
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrew Paul Sindone | -- | 2379 | 2022-06-17 04:24:04 | | | |
| 2 | Peter Tang | -35 word(s) | 2344 | 2022-06-17 05:04:28 | | | | |
| 3 | Peter Tang | Meta information modification | 2344 | 2022-06-17 05:05:40 | | | | |
| 4 | Peter Tang | Meta information modification | 2344 | 2022-06-20 02:57:35 | | |
Video Upload Options
Iron deficiency (ID) is a comorbid condition frequently seen in patients with heart failure (HF). Iron has an important role in the transport of oxygen, and is also essential for skeletal and cardiac muscle, which depend on iron for oxygen storage and cellular energy production. Thus, ID per se, even without anaemia, can be harmful. In patients with HF, ID is associated with a poorer quality of life (QoL) and exercise capacity, and a higher risk of hospitalisations and mortality, even in the absence of anaemia.
1. Introduction
2. Role of Iron and the Impact of Iron Deficiency

3. Iron Deficiency Prevalence in Patients with Heart Failure
4. Iron Deficiency Causes in Patients with Heart Failure
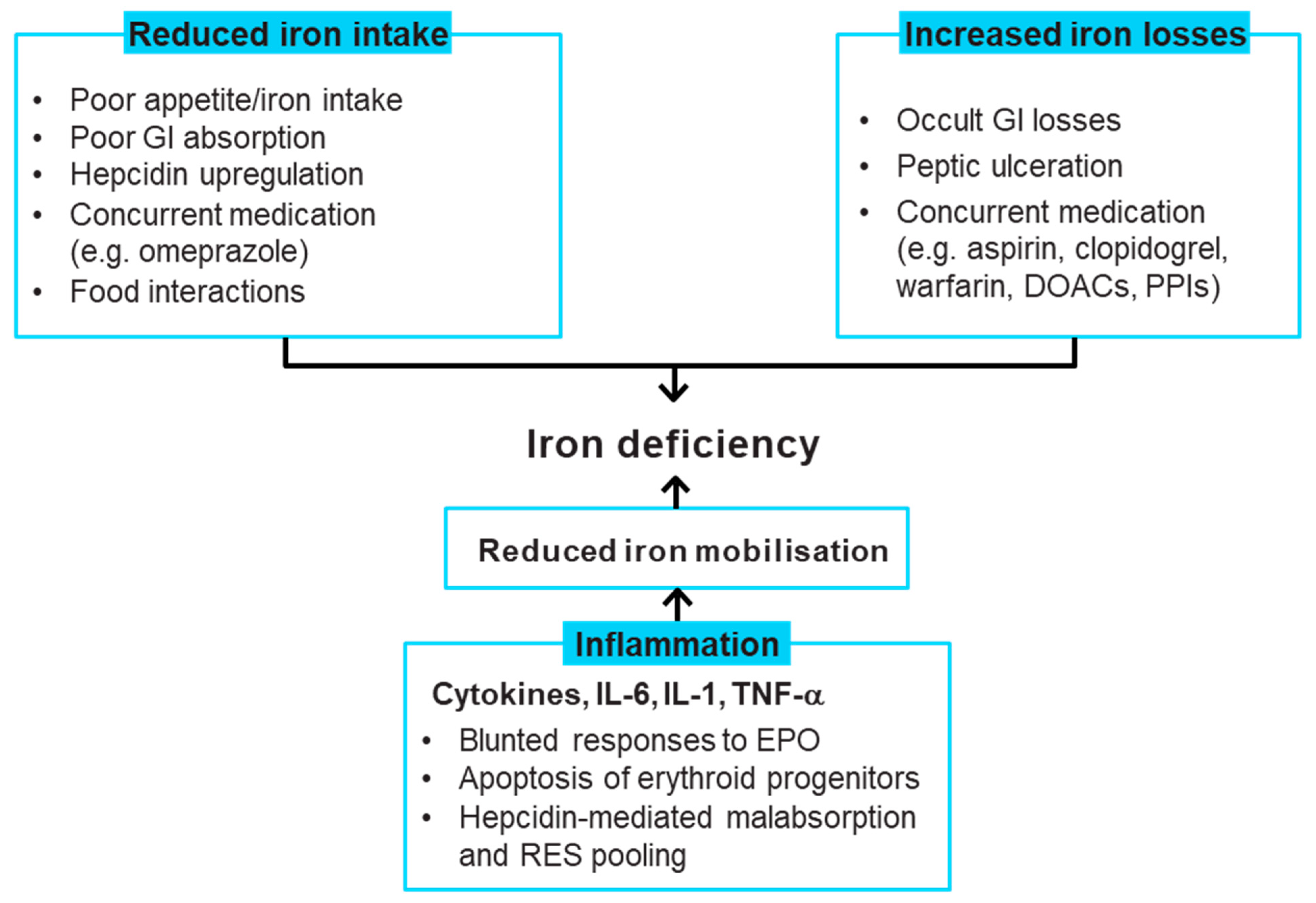
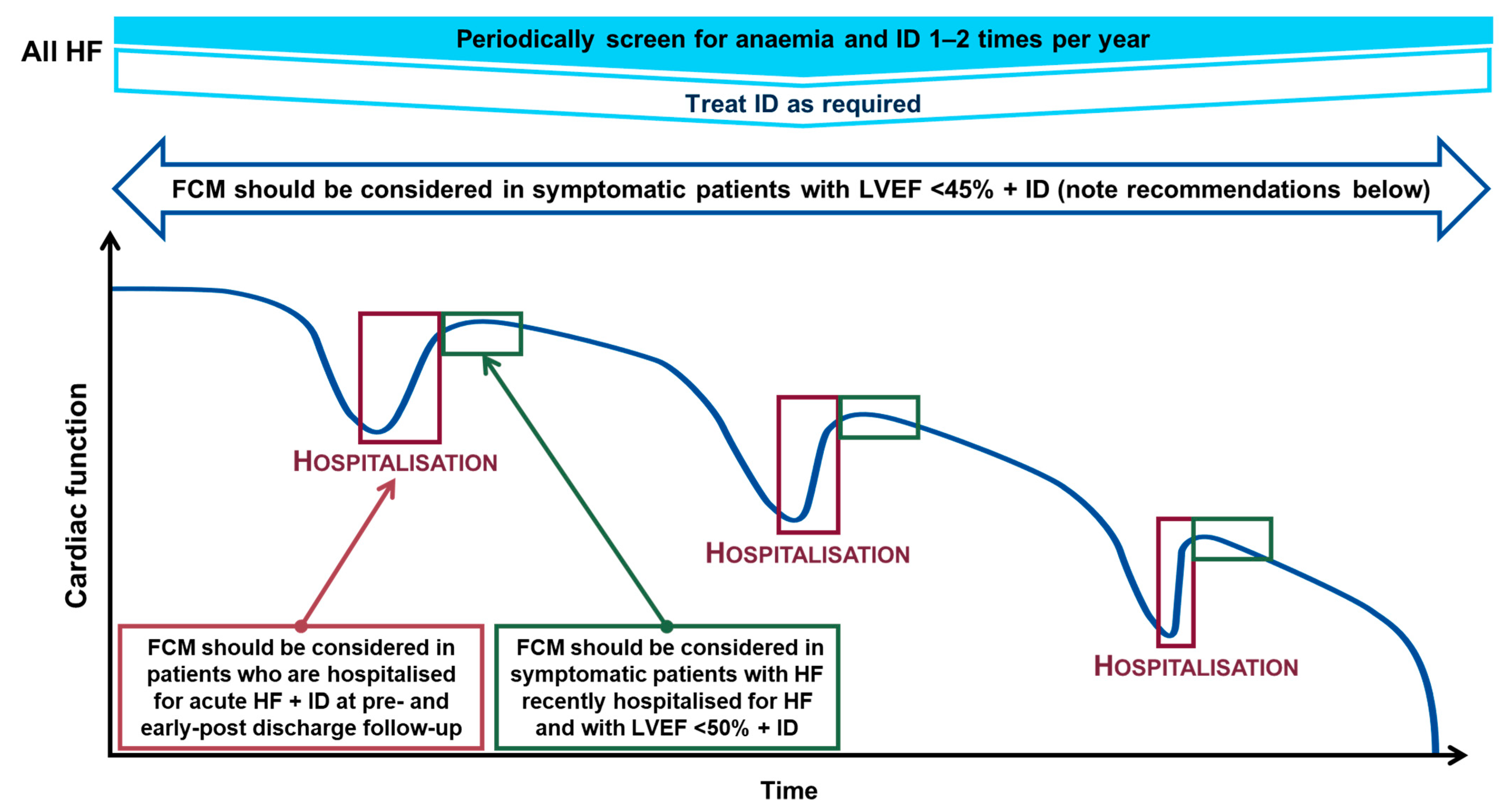
5. Recommendations for Correcting Iron Deficiency
- Symptomatic patients who have a left ventricular ejection fraction (LVEF) < 45% to alleviate symptoms, improve exercise capacity and QoL (recommendation class IIa, evidence level A)
- Pre- and post-discharge follow-up of patients hospitalised for AHF to improve symptoms and reduce rehospitalisation (recommendation class IIa, evidence level B)
- Symptomatic patients recently hospitalised for HF with LVEF < 50% to lessen the risk of HF hospitalisation (recommendation class IIa, evidence level B) [3].
6. Evidence on the Therapeutic Management of Iron Deficiency
|
FAIR-HF [15] |
CONFIRM-HF [17] |
EFFECT-HF [18] |
AFFIRM-AHF [16] |
|
|---|---|---|---|---|
|
Design, duration and number of patients who received treatment per arm |
Double-blind, placebo-controlled, randomised; 24 weeks FCM: 305 Placebo: 154 |
Double-blind, placebo-controlled, randomised; 52 weeks FCM: 152 Placebo: 152 |
Open-label, SoC-controlled, randomised; 24 weeks FCM: 88 SoC: 86 |
Double-blind, placebo-controlled, randomised; 52 weeks FCM: 559 Placebo: 551 |
|
Key inclusion criteria |
NYHA class II (LVEF ≤ 40%) or III (LVEF ≤45%) Hb 9.5–13.5 g/dL ID (ferritin <100 µg/L or 100–299 µg/L + TSAT <20%) |
NYHA class II/III (LVEF ≤ 45%) BNP >100 pg/mL and/or NT-proBNP >400 pg/ml Hb <15 g/dL ID (ferritin <100 µg/L or 100–300 µg/L + TSAT < 20%) |
NYHA class II/III (LVEF ≤ 45%) BNP >100 pg/mL and/or NT-proBNP >400 pg/ml Hb <15 g/dL ID (ferritin <100 µg/L or 100–300 µg/L + TSAT < 20%) Peak VO2 10–20 mL/kg/min (reproducible) |
Hospitalised for acute HF, treated with at least 40 mg IV furosemide (or equivalent) LVEF < 50% ID (ferritin <100 µg/L or 100–299 µg/L + TSAT <20%) |
|
Dosing regimen |
Dose determined by Ganzoni formula [50] FCM equivalent to 200 mg iron/week for iron repletion then Q4W for maintenance |
FCM equivalent to 500–3500 mg iron for iron repletion (baseline and Week 6); 500 mg iron for maintenance (Weeks 12, 24, 36) if iron deficiency still present |
FCM equivalent to 500–1000 mg iron for iron repletion (baseline and Week 6) based on screening Hb and weight; only given at Week 6 if <70 kg and Hb <10 g/dL or ≥70 kg and Hb <14 g/dL; 500 mg iron for maintenance (Week 12) if iron deficiency still present |
FCM equivalent to 500–1000 mg at baseline and Week 6 for iron repletion; 500 mg iron for maintenance at Weeks 12 and 24 for patients in whom ID persisted and for whom Hb was 8–15 g/dL |
|
Mean cumulative iron dose/ total number of injections |
NA/ Median 6 (3–7) during iron repletion phase |
1500 mg/>75% of patients receiving FCM needed 2 injections maximum to correct and sustain iron parameters during the study |
1204 mg/42% received 1, 55% received 2, and 3.3% received 3 FCM administrations |
1352 mg/80% of patients received 1 or 2 FCM administrations during the treatment phase (i.e., up to Week 24) |
|
Treatment effect on iron-related parameters |
FCM vs. placebo at Week 24 (mean ± SE)
(p < 0.001 for all) |
Mean treatment effect (baseline-adjusted) difference for FCM vs. placebo at Week 52:
(p < 0.001 for all) |
FCM vs. control (SoC) at Week 24:
|
Compared with placebo, serum ferritin and TSAT both rose with FCM by week 6 and continued to be significantly higher at week 52 |
|
Primary endpoint results |
Changes in PGA and NYHA functional class at Week 24 for FCM vs. placebo
|
LS means ± SE 6 MWT distance at Week 24 for FCM vs. placebo
|
Primary analysis LS means change from baseline in peak VO2 at Week 24 for FCM vs. control (SoC)
|
Composite of total HF hospitalisations and CV deaths up to 52 weeks after randomisation for FCM vs. placebo:
|
|
Key secondary endpoint results |
Significant improvement (p < 0.001) with FCM vs. placebo in:
|
Significant improvements in PGA, NYHA class and 6 MWT with FCM vs. placebo:
|
Significant improvements in NYHA class and PGA with FCM vs. control:
Note: effect of FCM vs. control on NYHA class and PGA without imputation (observed values) were similar |
Total CV hospitalisations and CV deaths with FCM vs. placebo
|
|
Safety endpoint results |
FCM vs. placebo (incidence per 100 patient-years at risk)
|
FCM vs. placebo (incidence per 100 patient-years at risk)
|
FCM vs. control (SoC)
|
FCM vs. placebo
|
6 MWT, 6-min walk test; AFFIRM-AHF, Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency; BNP, brain natriuretic peptide; CONFIRM-HF, Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure; CI, confidence interval; CV, cardiovascular; EFFECT-HF, Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Iron Deficiency and Chronic Heart Failure; EQ-5D, EuroQol-5 Dimension; FAIR-HF, Ferinject assessment in patients with IRon deficiency and chronic Heart Failure; FCM, ferric carboxymaltose; Hb, haemoglobin; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ID, iron deficiency; IV, intravenous; KCCQ, Kansas City Cardiomyopathy Questionnaire; LS, least squares; LVEF, left ventricular ejection fraction; NA, not available; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; PGA, patient global assessment; Q4W, every four weeks; OR, odds ratio; QoL, quality of life; RR, rate ratio; SE, standard error; SoC, standard of care; TSAT, transferrin saturation.
References
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11.
- Vitale, C.; Ilaria, S.; Rosano, G.M. Pharmacological interventions effective in improving exercise capacity in heart failure. Card. Fail. Rev. 2018, 4, 25–27.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 1872–1880.
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Heart J. 2013, 165, 575–582.e3.
- Núñez, J.; Comín-Colet, J.; Miñana, G.; Nunez, E.; Santas, E.; Mollar, A.; Valero, E.; Garcia-Blas, S.; Cardells, I.; Bodi, V.; et al. Iron deficiency and risk of early readmission following a hospitalization for acute heart failure. Eur. J. Heart Fail. 2016, 18, 798–802.
- Okonko, D.O.; Mandal, A.K.; Missouris, C.G.; Poole-Wilson, P.A. Disordered iron homeostasis in chronic heart failure: Prevalence, predictors, and relation to anemia, exercise capacity, and survival. J. Am. Coll. Cardiol. 2011, 58, 1241–1251.
- Alcaide-Aldeano, A.; Garay, A.; Alcoberro, L.; Jimenez-Marrero, S.; Yun, S.; Tajes, M.; Garcia-Romero, E.; Diez-Lopez, C.; Gonzalez-Costello, J.; Mateus-Porta, G.; et al. Iron deficiency: Impact on functional capacity and quality of life in heart failure with preserved ejection fraction. J. Clin. Med. 2020, 9, 1199.
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2018, 73, 115–123.
- Cohen-Solal, A.; Damy, T.; Terbah, M.; Kerebel, S.; Baguet, J.P.; Hanon, O.; Zannad, F.; Laperche, T.; Leclercq, C.; Concas, V.; et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur. J. Heart Fail. 2014, 16, 984–991.
- Wienbergen, H.; Pfister, O.; Hochadel, M.; Michel, S.; Bruder, O.; Remppis, B.A.; Maeder, M.T.; Strasser, R.; von Scheidt, W.; Pauschinger, M.; et al. Usefulness of iron deficiency correction in management of patients with heart failure . Am. J. Cardiol. 2016, 118, 1875–1880.
- Belmar Vega, L.; de Francisco, A.; Albines Fiestas, Z.; Serrano Soto, M.; Kislikova, M.; Seras Mozas, M.; Unzueta, M.G.; Arias Rodriguez, M. Investigation of iron deficiency in patients with congestive heart failure: A medical practice that requires greater attention. Nefrologia 2016, 36, 249–254.
- Mistry, R.; Hosoya, H.; Kohut, A.; Ford, P. Iron deficiency in heart failure, an underdiagnosed and undertreated condition during hospitalization. Ann. Hematol. 2019, 98, 2293–2297.
- Becher, P.M.; Schrage, B.; Benson, L.; Fudim, M.; Corovic Cabrera, C.; Dahlstrom, U.; Rosano, G.M.C.; Jankowska, E.A.; Anker, S.D.; Lund, L.H.; et al. Phenotyping heart failure patients for iron deficiency and use of intravenous iron therapy: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2021, 23, 1844–1854.
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448.
- Ponikowski, P.; Kirwan, B.A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Gohring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 2020, 396, 1895–1904.
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668.
- van Veldhuisen, D.J.; Ponikowski, P.; van der Meer, P.; Metra, M.; Bohm, M.; Doletsky, A.; Voors, A.A.; Macdougall, I.C.; Anker, S.D.; Roubert, B.; et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017, 136, 1374–1383.
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078.
- Stugiewicz, M.; Tkaczyszyn, M.; Kasztura, M.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. The influence of iron deficiency on the functioning of skeletal muscles: Experimental evidence and clinical implications. Eur. J. Heart Fail. 2016, 18, 762–773.
- Bakogiannis, C.; Briasoulis, A.; Mouselimis, D.; Tsarouchas, A.; Papageorgiou, N.; Papadopoulos, C.; Fragakis, N.; Vassilikos, V. Iron deficiency as therapeutic target in heart failure: A translational approach. Heart Fail. Rev. 2020, 25, 173–182.
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919.
- Enjuanes, C.; Bruguera, J.; Grau, M.; Cladellas, M.; Gonzalez, G.; Merono, O.; Moliner-Borja, P.; Verdu, J.M.; Farre, N.; Comin-Colet, J. Iron status in chronic heart failure: Impact on symptoms, functional class and submaximal exercise capacity. Rev. Esp. Cardiol. 2016, 69, 247–255.
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; von Haehling, S.; Doehner, W.; Banasiak, W.; et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J. Card. Fail. 2011, 17, 899–906.
- Comín-Colet, J.; Enjuanes, C.; González, G.; Torrens, A.; Cladellas, M.; Merono, O.; Ribas, N.; Ruiz, S.; Gomez, M.; Verdu, J.M.; et al. Iron deficiency is a key determinant of health-related quality of life in patients with chronic heart failure regardless of anaemia status. Eur. J. Heart Fail. 2013, 15, 1164–1172.
- Enjuanes, C.; Klip, I.T.; Bruguera, J.; Cladellas, M.; Ponikowski, P.; Banasiak, W.; van Veldhuisen, D.J.; van der Meer, P.; Jankowska, E.A.; Comin-Colet, J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter European study. Int. J. Cardiol. 2014, 174, 268–275.
- Drozd, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Iron therapy in patients with heart failure and iron deficiency: Review of iron preparations for practitioners. Am. J. Cardiovasc. Drugs 2017, 17, 183–201.
- Ebner, N.; von Haehling, S. Iron deficiency in heart failure: A practical guide. Nutrients 2013, 5, 3730–3739.
- Wong, C.C.Y.; Ng, A.C.C.; Kritharides, L.; Sindone, A.P. Iron deficiency in heart failure: Looking beyond anaemia. Heart Lung Circ. 2016, 25, 209–216.
- Anker, S.D.; Kirwan, B.A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Luscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: An individual patient data meta-analysis. Eur. J. Heart Fail. 2018, 20, 125–133.
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50.
- Nanas, J.N.; Matsouka, C.; Karageorgopoulos, D.; Leonti, A.; Tsolakis, E.; Drakos, S.G.; Tsagalou, E.P.; Maroulidis, G.D.; Alexopoulos, G.P.; Kanakakis, J.E.; et al. Etiology of anemia in patients with advanced heart failure. J. Am. Coll. Cardiol. 2006, 48, 2485–2489.
- Parikh, A.; Natarajan, S.; Lipsitz, S.R.; Katz, S.D. Iron deficiency in community-dwelling US adults with self-reported heart failure in the National Health and Nutrition Examination Survey III: Prevalence and associations with anemia and inflammation. Circ. Heart Fail. 2011, 4, 599–606.
- von Haehling, S.; Gremmler, U.; Krumm, M.; Mibach, F.; Schon, N.; Taggeselle, J.; Dahm, J.B.; Angermann, C.E. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: The PrEP Registry. Clin. Res. Cardiol. 2017, 106, 436–443.
- Yeo, T.J.; Yeo, P.S.; Ching-Chiew Wong, R.; Ong, H.Y.; Leong, K.T.; Jaufeerally, F.; Sim, D.; Santhanakrishnan, R.; Lim, S.L.; Chan, M.M.; et al. Iron deficiency in a multi-ethnic Asian population with and without heart failure: Prevalence, clinical correlates, functional significance and prognosis. Eur. J. Heart Fail. 2014, 16, 1125–1132.
- Cohen-Solal, A.; Philip, J.L.; Picard, F.; Delarche, N.; Taldir, G.; Gzara, H.; Korichi, A.; Trochu, J.N.; Cacoub, P.; Group, C.S. Iron deficiency in heart failure patients: The French CARENFER prospective study. ESC Heart Fail. 2022, 9, 874–884.
- Van Aelst, L.N.L.; Abraham, M.; Sadoune, M.; Lefebvre, T.; Manivet, P.; Logeart, D.; Launay, J.M.; Karim, Z.; Puy, H.; Cohen-Solal, A. Iron status and inflammatory biomarkers in patients with acutely decompensated heart failure: Early in-hospital phase and 30-day follow-up. Eur. J. Heart Fail. 2017, 19, 1075–1076.
- Fitzsimons, S.; Doughty, R.N. Iron deficiency in patients with heart failure. Eur. Heart J. Cardiovasc. Pharmacother. 2015, 1, 58–64.
- Hughes, C.M.; Woodside, J.V.; McGartland, C.; Roberts, M.J.; Nicholls, D.P.; McKeown, P.P. Nutritional intake and oxidative stress in chronic heart failure. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 376–382.
- Hamano, H.; Niimura, T.; Horinouchi, Y.; Zamami, Y.; Takechi, K.; Goda, M.; Imanishi, M.; Chuma, M.; Izawa-Ishizawa, Y.; Miyamoto, L.; et al. Proton pump inhibitors block iron absorption through direct regulation of hepcidin via the aryl hydrocarbon receptor-mediated pathway. Toxicol. Lett. 2020, 318, 86–91.
- Ganz, T. Hepcidin and its role in regulating systemic iron metabolism. Hematol. Am. Soc. Hematol. Educ. Program 2006, 2006, 29–35.
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 2009, 122, 78–86.
- Jankowska, E.A.; Malyszko, J.; Ardehali, H.; Koc-Zorawska, E.; Banasiak, W.; von Haehling, S.; Macdougall, I.C.; Weiss, G.; McMurray, J.J.; Anker, S.D.; et al. Iron status in patients with chronic heart failure. Eur. Heart J. 2013, 34, 827–834.
- Jankowska, E.A.; Kasztura, M.; Sokolski, M.; Bronisz, M.; Nawrocka, S.; Oleskowska-Florek, W.; Zymlinski, R.; Biegus, J.; Siwolowski, P.; Banasiak, W.; et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur. Heart J. 2014, 35, 2468–2476.
- Jankowska, E.A.; von Haehling, S.; Anker, S.D.; Macdougall, I.C.; Ponikowski, P. Iron deficiency and heart failure: Diagnostic dilemmas and therapeutic perspectives. Eur. Heart J. 2013, 34, 816–829.
- Anand, I.S.; Gupta, P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation 2018, 138, 80–98.
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.P.; Sindone, A.; van der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: Putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur. J. Heart Fail. 2018, 20, 1664–1672.
- Gheorghiade, M.; De Luca, L.; Fonarow, G.C.; Filippatos, G.; Metra, M.; Francis, G.S. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am. J. Cardiol. 2005, 96, 11–17.
- Hertig, J.B.; Shah, V.P.; Flühmann, B.; Muhlebach, S.; Stemer, G.; Surugue, J.; Moss, R.; Di Francesco, T. Tackling the challenges of nanomedicines: Are we ready? Am. J. Health Syst. Pharm. 2021, 78, 1047–1056.
- Ganzoni, A.M. Intravenous iron-dextran: Therapeutic and experimental possibilities. Schweiz. Med. Wochenschr. 1970, 100, 301–303.




