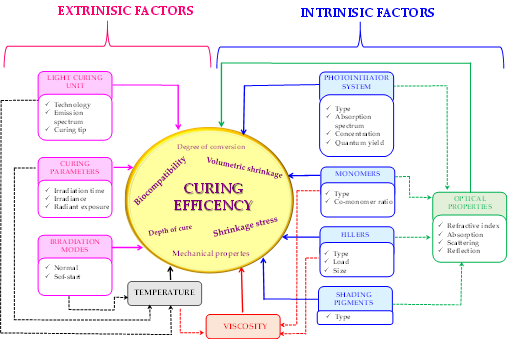
Nevertheless, obtaining polymer composites of the demanded properties, that is, above all, of favourable mechanical properties and reduction polymerization shrinkage, is still a significant challenge for the researchers. This is because many different factors, such as the selection of appropriate monomers, initiators, inorganic fillers, photopolymerization process time range, or the source and power of a light source, influence the quality of the composite obtained (Figure 1)[43].
Recently, iodonium salts have become of particular interest and are used as a component of initiating systems for the preparation of dental composites. This is directly due to the relatively good solubility of these salts in non-polar monomers. In addition, this group of compounds photodissociate with high initiation efficiency are thermally stable and show long-term stability under storage conditions[44][45].
In this paper, we aim to present commonly used monomers and photoinitiating systems for the photocurable dental composites and indicated their main disadvantages. Recent developments and progress in the future of photocurable resins have also been shown. A particular emphasis was placed on novel photoinitiating systems containing iodonium salts applied in dental adhesive resin.
Figure 1. Illustrative diagram showing the influence of factors on the quality of obtained dental composites.
2. Monomers Used for the Production of Dental Composites
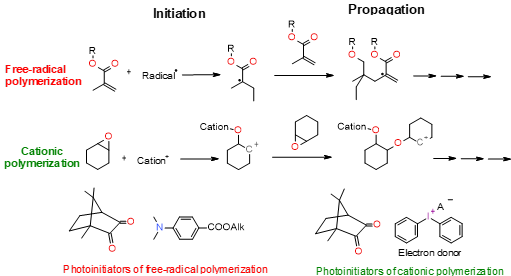
The mechanism of photopolymerization depends on using monomers. There are two main types of photopolymerization: radical polymerization and cationic polymerization (Figure 2). The type of organic matrix has a considerable impact on the properties of dental composites. It primarily affects mechanical strength, sorption, solubility, polymerization shrinkage, abrasion resistance, colour stability, and biocompatibility[43][46]. Generally, the organic components of a typical photocuring composition constitute about 10%–30% wt.[47] The remainder is inorganic filling in the form of microparticles (≥0.4 μm) or a mixture of micro and nanoparticles (50 nm > 400 nm)[47]. In addition to photoinitiators, adhesion promoters and possibly antibacterial compounds are also added to dental composites[48][49][50].
Figure 2. Mechanism of free radical polymerization and cationic ring-opening polymerization with their corresponding photoinitiation systems.
2.1. Monomers for Free Radical Photopolymerization Processes in Dental Adhesive Resin Application.
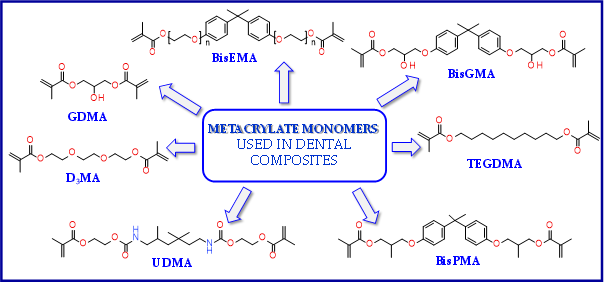
The most popular materials for obtaining dental composites through photopolymerization are (meth)acrylate monomers (RCB—resin-based composites) characterized by high reactivity, which form an organic matrix[51][52][53]. They guarantee obtaining networks with a high degree of crosslinking[53][54]. By free radical polymerization of the matrix monomers, a three-dimensional network is formed. Among the currently available dental composites, the most common are 2,2-bis[4-2-hydroxy-3-methacryloyloxypropyl)phenyl]propane (BisGMA) and triethylene glycol dimethacrylate (TEGDMA)[55].
The use of BisGMA in dental materials, due to the presence of the aromatic structure of Bisphenol A in the core of the molecule, ensures low volatility of the composition and high modulus of the light-cured composite[56][57][58]. In turn, the use of the low-viscosity TEGDMA monomer, which is the so-called active diluent, allows the introduction of an appropriate amount of inorganic filler[59]. The weight proportions of both monomers are usually 7/3 or 8/2, where BisGMA is the main component. Another commonly used acrylate monomer is 1,6-bis-[2-methacryloyloxyethoxycarbonylamino]-2,4,4-trimethylhexane (UDMA)[60]. The content of rigid urethane groups guarantees dental composites with favorable strength properties[61][62][63]. In addition to the aforementioned BisGMA, TEGDMA, and UDMA, other common dental monomers polymerized via the free radical process are also ethoxylated BisGMA (BisEMA). This monomer is used for reducing water absorption by the organic matrix. In addition, the lack of -OH also causes this monomer to be less viscous than BisGMA. An array of monomer structures for the base dimethacrylate materials, as well as new monomers, are given in Figure 3[64][65][66][67][68][69][70].
Figure 3. Examples of methacrylate monomers used in commercial and conventional dental composites based on free radical photopolymerization mechanism.
All dental composites based on crosslinking dimethacrylates exhibit an inherent problem of 2%–14% volumetric shrinkage during the photopolymerization process[71]. These stresses may produce defects in the composite–tooth bond, leading to bond failure, microleakage, postoperative sensitivity, and recurrent caries. Such shrinkage stresses could also cause deformation of the surrounding tooth structure when the composite–tooth bond is strong, predisposing the tooth to fracture[72]. The polymerization shrinkage of low molecular monomers is more pronounced when compared to that of high molecular monomers; however, high molecular monomers are very viscous (Table 1). For these reasons, polymerization shrinkage is dictated by a complex interplay among resin viscosity, polymerization rate, degree of conversion, and network structural evolution, where each of these properties cannot be individually manipulated and studied without having a significant impact on other properties.
Table 1. Properties of the popular free radical monomers to obtained dental composites[53].
|
Monomer |
Molecular Weight [g/mol] |
ρmona [g/cm3] |
ρpolb [g/cm3] |
ΔVp [%] |
Viscosity [mPa•s] |
|
TEGDMA |
286 |
1.072 |
1.250 |
−14.3 |
100 |
|
UDMA |
470 |
1.110 |
1.190 |
−6.7 |
5000–10,000 |
|
Bis-GMA |
512 |
1.151 |
1.226 |
−6.1 |
500,000–800,000 |
|
ρmona—density of monomer ρpolb—density of polymer |
|||||
Moreover, due to the inhomogeneous network architecture, which is obtained during a free radical photopolymerization process, the final materials tend to show a somewhat brittle behavior, and the occurring shrinkage stress could lead to delamination, deformation or mechanical failure of the final composites materials. The observed shrinkage stress evolves during polymerization reaction upon transitioning of the applied formulation from the liquid to solid-state (i.e., gel point) and is built up upon vitrification until the final conversion is reached. Before free radical photopolymerization, the monomers are situated at van der Waal’s distance towards each other (approximately 3.4 Å)[73]. The occurring shrinkage stress upon gelation is partially due to the formation of covalent bonds between the respective monomers, where the revealing distance is only 1.5 Å[74]. Incomplete free radical photopolymerization, volumetric shrinkage, and stress are some of the primary disadvantages of current methacrylates resin-based dental composites. Generally, attempts to increase the double-bond conversion and reduce polymerization shrinkage and stress have been conducted[74].
2.2. Monomers for Cationic Photopolymerization.
In recent years, the application of ring-opening cationic photopolymerizable epoxy–monomer-based compositions for dental fillings have found increasing attention in different articles and patent applications[53][75][76].
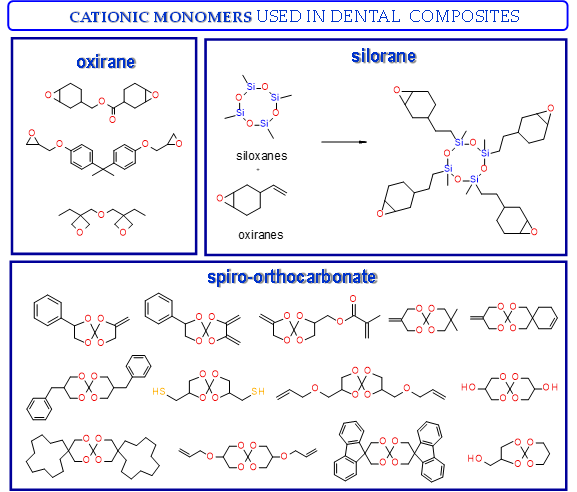
Thus, based on the cationic photopolymerization process, new-generation photocuring dental materials, including oxiranes[53], siloranes[77], oxetanes, and spiro-orthocarbonate [76], were developed (Figure 4). Dental materials based on these monomers have achieved clinical success because they have significantly reduced polymerization shrinkage to below 1% and minimized polymerization stress compared to traditional methacrylate materials[75]. The mechanism of compensation for systolic stress in this system was achieved by the phenomenon of opening the oxirane rings during the cationic photopolymerization process, which proceeds with a small change in the volume of the system[78].
Crosslinking cycloaliphatic epoxy compounds were particularly of interest because they demonstrate significantly lower shrinkage than dental methacrylate resins (e.g., cycloaliphatic epoxide 3,4-epoxycyclohexyl-methyl-3,4-epoxycyclohexane carboxylate and the diglycidyl ether of bisphenol A, which improved the mechanical properties of the cured composite). Moreover, these epoxy resins were reactive enough to be cured by cationic photopolymerization in an acceptable time frame and to an adequate depth using a dental Vis-LED light source. In addition to epoxy resins, oxetanes were evaluated for dental applications[79]. The reactivity of oxetanes is mainly controlled by the ring stress and the basicity of the ring oxygen. However, oxetanes demonstrate higher basicity. The ring-opening cationic photopolymerization of oxetanes was also characterized by a significantly lower shrinkage in comparison to methacrylates. From the investigated oxetanes, the hydroxy group containing monomer possessed the highest polymerization rate[80].
In turn, spiroorthoesters (SOEs) and spiroorthocarbonates (SOCs) (Figure 4) are other monomers that are polymerized via to the cationic mechanism and are increasingly used in dental applications. Spiroorthoesters (SOEs) and spiroorthocarbonates (SOCs) are the most widely studied expanding monomers. SOCs are double-cyclic acetals that polymerize under acidic catalysis but are stable under basic conditions. When these compounds polymerize by double ring-opening photopolymerization (ROP), poly(ether-carbonates) are produced. In general, bi-cyclic compounds cured by ROP shrink less as they harden because of an increase in the excluded free volume associated with the ring-opening process. Bailey[81] investigated bi-cyclic compounds, such as spiro-orthocarbonates (SOCs), that can be used as an expanding co-monomer in RBC formulations. Ring-opening reactions with SOCs produce expansion (3.5%), which could counteract normal shrinkage[82]. However, SOCs exhibit incomplete ring-opening, as well as limited solubility and minimal copolymerization in dimethacrylate resins, resulting in minimal shrinkage reduction.
Nevertheless, compared to traditional composite materials, spiroorthocarbonate-based composites show less polymerization shrinkage and twice as much adhesion to enamel[76]
However, the most recent modification on the polymer matrix is based on using ring-opening polymerization of the silorane molecules, instead of free radical polymerization of dimethacrylate monomers[83]. They are built of a siloxane backbone, which gives them hydrophobic and cycloaliphatic oxirane molecules responsible for low polymerization shrinkage. These monomers have provided particularly interesting and commercially viable results. Such monomers “open” their molecular structures with local volumetric expansion, and this may partly or totally compensate for volumetric shrinkage from C=C or similar polymerization[84][85]. Based on the literature reports, the use of siloranes has been shown to guarantee a reduction in the polymerization shrinkage to 0.94%[77].
Examples of monomers that polymerize thorough to the cationic mechanism and have reduced polymerization shrinkage are shown in Figure 4.
Figure 4. Examples of monomers used in cationic photopolymerization (a) oxirane, (b) spiro-orthocarbonate, (c) silorane: a merger of siloxanes and oxiranes.
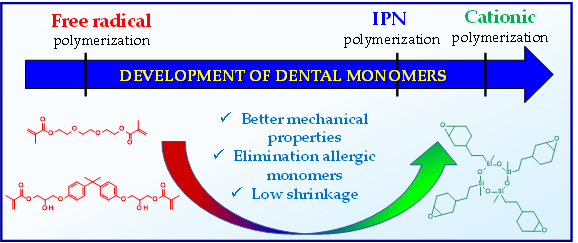
The development of new monomers polymerizing via the cationic mechanism contributed to a significant reduction in the polymerization shrinkage of dental composites and obtaining dental composites with better mechanical properties. Moreover, acrylate monomers, which often cause severe allergies, have been eliminated (Figure 5).
Figure 5. Comparison of the properties of monomers polymerizable via free radical mechanism with monomers polymerizable via cationic mechanism.
3. Commonly Used Photoinitiating Systems for Dental Application.
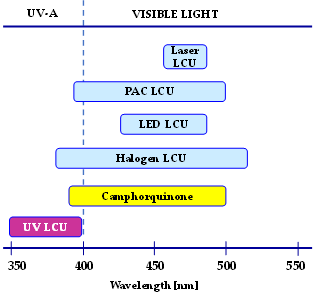
Photoinitiating systems for obtaining dental composites are particularly important. They affect such parameters as the efficiency of the photopolymerization process and the choice of a light source (Figure 6).
Figure 6. The range of emission spectra of UV and visible light-curing units and the range of the absorption of standard co-initiators camphorquinone used in the dental application (UV—ultraviolet, LCU—light-curing units, LED—light-emitting diode, PAC—plasma arc).
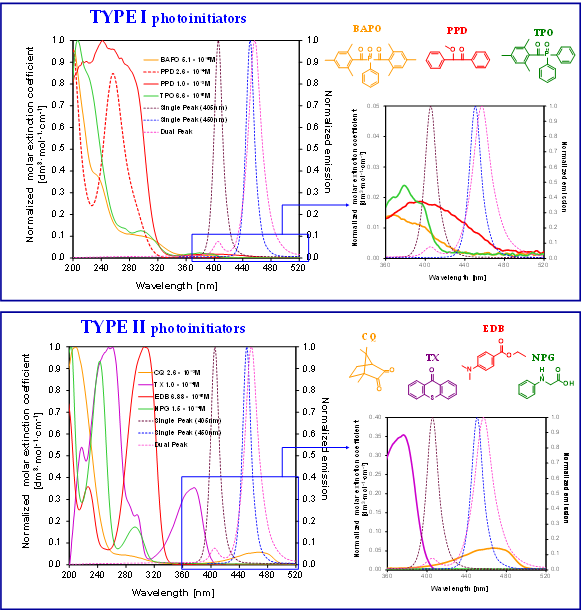
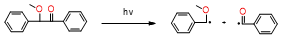
To date, several initiation systems for radical photopolymerization processes have been developed. In Figure 7, the absorption spectra of standard initiators in comparison with the emission characteristics of the commonly used light-curing units are presented. While table 2 shows the photoinitiators used in a dental application, their basic properties, and photoinduced cleavage of photoinitiators.
Figure 7. Comparison of the normalized molar extinction coefficient of standard type I initiators (top) and type II initiators together with amines (bottom) used in dental applications with the emission characteristics of standard light-curing units.
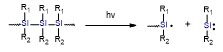
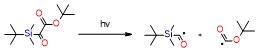
Table 2. Summary of the photoinitiators used in dental application, their basic properties, and photoinduced cleavage of photoinitiators.
|
Acronym of Photoinitiator |
Structure, Together with a Scheme of Photoinduced Cleavage of Photoinitiator |
Characteristic of Absorbance |
Advantages |
Disadvantages |
Ref. |
|
CQ |
|
λmax = 468nm |
wide absorption range based on the visible range |
molar extinction coefficient in the range of 400–500 nm is only 40 [dm3 · mol–1 · cm–1], strongly yellow color |
[86][87] |
|
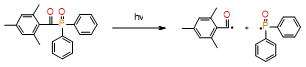
PPD |
|
λmax = 400nm |
improve the color stability |
necessary to use LED light sources with two violet emission bands (380–420 nm and blue 420–520 nm), dual peak LEDs |
|
|
TPO |
|
λmax = 382nm |
high efficiency of generating radicals, improve the color stability |
low initiation efficiency, the need for UV light sources |
|
|
IVO |
|
λmax = 445nm |
no cytotoxicity, high initiation rate and excellent bleaching |
initiators only for free radical polymerization |
|
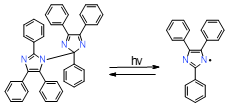
|
HABI |
|
display extended absorption tails well into the visible spectral region |
effective in initiating thiol-ene photopolymerization |
poor absorption in the visible spectrum, sometimes requiring a photosensitizer, low solubility in standard resins used in a dental application and low solubility in organic solvents |
|
|
Silane derivatives |
|
ultraviolent strong absorption in the 300–350 nm region |
very effective in free radical photopolymerization, witch iodonium salts or pyridinium can be used for cationic photopolymerization |
need for UV light sources |
|
|
|
λmax = 425 nm
|
excellent bleaching properties, in combination with an iodonium salt, can be useful for initiating cationic photopolymerization |
low value of molar extinction coefficient ε: 120 dm3 · mol–1 · cm–1 in toluene and 100 dm3 · mol–1 · cm–1 in acetonitrile |
[103][104] | |
|
|
λmax = 486nm for SED1 λmax = 468 nm for SED2 in toluene |
suitable for free radical photopolymerization exposure to blue (@ 455 nm) and even green (@ 520 nm) LED |
not suitable for methacrylate photopolymerization |
[105] | |
|
|
λmax = 442 nm in acetonitirle |
excellent bleaching properties, a high water solubility, and a very good stability in acidic |
not suitable for methacrylate photopolymerization |
[106] |
|
|
TX |
|
λmax = ∼378 nm |
water-soluble co-initiators, instant bond strength to dentin minimize the effects of concentration stress and phase separation in the aquatic environment |
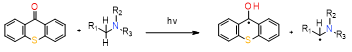
generally, less reactive than CQ/amine system |
3. Onium Salts as an Innovative Component of Photoinitiating Systems for Photopolymerization Processes in Dental Applications.
In recent times, onium salts, i.e., sulfonium and iodonium salts, particularly in the form of diaryliodonium salts, have been playing an increasingly important role in initiating photopolymerization processes[109][110][111][112][113][114][115][116].
All the properties of ionic compounds supporting their commercial use as photoinitiators depend only on their structure. It has been shown that the cation of iodonium salt, absorbing electromagnetic radiation, is responsible for the photochemical properties of these compounds as photoinitiators. Thus, the structure of the cation determines the initiator’s properties, such as the location of the maximum absorption (λmax), molar absorption coefficient (ε), the quantum efficiency of the initiator, and even thermal stability. On the other hand, the nature of anion has a decisive influence on the suitability of the initiating system as a photoinitiator. The type of anion determines the power of protic acid generated during photolysis, directly affecting the efficiency of initiation and the kinetics of the polymerization process. However, the essential properties of iodonium salts, from their applications in cationic polymerization processes (in addition to solubility in monomers) are their optical properties, i.e., the location of the maximum absorption (λmax) and molar extinction coefficient (ε)[44][117]
3.1 Two- or Three-component Photoinitiating Systems Containing Iodonium Salt for Initiating Free radical Photopolymerization Processes for an Obtained Dental Composites.
The hydrophobicity of commonly used photoinitiating systems based on camphorquinone (CQ) and ethyl 4-(dimethylamino)benzoate (EDMAB) has limited their performance in the wet, oral environment. Therefore, to eliminate this limitation, a water-soluble iodonium salt is added mainly diphenyliodonium hexafluorophosphate (DPIHP)[118]. Iodonium salt as an accelerator in dental applications is usually found in a ternary initiating system containing CQ and a tertiary aromatic amine. However, it is also possible to use a two-component initiating system based on CQ and an iodonium salt (without a tertiary aromatic amine), except that, compared to the three-component system, slightly lower conversion rates are usually obtained.
In a two-component initiating system based on CQ/onium salt, after being irradiated with blue light, the exciplex state is formed; next, the onium salt is reduced by electron transfer. The resulting diphenyliodine free radical is unstable and quickly degrades to phenyliodine and phenyl free radical, which causes the reaction to be irreversible. These reactive phenyl forms are useful in initiating the photopolymerization. Radicals generated during polymerization propagation effectively cleave the C–I bond, releasing another radical and allowing the photopolymerization[119].
The three-component initiating system is usually based on CQ/aromatic amine/iodonium salt, and this system is characterized that the additional amine radicals are produced. In addition, CQ is regenerated through substitution of inactive and also termination radicals to active radicals in the form of phenyl radicals and the generation of positive active phenyl radicals[115].
This makes the photopolymerization process initiated by the ternary initiation system extremely efficient and fast. A similar degree of conversion and rate of polymerization compared to acylphosphine oxide (MAPO) or bis-acylphosphine oxide (BAPO) photoinitiators is even obtained[120][121][122].
The photoinitiating system based on CQ/iodonium salt or CQ/iodonium salt/amine have mainly found application in initiating traditional methacrylate monomers used in the production of dental composites[120][121][123][124][125][126]. The addition of iodonium salt to the photoinitiating systems used to prepare dental composites brings many benefits. The most important are the
- Increase conversion in short photo-activation time;
- Reduced inhibitory polymerization effect from an organic solvent;
- Improved dentin bonding performance;
- Improved reactivity and mechanical properties;
- Decreased sorption and water solubility;
- Reduced initial color and improved color stability.
3.2. Iodonium Salts as Photoinitiators for Cationic and IPN Photopolymerization to Obtain a Dental Composites.
In recent years, researchers from around the world have designed new initiating systems based on onium salts for cationic and thiol-ene photopolymerization processes for obtained dental composites.
In 2014, it was first described using composites based on cationic systems for dental applications[75]. It has been shown that the two-component system of the CQ/[4(1-methylethyl)phenyl][4-methylphenyl] iodonium tetrakis (pentafluorophenyl)borate, Rhodorsil 2074 is useful for initiating the cationic photopolymerization process of bis[2-(3,4-epoxycyclohexyl)ethyl]tetramethyldisiloxane, UV30. CQ promotes the photopolymerization process even in the absence of amines as a hydrogen donor. It can separate labile hydrogen from an epoxy monomer; carbon-concentrated radicals are formed, which are oxidized by onium salt. The complete conversion of the epoxy group was achieved after 50 s with blue irradiation.
Fu et al. proposed the use a three-component photoinitiation system comprising 1 wt.% CQ (camphorquinone), 2 wt.% DMAEMA (2-(dimethylamino) ethyl meth acrylate) and 2 wt.% diphenyliodonium hexafluorophosphate to initiate the copolymerization of the matrix resins which combine bisphenol-S-bis(3-methacrylate-2-hydroxy propyl) ether (BisS-GMA) with the expanding monomer unsaturated spiro orthoesters 2-methylene-1,4,6-trispiro[4,4] nonane (MTOSN), for minimizing the volumetric shrinkage that generally occurs during polymerization. The results supported that the dental composites based on the expanding monomer and three-component photoinitiator system engendered a more significant decrease of volumetric shrinkage and better mechanical properties[127].
Danso, R. et al. proposed new resins (Oxirane-Acrylate IPN System—OASys) based on p-Cycloaliphatic diepoxide EPALLOY 5000TM (EP5000) and dipenta erythritol hexaacrylate (DPHA). A three-component initiating system in the form of (4-octyloxyphenyl)phenyliodonium hexafluoroantimonate (OPPI), CQ, and a co-reactant oligomeric diol 250 Mn poly(tetrahydrofuran) was used. These results demonstrate that OASys resins cure well, are more hydrophobic, and have lower shrinkage stress than BisGMA-based resins. However, they are mechanically weaker[128].
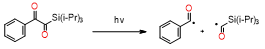
This new class of photoinitiators based on silyl glyoxylates to initiate cationic polymerization combined with an iodonium salt was presented in an article by Kirschner[129]. This system can be used to initiate free radical/cationic hybrid polymerization and for the synthesis of interpenetrating polymer networks. The system silyl glyoxylate/iodonium exhibits excellent polymerization performances and exceptional bleaching properties compared to other well-established photoinitiators (e.g., camphorquinone)[129]. This system is also suitable for initiating a hybrid monomer (2-vinyloxyethoxyethyl methacrylate [VEEM]). This monomer leads to a considerable improvement of the mechanical properties of the final polymer through hybrid polymerization[129].
Zang et al. proposed the use 1,2-diketone/iodonium salt (and optional NVK) systems to initiate cationic photopolymerization of epoxides or free radical photopolymerization of methacrylates. Most of the photoinitiating systems have exhibited higher initiation ability than the well-known CQ-based systems. Nevertheless, the study of the biocompatibility indicates that these materials exhibit cytotoxicity[130].
4. Challenges of Photoinitiator Systems for Dental Applications, Future Trends and Practical Aspects
In recent years, several new initiating systems for dental composites have been developed. However, these are mainly photoinitiating systems used to obtain dental composites by radical photopolymerization[131]. Most of them have several of the significant disadvantages mentioned earlier in this article. Despite such significant progress, new initiating systems with improved properties are still being sought, mainly to produce dental composites obtained by cationic photopolymerization, which
- Are entirely safe for humans, eliminating the cytotoxic amines and acrylate monomers that often cause severe allergies;
- Do not generate yellow color-eliminating camphorquinone, greater aesthetics, and quality of the final product;
- Have better and/or comparable mechanical properties and, due to the use of polymerizable monomers via to the cationic mechanism, have reduced polymerization shrinkage;
- Is possible to be used with dental lamps emitting radiation in the visible light range for the curing process, eliminating harmful UV radiation.
5. Conclusion
In conclusion, it can be stated that in the scope of initiating systems for photocuring dental composites according to the radical mechanism[131], a significant milestone towards solutions guaranteeing the active initiation of this type of process has now been realized. In addition, in recent years, new initiating systems containing iodonium salt to initiate cationic and/or IPN photopolymerization processes have been developed. In this way, dental composites with better mechanical properties and reduced polymerization shrinkage were obtained. Nevertheless, in most cases, these are camphorquinone-containing systems that generate yellow color or toxic co-initiators. In addition, the complete elimination of acrylate monomers that often cause severe allergies is still a significant challenge for researchers.
The literature review has presented previous achievements in the field of radical photoinitiators dedicated to the preparation of dental composites; their advantages and disadvantages are discussed. The advantages of iodonium salts and their potential to initiate cationic photopolymerization processes of silorane monomers to obtain new-generation dental composites were also indicated.
References
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912, doi:10.1039/c5py00258c.
- Funke, W. UV Curing: Science and Technology. Prog. Org. Coat. 1980, 8, 110, doi:10.1016/0300-9440(80)80007-5.
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 577–589, doi:10.1021/cr3005197.
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260, doi:10.1021/ma1007545.
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793, doi:10.1002/mame.201300349.
- Ortyl, J.; Popielarz, R. The performance of 7-hydroxycoumarin-3-carbonitrile and 7-hydroxycoumarin-3-carboxylic acid as fluorescent probes for monitoring of cationic photopolymerization processes by FPT. J. Appl. Polym. Sci. 2013, 128, 1974–1978, doi:10.1002/app.38378.
- Ortyl, J.; Galek, M.; Milart, P.; Popielarz, R. Aminophthalimide probes for monitoring of cationic photopolymerization by fluorescence probe technology and their effect on the polymerization kinetics. Polym. Test. 2012, 31, 466–473, doi:10.1016/j.polymertesting.2012.01.008.
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coat. 2019, 133, 131–138, doi:10.1016/j.porgcoat.2019.03.054.
- Ortyl, J.; Galica, M.; Popielarz, R.; Bogdał, D. Application of a carbazole derivative as a spectroscopic fluorescent probe for real time monitoring of cationic photopolymerization. Polish J. Chem. Technol. 2014, 16, 75–80, doi:10.2478/pjct-2014-0013.
- Ortyl, J.; Topa, M.; Kamińska-Borek, I.; Popielarz, R. Mechanism of interaction of aminocoumarins with reaction medium during cationic photopolymerization of triethylene glycol divinyl ether. Eur. Polym. J. 2019, 116, 45–55, doi:10.1016/j.eurpolymj.2019.03.060.
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications, 1st ed.; Elsevier Science: Amsterdam, Netherlands, 2006.
- Schnabel, W. Polymers and Light: Fundamentals and Technical Applications; Wiley VCH: New Jersey, NJ, USA, 2007.
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665, doi:10.1016/S0079-6700(01)00004-1.
- Hola, E.; Topa, M.; Chachaj-Brekiesz, A.; Pilch, M.; Fiedor, P.; Galek, M.; Ortyl, J. New, highly versatile bimolecular photoinitiating systems for free-radical, cationic and thiol-ene photopolymerization processes under low light intensity UV and visible LEDs for 3D printing application. RSC Adv. 2020, 10, 7509–7522, doi:10.1039/c9ra10212d.
- Weems, A.C.; Delle Chiaie, K.R.; Yee, R.; Dove, A.P. Selective Reactivity of Myrcene for Vat Photopolymerization 3D Printing and Postfabrication Surface Modification. Biomacromolecules 2020, 21, 163–170, doi:10.1021/acs.biomac.9b01125.
- Xu, Y.; Noirbent, G.; Brunel, D.; Liu, F.; Gigmes, D.; Sun, K.; Zhang, Y.; Liu, S.; Morlet-Savary, F.; Xiao, P.; et al. Ketone derivatives as photoinitiators for both radical and cationic photopolymerizations under visible LED and application in 3D printing. Eur. Polym. J. 2020, 132,109737, doi:10.1016/j.eurpolymj.2020.109737.
- Tang, L.; Nie, J.; Zhu, X. A high performance phenyl-free LED photoinitiator for cationic or hybrid photopolymerization and its application in LED cationic 3D printing. Polym. Chem. 2020, 11, 2855–2863, doi:10.1039/d0py00142b.
- Sivasankar, V.S.; Sachar, H.S.; Sinha, S.; Hines, D.R.; Das, S. 3D Printed Microdroplet Curing: Unravelling the Physics of On-Spot Photopolymerization. ACS Appl. Polym. Mater. 2020, 2, 966–976, doi:10.1021/acsapm.9b01181.
- You, S.; Wang, P.; Schimelman, J.; Hwang, H.H.; Chen, S. High-fidelity 3D printing using flashing photopolymerization. Addit. Manuf. 2019, 30, 100834, doi:10.1016/j.addma.2019.100834.
- Lin, J.T.; Cheng, D.C.; Chen, K.T.; Liu, H.W. Dual-wavelength (UV and blue) controlled photopolymerization confinement for 3D-printing: Modeling and analysis of measurements. Polymers 2019, 11, 1819, doi:10.3390/polym11111819.
- Malas, A.; Isakov, D.; Couling, K.; Gibbons, G.J. Fabrication of high permittivity resin composite for vat photopolymerization 3D printing: Morphology, thermal, dynamic mechanical and dielectric properties. Materials 2019, 12, 3818, doi:10.3390/ma12233818.
- Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free radical photopolymerization and 3D printing using newly developed dyes: Indane-1,3-dione and 1H-cyclopentanaphthalene-1,3-dione derivatives as photoinitiators in three-component systems. Catalysts 2020, 10, 463, doi:10.3390/catal10040463.
- Sirrine, J.M.; Zlatanic, A.; Meenakshisundaram, V.; Messman, J.M.; Williams, C.B.; Dvornic, P.R.; Long, T.E. 3D printing amorphous polysiloxane terpolymers via vat photopolymerization. Macromol. Chem. Phys. 2019, 220, 1800425, doi:10.1002/macp.201800425.
- Corcione, C.E.; Greco, A.; Maffezzoli, A. Photopolymerization kinetics of an epoxy-based resin for stereolithography. J. Appl. Polym. Sci. 2004, 92, 3484–3491, doi:10.1002/app.20347.
- Lalevée, J.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Fouassier, J.P. Photochemistry and radical chemistry under low intensity visible light sources: Application to photopolymerization reactions. Molecules 2014, 19, 15026–15041, doi:10.3390/molecules190915026
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320, doi:10.1016/j.polymertesting.2017.10.020.
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers, Part II: Determination of relative quantum efficiency of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2018, 67, 144–150, doi:10.1016/j.polymertesting.2018.02.025.
- Topa, M.; Petko, F.; Galek, M.; Machowski, K.; Pilch, M.; Szymaszek, P.; Ortyl, J. Applicability of 1,6-Diphenylquinolin-2-one derivatives as fluorescent sensors for monitoring the progress of photopolymerisation processes and as photosensitisers for bimolecular photoinitiating systems. Polymers 2019, 11, 1756, doi:10.3390/polym11111756.
- Ortyl, J.; Milart, P.; Popielarz, R. Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polym. Test. 2013, 32, 708–715, doi:10.1016/j.polymertesting.2013.03.009.
- Hola, E.; Ortyl, J.; Jankowska, M.; Pilch, M.; Galek, M.; Morlet-Savary, F.; Graff, B.; Dietlin, C.; Lalevée, J. New bimolecular photoinitiating systems based on terphenyl derivatives as highly efficient photosensitizers for 3D printing application. Polym. Chem. 2020, 11, 922–935, doi:10.1039/c9py01551e.
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoredox catalyst: Applications of free radical/cationic hybrid photopolymerization in the composites and 3D printing fields. Prog. Org. Coat. 2019, 132, 50–61, doi:10.1016/j.porgcoat.2019.02.044.
- Ortyl, J.; Wilamowski, J.; Milart, P.; Galek, M.; Popielarz, R. Relative sensitization efficiency of fluorescent probes/sensitizers for monitoring and acceleration of cationic photopolymerization of monomers. Polym. Test. 2015, 48, 151–159, doi:10.1016/j.polymertesting.2015.10.006.
- Kamińska, I.; Ortyl, J.; Popielarz, R. Applicability of quinolizino-coumarins for monitoring free radical photopolymerization by fluorescence spectroscopy. Polym. Test. 2015, 42, 99–107, doi:10.1016/j.polymertesting.2014.12.013.
- Kamińska, I.; Ortyl, J.; Popielarz, R. Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polym. Test. 2016, 55, 310–317, doi:10.1016/j.polymertesting.2016.09.013.
- Topa, M.; Ortyl, J.; Chachaj-Brekiesz, A.; Kamińska-Borek, I.; Pilch, M.; Popielarz, R. Applicability of samarium(III) complexes for the role of luminescent molecular sensors for monitoring progress of photopolymerization processes and control of the thickness of polymer coatings. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2018, 199, 430–440, doi:10.1016/j.saa.2018.03.050.
- Zuo, X.; Morlet-Savary, F.; Schmitt, M.; Le Nouën, D.; Blanchard, N.; Goddard, J.P.; Lalevée, J. Novel applications of fluorescent brighteners in aqueous visible-light photopolymerization: High performance water-based coating and LED-assisted hydrogel synthesis. Polym. Chem. 2018, 9, 3952–3958, doi:10.1039/c8py00584b.
- Staneva, D.; Grabchev, I.; Bosch, P. Fluorescent hydrogel-textile composite material synthesized by photopolymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 838–847, doi:10.1080/00914037.2015.1030654.
- Xia, B.; Jiang, Z.; Debroy, D.; Li, D.; Oakey, J. Cytocompatible cell encapsulation via hydrogel photopolymerization in microfluidic emulsion droplets. Biomicrofluidics 2017, 11, 044102, doi:10.1063/1.4993122.
- Larsen, E.K.U.; Larsen, N.B.; Almdal, K.; Larsen, E.K.U.; Larsen, N.B.; Almdal, K. Multimaterial hydrogel with widely tunable elasticity by selective photopolymerization of PEG diacrylate and epoxy monomers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1195–1201, doi:10.1002/polb.24007.
- Ingrosso, C.; Esposito Corcione, C.; Striani, R.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Frigione, M. UV-Curable Nanocomposite Based on Methacrylic-Siloxane Resin and Surface-Modified TiO2 Nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 15494–15505, doi:10.1021/acsami.5b03731.
- Liu, D.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Synthesis of a novel tertiary amine containing urethane dimethacrylate monomer (UDMTA) and its application in dental resin. J. Mater. Sci. Mater. Med. 2013, 24, 1595–1603, doi:10.1007/s10856-013-4897-2.
- Fu, J.; Jia, F.; Xu, H.; Ji, B.; Liu, X. Properties of a new dental photocurable matrix resin with low shrinkage. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 236–241, doi:10.1007/s11595-011-0204-6.
- Rüttermann, S.; Dluzhevskaya, I.; Großsteinbeck, C.; Raab, W.H.M.; Janda, R. Impact of replacing Bis-GMA and TEGDMA by other commercially available monomers on the properties of resin-based composites. Dent. Mater. 2010, 26, 353–359, doi:10.1016/j.dental.2009.12.006.
- Ortyl, J. Chapter 3: Cationic Photoinitiators. In Photopolymerisation Initiating Systems; Lalevée, J., Fouassier, J.-P., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 74–130.
- Crivello, J.V. Cationic polymerization—Iodonium and sulfonium salt photoinitiators. In Initiators—Poly-Reactions—Optical Activity; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–48.
- Jandt, K.D.; Sigusch, B.W. Future perspectives of resin-based dental materials. Dent. Mater. 2009, 25, 1001–1006, doi:10.1016/j.dental.2009.02.009.
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38, doi:10.1016/j.dental.2010.10.020.
- Shin, D.H.; Rawls, H.R. Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent. Mater. 2009, 25, 1030–1038, doi:10.1016/j.dental.2009.03.004.
- Oxman, J.D.; Jacobs, D.W. Ternary Photoinitiator System for Curing of Epoxy Resns. U.S. Patent 6,043,295, 28.03.2000.
- Rabek, J.F. Photosensitized Processes in Polymer Chemistry: A Review. Photochem. Photobiol. 1968, 7, 5–57, doi:10.1111/j.1751-1097.1968.tb05828.x.
- Marghalani, H.Y. Handbook of Bioceramics and Biocomposites, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783319092300.
- Linden, L.A. Photocuring of polymericdental materials, plastics compositeresins. In Radiationcuring in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier Applied Science Publisher: Amsterdam, The Netherlands, 1993; Volume 4, pp. 387–466.
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576, doi:10.1016/S0079-6700(01)00005-3.
- Fouassier, J.P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology: Practical Aspects and and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993.
- Xu, X.; He, L.; Zhu, B.; Li, J.; Li, J. Advances in polymeric materials for dental applications. Polym. Chem. 2017, 8, 807–823, doi:10.1039/c6py01957a.
- Lovell, L.G.; Lu, H.; Elliott, J.E.; Stansbury, J.W.; Bowman, C.N. The effect of cure rate on the mechanical properties of dental resins. Dent. Mater. 2001, 17, 504–511, doi:10.1016/S0109-5641(01)00010-0.
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22, doi:10.1016/j.dental.2011.09.005.
- Zandinejad, A.A.; Atai, M.; Pahlevan, A. The effect of ceramic and porous fillers on the mechanical properties of experimental dental composites. Dent. Mater. 2006, 22, 382–387, doi:10.1016/j.dental.2005.04.027.
- Lee, J.H.; Um, C.M.; Lee, I. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526, doi:10.1016/j.dental.2005.05.008.
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156, doi:10.1016/j.dental.2012.11.005.
- Buruiana, T.; Melinte, V.; Stroea, L.; Buruiana, E.C. Urethane dimethacrylates with carboxylic groups as potential dental monomers. Synthesis and properties. Polym. J. 2009, 41, 978–987, doi:10.1295/polymj.PJ2009131.
- Nguyen, J.F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Properties of experimental urethane dimethacrylate-based dental resin composite blocks obtained via thermo-polymerization under high pressure. Dent. Mater. 2013, 29, 535–541, doi:10.1016/j.dental.2013.02.006.
- Polydorou, O.; König, A.; Hellwig, E.; Kümmerer, K. Uthethane dimethacrylate: A molecule that may cause confusion in dental research. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2009, 91, 1–4, doi:10.1002/jbm.b.31383.
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665, doi:10.1016/S0142-9612(02)00380-0.
- Charton, C.; Falk, V.; Marchal, P.; Pla, F.; Colon, P. Influence of Tg, viscosity and chemical structure of monomers on shrinkage stress in light-cured dimethacrylate-based dental resins. Dent. Mater. 2007, 23, 1447–1459, doi:10.1016/j.dental.2007.05.017.
- Ellakwa, A.; Cho, N.; Lee, I.B. The effect of resin matrix composition on the polymerization shrinkage and rheological properties of experimental dental composites. Dent. Mater. 2007, 23, 1229–1235, doi:10.1016/j.dental.2006.11.004.
- Gonçalves, F.; Pfeifer, C.S.; Ferracane, J.L.; Braga, R.R. Contraction stress determinants in dimethacrylate composites. J. Dent. Res. 2008, 87, 367–371, doi:10.1177/154405910808700404.
- Goņalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446, doi:10.1111/j.1600-0722.2009.00636.x.
- Pfeifer, C.S.; Silva, L.R.; Kawano, Y.; Braga, R.R. Bis-GMA co-polymerizations: Influence on conversion, flexural properties, fracture toughness and susceptibility to ethanol degradation of experimental composites. Dent. Mater. 2009, 25, 1136–1141, doi:10.1016/j.dental.2009.03.010.
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416, doi:10.1177/0022034510381263.
- Price, R.B.; Rizkalla, A.S.; Hall, G.C. Effect of stepped light exposure on the volumetric polymerization shrinkage and bulk modulus of dental composites and an unfilled resin. Am. J. Dent. 2000, 13, 176–180.
- Sheth, J.J.; Fuller, J.L.; Jensen, M.E. Cuspal deformation and fracture resistance of teeth with dentin adhesives and composites. J. Prosthet. Dent. 1988, 60, 560–569, doi:10.1016/0022-3913(88)90215-6.
- Schoerpf, S.; Catel, Y.; Moszner, N.; Gorsche, C.; Liska, R. Enhanced reduction of polymerization-induced shrinkage stress: Via combination of radical ring opening and addition fragmentation chain transfer. Polym. Chem. 2019, 10, 1357–1366, doi:10.1039/c8py01540f.
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970, doi:10.1016/j.dental.2005.04.018.
- Vitale, A.; Sangermano, M.; Bongiovanni, R.; Burtscher, P.; Moszner, N. Visible light curable restorative composites for dental applications based on epoxy monomer. Materials 2014, 7, 554–562, doi:10.3390/ma7010554.
- Oxman, J.D.; Jacobs, D.W. Ternary Photoinitiator System for Curing of Epoxy Resins. WO 1998047046 A1, 22 October 1998.
- Hamano, N.; Vitale, A.; Sangermano, M.; Bongiovanni, R.; Burtscher, P.; Moszner, N. Visible light curable restorative composites for dental applications based on epoxy monomer. Materials 2014, 7, 554–562, doi:10.3390/ma7010554.
- Chiang, Y.C.; Nyamaa, I.; Yamaguchi, H.; Ino, S.; Hickel, R.; Kunzelmann, K.H. Repair of silorane-based dental composites: Influence of surface treatments. Dent. Mater. 2012, 28, 894–902, doi:10.1016/j.dental.2012.04.014.
- Lavigueur, C.; Zhu, X.X. Recent advances in the development of dental composite resins. RSC Adv. 2012, 2, 59–63, doi:10.1039/c1ra00922b.
- Weinmann, W.; Thalacker, C.; Guggenberger, R. Siloranes in dental composites. Dent. Mater. 2005, 21, 68–74, doi:10.1016/j.dental.2004.10.007.
- Nuyken, O.; Böhner, R.; Erdmann, C. Oxetane photopolymerization—A system with low volume shrinkage. Macromol. Symp. 1996, 107, 125–138, doi:10.1002/masy.19961070113.
- Moszner, N.; Salz, U. Composites for Dental Restoratives. In Polymers for Dental and Orthopedic Application, 1st ed.; Salz, U., Shalaby, W.S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 13–68.
- Bailey, W.J. Polycyclic Ring-Opened Polymers. U.S. Patent 4,387,215, 07.06.1983 .
- Stansbury, J.W.; Bailey, W.J. Evaluation of spiro orthocarbonate monomers capable of polymerization with expansion as ingredients in dental composite materials. In Progress in Biomedical Polymers; Gebelein, C.G., Dunn, R.L., Eds.; Springer: Boston, MA, USA, 1988; pp. 402–406.
- Eick, J.D.; Kotha, S.P.; Chappelow, C.C.; Kilway, K.V.; Giese, G.J.; Glaros, A.G.; Pinzino, C.S. Properties of silorane-based dental resins and composites containing a stress-reducing monomer. Dent. Mater. 2007, 23, 1011–1017, doi:10.1016/j.dental.2006.09.002.
- Nie, J.; Andrzejewska, E.; Rabek, J.F.; Lindén, L.Å.; Fouassier, J.P.; Paczkowski, J.; Scigalski, F.; Wrzyszczynski, A. Effect of peroxides and hydroperoxides on the camphorquinoneinitiated photopolymerization. Macromol. Chem. Phys. 1999, 200, 1692–1701, doi:10.1002/(SICI)1521-3935(19990701)200:7<1692::AID-MACP1692>3.0.CO;2-R.
- Neumann, M.G.; Miranda, W.G.; Schmitt, C.C.; Rueggeberg, F.A.; Correa, I.C. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J. Dent. 2005, 33, 525–532, doi:10.1016/j.jdent.2004.11.013.
- Asmussen, S.; Vallo, C. Light absorbing products during polymerization of methacrylate monomers photoinitiated with phenyl-1,2-propanedione/amine. J. Photochem. Photobiol. A Chem. 2009, 202, 228–234, doi:10.1016/j.jphotochem.2008.12.007.
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Lima, A.F. Effect of diphenyliodonium hexafluorophosphate on the physical and chemical properties of ethanolic solvated resins containing camphorquinone and 1-phenyl-1,2-propanedione sensitizers as initiators. Dent. Mater. 2016, 32, 756–764, doi:10.1016/j.dental.2016.03.010.
- Sun, G.J.; Chae, K.H. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer 2000, 41, 6205–6212, doi:10.1016/S0032-3861(99)00832-0.
- Kunio, I.; Takeshi, E. A review of the development of radical photopolymerization initiators used for designing light-curing dental adhesives and resin composites. Dent. Mater. J. 2010, 29, 481–501, doi:10.4012/dmj.2009-137.
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785, doi:10.1016/j.biomaterials.2007.04.044.
- Wu, N.; Zhang, Y.; Wang, Y. Photo-polymerization efficiency of self-etch dental adhesives composed of camphorquinone or trimethylbenzoyl-diphenyl-phosphine oxide. Int. J. Adhes. Adhes. 2013, 45, 53–58, doi:10.1016/j.ijadhadh.2013.04.002.
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New photocleavable structures, 4a acylgermane-based photoinitiator for visible light curing. Macromol. Rapid Commun. 2008, 29, 57–62, doi:10.1002/marc.200700620.
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New photocleavable structures. Diacylgermane-based photoinitiators for visible light curing. Macromolecules 2008, 41, 2394–2400, doi:10.1021/ma702418q.
- Ely, C.; Ottoboni, T.D.; Kumagai, R.Y.; de Souza, N.A.; da Ramos, T.S.; Arrais, C.A.G.; Piva, E.; Reis, A.F. Bond Strength of Methacrylate-based Blends Containing Elastomeric Monomers and Alternative Initiators after Thermomechanical Cycling. J. Adhes. Dent. 2019, 21, 281–286, doi:10.3290/j.jad.a42549.
- Li, Y.H.; Chen, Y.C. Triphenylamine-hexaarylbiimidazole derivatives as hydrogen-acceptor photoinitiators for free radical photopolymerization under UV and LED light. Polym. Chem. 2020, 11, 1504–1513, doi:10.1039/c9py01605h.
- Wu, X.; Wang, Y.; Liu, J.; He, S.; Zhang, L.; Polymers, F. Improved Crack Growth Resistance and Its Molecular Origin of Natural Rubber / Carbon Black by Nanodispersed Clay. Polym. Eng. Sci. 2012, 52, 1027–1036, doi:10.1002/pen.22171 .
- Shi, Y.; Yin, J.; Kaji, M.; Yori, H. Photopolymerization of acrylate derivatives initiated by hexaarylbiimidazole with ether groups. Polym. Int. 2006, 55, 330–339, doi:10.1002/pi.1968.
- West, R. The polysilane high polymers. J. Organomet. Chem. 1986, 300, 327–346, doi:10.1016/0022-328X(86)84068-2.
- Wolff, A.R.; West, R. Photoinitiation of vinyl polymerization by polysilanes. Appl. Organomet. Chem. 1987, 1, 7–14, doi:10.1002/aoc.590010103.
- Yagci, Y.; Kminek, I.; Schnabel, W. Long wavelength photoinitiated cationic polymerization using diphenyliodonium salt and catena-poly (phenyl-4-phenylphenylsilicon). Polymer 1993, 34, 426–428, doi:10.1016/0032-3861(93)90101-F.
- Bouzrati-Zerelli, M.; Kirschner, J.; Fik, C.P.; Maier, M.; Dietlin, C.; Morlet-Savary, F.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl Glyoxylates as a New Class of High Performance Photoinitiators: Blue LED Induced Polymerization of Methacrylates in Thin and Thick Films. Macromolecules 2017, 50, 6911–6923, doi:10.1021/acs.macromol.7b01370.
- Kirschner, J.; Bouzrati-Zerelli, M.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl glyoxylates as high-performance photoinitiators for cationic and hybrid polymerizations: Towards better polymer mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1420–1429, doi:10.1002/pola.29404.
- Kirschner, J.; Baralle, A.; Graff, B.; Becht, J.M.; Klee, J.E.; Lalevée, J. 1-Aryl-2-(triisopropylsilyl)ethane-1,2-diones: Toward a New Class of Visible Type I Photoinitiators for Free Radical Polymerization of Methacrylates. Macromol. Rapid Commun. 2019, 40, 2–7, doi:10.1002/marc.201900319.
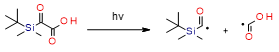
- Kirschner, J.; Paillard, J.; Graff, B.; Becht, J.M.; Klee, J.E.; Lalevée, J. 2-Oxo-2(tert-butyldimethylsilyl)Acetic Acid (DKSi-COOH) as a New Water-Soluble Visible Light Type I Photoinitiator for Free Radical Polymerization. Macromol. Chem. Phys. 2020, 221, 5–9, doi:10.1002/macp.201900495.
- Hayakawa, T.; Horie, K. Effect of water-soluble photoinitiator on the adhesion between composite and tooth substrate. Dent. Mater. 1992, 8, 351–353, doi:10.1016/0109-5641(92)90017-7.
- Breloy, L.; Losantos, R.; Sampedro, D.; Marazzi, M.; Malval, J.-P.; Heo, Y.; Akimoto, J.; Ito, Y.; Brezová, V.; Versace, D.-L. Allyl amino-thioxanthone derivatives as highly efficient visible light H-donors and co-polymerizable photoinitiators. Polym. Chem. 2020, 11, 4297–4312, doi:10.1039/d0py00551g.
- Kabatc, J.; Ortyl, J.; Kostrzewska, K. New kinetic and mechanistic aspects of photosensitization of iodonium salts in photopolymerization of acrylates. RSC Adv. 2017, 7, 41619–41629, doi:10.1039/c7ra05978g.
- Baralle, A.; Garra, P.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Polymeric Iodonium Salts to Trigger Free Radical Photopolymerization. Macromol. Rapid Commun. 2020, 41, doi:10.1002/marc.201900644.
- Abedin, F.; Ye, Q.; Song, L.; Ge, X.; Camarda, K.; Spencer, P. Effect of Partition of Photo-Initiator Components and Addition of Iodonium Salt on the Photopolymerization of Phase-Separated Dental Adhesive. JOM 2016, 68, 1090–1099, doi:10.1007/s11837-016-1816-2.
- Shiraishi, A.; Ueda, Y.; Schläpfer, M.; Schmitz, C.; Brömme, T.; Oprych, D.; Strehmel, B. Nir-sensitized photopolymerization with iodonium salts bearing weak coordinating anions. J. Photopolym. Sci. Technol. 2016, 29, 609–615, doi:10.2494/photopolymer.29.609.
- Cook, W.D.; Chen, F. Enhanced visible radiation photopolymerization of dimethacrylates with the three component thioxanthone (CPTXO)-amine-iodonium salt system. Polym. Chem. 2015, 6, 1325–1338, doi:10.1039/c4py01561d.
- Podsiadły, R.; Maruszewska, A.; Michalski, R.; Marcinek, A.; Kolińska, J. Naphthoylenebenzimidazolone dyes as electron transfer photosensitizers for iodonium salt induced cationic photopolymerizations. Dye. Pigment. 2012, 95, 252–259, doi:10.1016/j.dyepig.2012.04.004.
- 193. Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315, doi:10.1021/ma60060a028.
- Asmussen, S.; Arenas, G.; Vallo, C. Photopolymerization of pyrrole/methacrylate mixtures using α-cleavage type photoinitiators in combination with iodonium salt. Synth. Met. 2015, 209, 304–312, doi:10.1016/j.synthmet.2015.08.004.
- Crivello, J.V. Cationic polymerization—Iodonium and sulfonium salt photoinitiators. In Initiators—Poly-Reactions—Optical Activity; Springer: Berlin/Heidelberg, Germany , 2005; pp. 1–48.
- 129. Lin, Y.; Stansbury, J.W. Kinetics studies of hybrid structure formation by controlled photopolymerization. Polymer 2003, 44, 4781–4789, doi:10.1016/S0032-3861(03)00469-5.
- 200. Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754, doi:10.1016/j.arabjc.2014.03.008.
- Lima, A.F.; Salvador, M.V.O.; Dressano, D.; Saraceni, C.H.C.; Gonçalves, L.S.; Hadis, M.; Palin, W.M. Increased rates of photopolymerisation by ternary type II photoinitiator systems in dental resins. J. Mech. Behav. Biomed. Mater. 2019, 98, 71–78, doi:10.1016/j.jmbbm.2019.06.005.
- Guo, X.; Wang, Y.; Spencer, P.; Ye, Q.; Yao, X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent. Mater. 2008, 24, 824–831, doi:10.1016/j.dental.2007.10.003.
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875, doi:10.1016/j.jmbbm.2020.103875.
- Baena Lopes, M.; Tirado Dos Santos, A.M.; Coelho, D.; Gonini Júnior, A.; Aulo Ogliari, F.; Ratto De Moraes, R. Influence of diphenyliodonium hexafluorophosphate on the bond strength and mechanical properties of model resin cements. Int. J. Adhes. Adhes. 2013, 47, 125–128, doi:10.1016/j.ijadhadh.2013.08.006.
- Leal, F.B.; Lima, G.S.; Collares, F.M.; Samuel, S.M.; Petzhold, C.L.; Piva, E.; Ogliari, F.A. Iodonium salt improves the dentin bonding performance in an experimental dental adhesive resin. Int. J. Adhes. Adhes. 2012, 38, 1–4, doi:10.1016/j.ijadhadh.2012.05.008.
- Augusto, C.R.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Influence of an iodonium salt on the properties of dual-polymerizing self-adhesive resin cements. J. Prosthet. Dent. 2017, 118, 228–234, doi:10.1016/j.prosdent.2016.10.013.
- Chloride, D.; Padon, K.S.; Scranton, A.B. A mechanistic investigation of the three-component radical photoinitiator system Eosin Y spirit soluble, n-methyldiethanolamine. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 715–723, doi:10.1002/1099-0518(20010301)39:5<715::aid-pola1043>3.0.co;2-o.
- Fu, J.; Liu, W.; Liu, X.; Tuladhar, S.L.; Wan, Q.; Wang, H. Properties of a new dental photocurable resin based on the expanding monomer and three-component photoinitiator system. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 384–390, doi:10.1007/s11595-014-0926-3.
- Danso, R.; Hoedebecke, B.; Whang, K.; Sarrami, S.; Johnston, A.; Flipse, S.; Wong, N.; Rawls, H.R. Development of an oxirane/acrylate interpenetrating polymer network (IPN) resin system. Dent. Mater. 2018, 34, 1459–1465, doi:10.1016/j.dental.2018.06.013.
- Kirschner, J.; Bouzrati-Zerelli, M.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl glyoxylates as high-performance photoinitiators for cationic and hybrid polymerizations: Towards better polymer mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1420–1429.
- Zhang, J.; Wang, S.; Lalevée, J.; Morlet-Savary, F.; Lam, E.S.-H.; Graff, B.; Liu, J.; Xing, F.; Xiao, P. 1,2-Diketones as photoinitiators of both cationic and free-radical photopolymerization under UV (392 nm) or Blue (455 nm) LEDs. J. Polym. Sci. 2020, 58, 792–802.
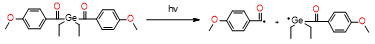
- Moszner, N.; Zeuner, F.; Lamparth, I.; Fischer, U.K. Benzoylgermanium derivatives as novel visible-light photoinitiators for dental composites. Macromol. Mater. Eng. 2009, 294, 877–886, doi:10.1002/mame.200900181.
- Moszner, N.; Fischer, U.K.; Ganster, B.; Liska, R.; Rheinberger, V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent. Mater. 2008, 24, 901–907.
- Al Mousawi, A.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Chachaj-Brekiesz, A.; Ortyl, J.; Lalevée, J. Meta-Terphenyl Derivative/Iodonium Salt/9H-Carbazole-9-ethanol Photoinitiating Systems for Free Radical Promoted Cationic Polymerization upon Visible Lights. Macromol. Chem. Phys. 2016, 217, 1955–1965, doi:10.1002/macp.201600224.
- Brömme, T.; Oprych, D.; Horst, J.; Pinto, P.S.; Strehmel, B. New iodonium salts in NIR sensitized radical photopolymerization of multifunctional monomers. RSC Adv. 2015, 5, 69915–69924, doi:10.1039/c5ra12236h.
- Karaca, N.; Ocal, N.; Arsu, N.; Jockusch, S. Thioxanthone-benzothiophenes as photoinitiator for free radical polymerization. J. Photochem. Photobiol. A Chem. 2016, 331, 22–28, doi:10.1016/j.jphotochem.2016.01.017.
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754, doi:10.1016/j.arabjc.2014.03.008.
- Lima, A.F.; Salvador, M.V.O.; Dressano, D.; Saraceni, C.H.C.; Gonçalves, L.S.; Hadis, M.; Palin, W.M. Increased rates of photopolymerisation by ternary type II photoinitiator systems in dental resins. J. Mech. Behav. Biomed. Mater. 2019, 98, 71–78, doi:10.1016/j.jmbbm.2019.06.005.
- Guo, X.; Wang, Y.; Spencer, P.; Ye, Q.; Yao, X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent. Mater. 2008, 24, 824–831, doi:10.1016/j.dental.2007.10.003.
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875, doi:10.1016/j.jmbbm.2020.103875.
- Baena Lopes, M.; Tirado Dos Santos, A.M.; Coelho, D.; Gonini Júnior, A.; Aulo Ogliari, F.; Ratto De Moraes, R. Influence of diphenyliodonium hexafluorophosphate on the bond strength and mechanical properties of model resin cements. Int. J. Adhes. Adhes. 2013, 47, 125–128, doi:10.1016/j.ijadhadh.2013.08.006.
- Leal, F.B.; Lima, G.S.; Collares, F.M.; Samuel, S.M.; Petzhold, C.L.; Piva, E.; Ogliari, F.A. Iodonium salt improves the dentin bonding performance in an experimental dental adhesive resin. Int. J. Adhes. Adhes. 2012, 38, 1–4, doi:10.1016/j.ijadhadh.2012.05.008.
- Fu, J.; Liu, W.; Liu, X.; Tuladhar, S.L.; Wan, Q.; Wang, H. Properties of a new dental photocurable resin based on the expanding monomer and three-component photoinitiator system. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 384–390, doi:10.1007/s11595-014-0926-3.
- Danso, R.; Hoedebecke, B.; Whang, K.; Sarrami, S.; Johnston, A.; Flipse, S.; Wong, N.; Rawls, H.R. Development of an oxirane/acrylate interpenetrating polymer network (IPN) resin system. Dent. Mater. 2018, 34, 1459–1465, doi:10.1016/j.dental.2018.06.013.
- Zhang, J.; Wang, S.; Lalevée, J.; Morlet-Savary, F.; Lam, E.S.-H.; Graff, B.; Liu, J.; Xing, F.; Xiao, P. 1,2-Diketones as photoinitiators of both cationic and free-radical photopolymerization under UV (392 nm) or Blue (455 nm) LEDs. J. Polym. Sci. 2020, 58, 792–802.
- Kirschner, J.; Bouzrati-Zerelli, M.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl glyoxylates as high-performance photoinitiators for cationic and hybrid polymerizations: Towards better polymer mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1420–1429.
- Moszner, N.; Fischer, U.K.; Ganster, B.; Liska, R.; Rheinberger, V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent. Mater. 2008, 24, 901–907.
- Nie, J.; Andrzejewska, E.; Rabek, J.F.; Lindén, L.Å.; Fouassier, J.P.; Paczkowski, J.; Scigalski, F.; Wrzyszczynski, A. Effect of peroxides and hydroperoxides on the camphorquinoneinitiated photopolymerization. Macromol. Chem. Phys. 1999, 200, 1692–1701, doi:10.1002/(SICI)1521-3935(19990701)200:7<1692::AID-MACP1692>3.0.CO;2-R.
- Neumann, M.G.; Miranda, W.G.; Schmitt, C.C.; Rueggeberg, F.A.; Correa, I.C. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J. Dent. 2005, 33, 525–532, doi:10.1016/j.jdent.2004.11.013.
- Asmussen, S.; Vallo, C. Light absorbing products during polymerization of methacrylate monomers photoinitiated with phenyl-1,2-propanedione/amine. J. Photochem. Photobiol. A Chem. 2009, 202, 228–234, doi:10.1016/j.jphotochem.2008.12.007.
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Lima, A.F. Effect of diphenyliodonium hexafluorophosphate on the physical and chemical properties of ethanolic solvated resins containing camphorquinone and 1-phenyl-1,2-propanedione sensitizers as initiators. Dent. Mater. 2016, 32, 756–764, doi:10.1016/j.dental.2016.03.010.
- Sun, G.J.; Chae, K.H. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer 2000, 41, 6205–6212, doi:10.1016/S0032-3861(99)00832-0.
- Kunio, I.; Takeshi, E. A review of the development of radical photopolymerization initiators used for designing light-curing dental adhesives and resin composites. Dent. Mater. J. 2010, 29, 481–501, doi:10.4012/dmj.2009-137.
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785, doi:10.1016/j.biomaterials.2007.04.044.
- Wu, N.; Zhang, Y.; Wang, Y. Photo-polymerization efficiency of self-etch dental adhesives composed of camphorquinone or trimethylbenzoyl-diphenyl-phosphine oxide. Int. J. Adhes. Adhes. 2013, 45, 53–58, doi:10.1016/j.ijadhadh.2013.04.002.
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New photocleavable structures, 4a acylgermane-based photoinitiator for visible light curing. Macromol. Rapid Commun. 2008, 29, 57–62, doi:10.1002/marc.200700620.
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New photocleavable structures. Diacylgermane-based photoinitiators for visible light curing. Macromolecules 2008, 41, 2394–2400, doi:10.1021/ma702418q.
- Ely, C.; Ottoboni, T.D.; Kumagai, R.Y.; de Souza, N.A.; da Ramos, T.S.; Arrais, C.A.G.; Piva, E.; Reis, A.F. Bond Strength of Methacrylate-based Blends Containing Elastomeric Monomers and Alternative Initiators after Thermomechanical Cycling. J. Adhes. Dent. 2019, 21, 281–286, doi:10.3290/j.jad.a42549.
- Li, Y.H.; Chen, Y.C. Triphenylamine-hexaarylbiimidazole derivatives as hydrogen-acceptor photoinitiators for free radical photopolymerization under UV and LED light. Polym. Chem. 2020, 11, 1504–1513, doi:10.1039/c9py01605h.
- Wu, X.; Wang, Y.; Liu, J.; He, S.; Zhang, L.; Polymers, F. Improved Crack Growth Resistance and Its Molecular Origin of Natural Rubber / Carbon Black by Nanodispersed Clay. Polym. Eng. Sci. 2012, 52, 1027–1036, doi:10.1002/pen.22171 .
- Shi, Y.; Yin, J.; Kaji, M.; Yori, H. Photopolymerization of acrylate derivatives initiated by hexaarylbiimidazole with ether groups. Polym. Int. 2006, 55, 330–339, doi:10.1002/pi.1968.
- West, R. The polysilane high polymers. J. Organomet. Chem. 1986, 300, 327–346, doi:10.1016/0022-328X(86)84068-2.
- Wolff, A.R.; West, R. Photoinitiation of vinyl polymerization by polysilanes. Appl. Organomet. Chem. 1987, 1, 7–14, doi:10.1002/aoc.590010103.
- Yagci, Y.; Kminek, I.; Schnabel, W. Long wavelength photoinitiated cationic polymerization using diphenyliodonium salt and catena-poly (phenyl-4-phenylphenylsilicon). Polymer 1993, 34, 426–428, doi:10.1016/0032-3861(93)90101-F.
- Bouzrati-Zerelli, M.; Kirschner, J.; Fik, C.P.; Maier, M.; Dietlin, C.; Morlet-Savary, F.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl Glyoxylates as a New Class of High Performance Photoinitiators: Blue LED Induced Polymerization of Methacrylates in Thin and Thick Films. Macromolecules 2017, 50, 6911–6923, doi:10.1021/acs.macromol.7b01370.
- Kirschner, J.; Bouzrati-Zerelli, M.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl glyoxylates as high-performance photoinitiators for cationic and hybrid polymerizations: Towards better polymer mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1420–1429, doi:10.1002/pola.29404.
- Kirschner, J.; Baralle, A.; Graff, B.; Becht, J.M.; Klee, J.E.; Lalevée, J. 1-Aryl-2-(triisopropylsilyl)ethane-1,2-diones: Toward a New Class of Visible Type I Photoinitiators for Free Radical Polymerization of Methacrylates. Macromol. Rapid Commun. 2019, 40, 2–7, doi:10.1002/marc.201900319.
- Kirschner, J.; Paillard, J.; Graff, B.; Becht, J.M.; Klee, J.E.; Lalevée, J. 2-Oxo-2(tert-butyldimethylsilyl)Acetic Acid (DKSi-COOH) as a New Water-Soluble Visible Light Type I Photoinitiator for Free Radical Polymerization. Macromol. Chem. Phys. 2020, 221, 5–9, doi:10.1002/macp.201900495.
- Hayakawa, T.; Horie, K. Effect of water-soluble photoinitiator on the adhesion between composite and tooth substrate. Dent. Mater. 1992, 8, 351–353, doi:10.1016/0109-5641(92)90017-7.
- Asmussen, S.; Arenas, G.; Vallo, C. Photopolymerization of pyrrole/methacrylate mixtures using α-cleavage type photoinitiators in combination with iodonium salt. Synth. Met. 2015, 209, 304–312, doi:10.1016/j.synthmet.2015.08.004.
- Crivello, J.V. Cationic polymerization—Iodonium and sulfonium salt photoinitiators. In Initiators—Poly-Reactions—Optical Activity; Springer: Berlin/Heidelberg, Germany , 2005; pp. 1–48.
- Augusto, C.R.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Influence of an iodonium salt on the properties of dual-polymerizing self-adhesive resin cements. J. Prosthet. Dent. 2017, 118, 228–234, doi:10.1016/j.prosdent.2016.10.013.
- Chloride, D.; Padon, K.S.; Scranton, A.B. A mechanistic investigation of the three-component radical photoinitiator system Eosin Y spirit soluble, n-methyldiethanolamine. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 715–723, doi:10.1002/1099-0518(20010301)39:5<715::aid-pola1043>3.0.co;2-o.
- Moszner, N.; Zeuner, F.; Lamparth, I.; Fischer, U.K. Benzoylgermanium derivatives as novel visible-light photoinitiators for dental composites. Macromol. Mater. Eng. 2009, 294, 877–886, doi:10.1002/mame.200900181.