1. Graphene Oxide (GO) as Antimicrobial Agent
Infections caused by bacteria can result in acute or chronic illness, and the decontrolled use of antibiotics leads to the emergence of bacteria more resistant to them, which manifests a serious health problem around the world
[1]. Graphene-based materials (including graphite, graphite oxide, GO, and reduced graphene oxide (rGO)) possess a wide range of antibacterial activities toward bacteria, fungi, and viruses
[2]. Specifically, GO has been reported as a promising material that can be used as an antimicrobial agent and, hence, applied in applications where it is necessary to inhibit microbial growth, such as in the treatment of infections, the coating of medical devices, packaging, and fabrics, among others
[3]. Chang et al. reported that GO did not have a significant cytotoxicity effect on lung adenocarcinoma human cells (A549). However, it could produce dose-dependent oxidative stress in cells and a slight loss in cell viability at high concentrations. The cytotoxic effects were concentration- and size-dependent for GO, which is important to consider during the fabrication of antimicrobial GO therapeutics and bio-applications
[4].
1.1. Antimicrobial Mechanism of GO
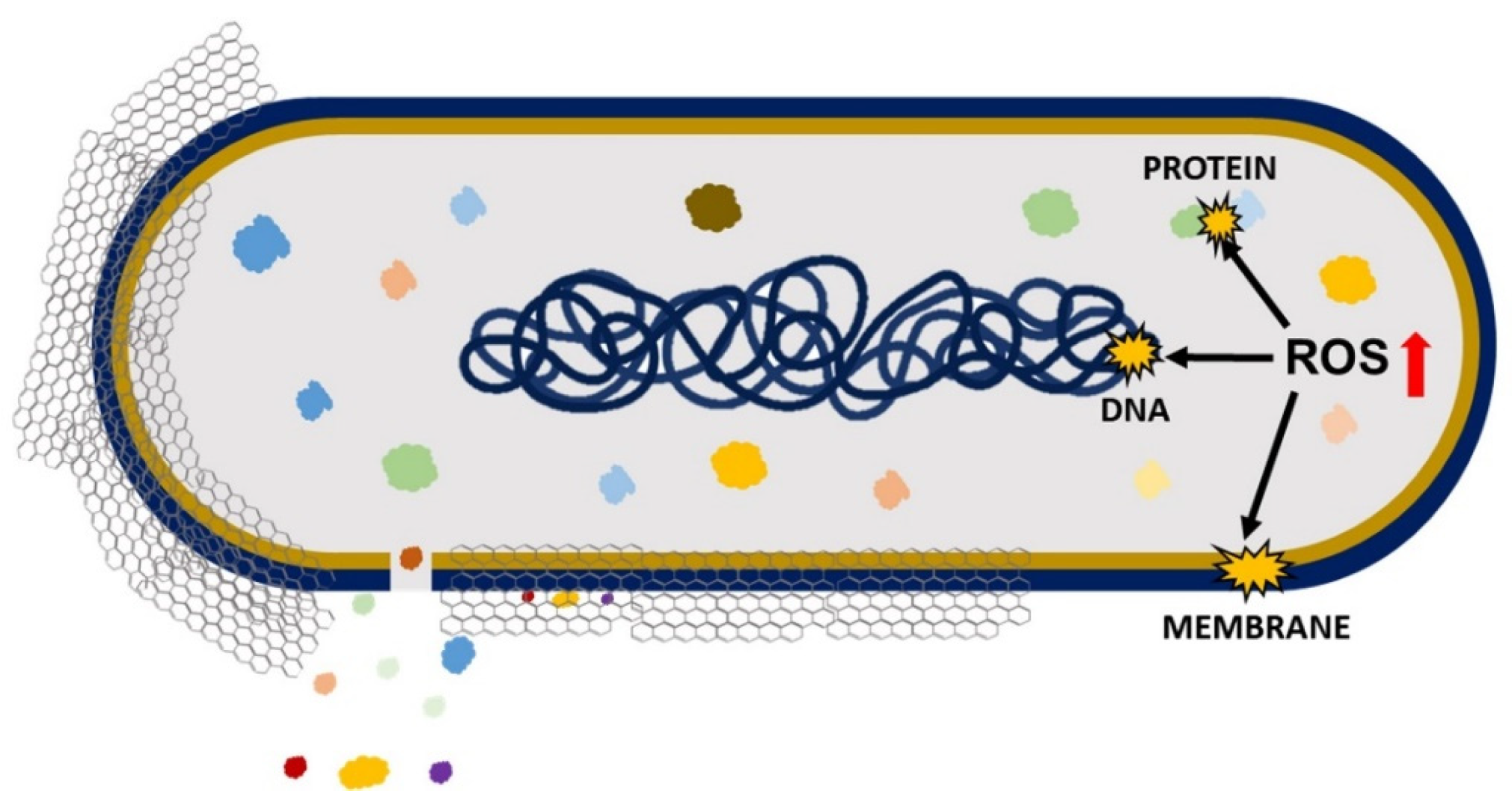
Antimicrobial effects cause a loss in cell viability, oxidative stress, and membrane stress that results in DNA fragmentation involving three steps: (i) cell deposition on graphene materials; (ii) membrane stress due to direct contact with sharp GO nanosheets; and (iii) the production of superoxide radical anions when the cell is exposed to GO and rGO (
Figure 1)
[5]. Liu et al. compared the antibacterial activity of graphite, graphite oxide, GO, and rGO on
E. coli under the same concentration and incubation duration. The obtained results demonstrated that GO had the highest antibacterial activity because it caused the highest oxidative stress level (had the most functional groups containing oxygen), followed by rGO, graphite, and graphite oxide
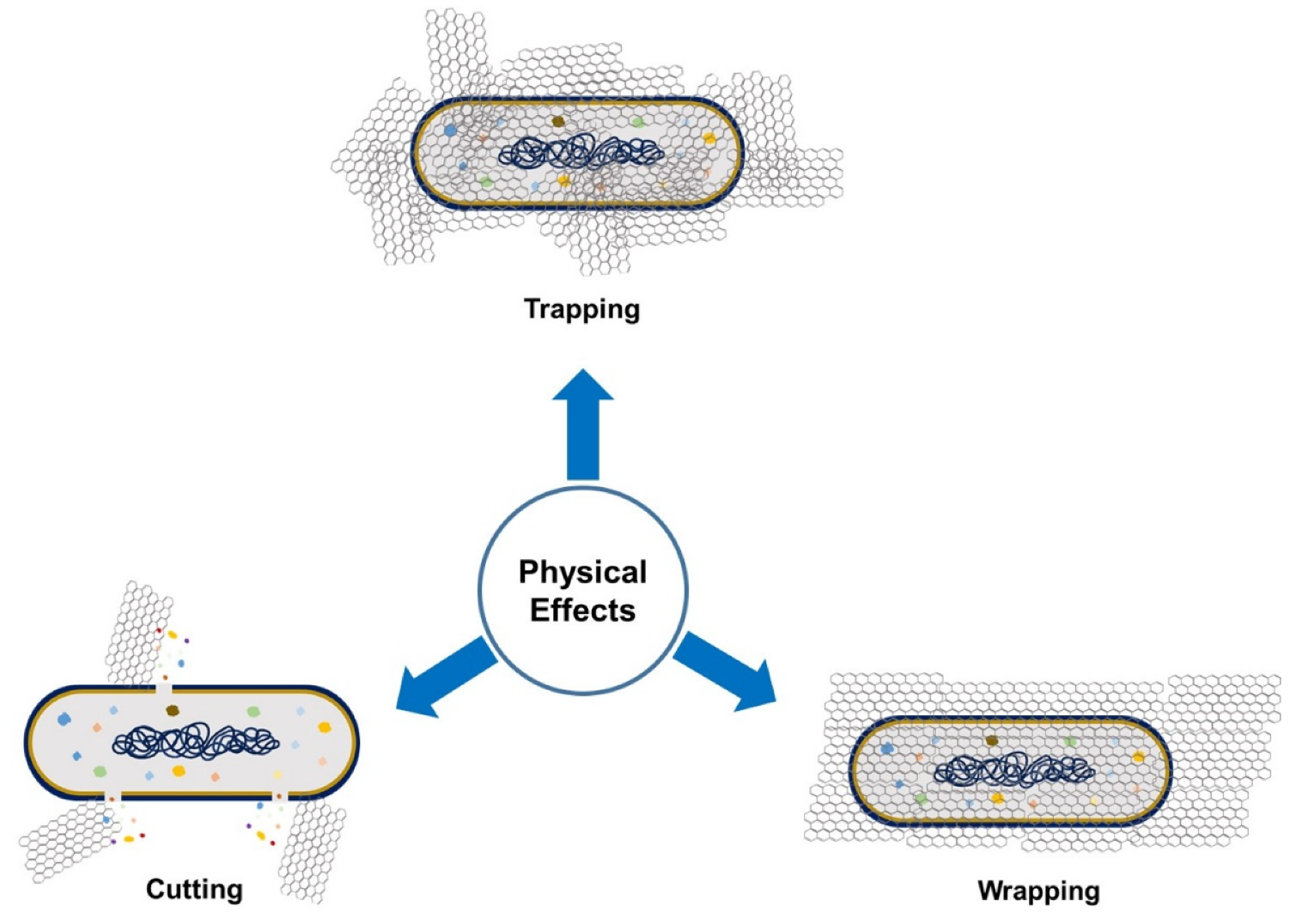
[6]. The antimicrobial activity of GO involves physical and chemical factors. The physical factor (
Figure 2) involves damage of the cell membrane by the direct contact of the sharp edges of GO nanosheets with the membrane, piercing through it, which causes leakage of the intracellular matrix eventually causing the death of the bacterial cell (cutting effect)
[7][8]. The connection between nanosheets and cells occurs through three mechanisms: (i) the swing mechanism, where nanosheets collide with the cell membrane multiple time; (ii) the nanosheets trap the cell membrane by Van der Waals forces and hydrophobic interaction, causing membrane damage; and (iii) the extraction mechanism, which results in distortion of the membrane and the loss of its integrity
[9].
Figure 1. Antimicrobial mechanisms of GO and rGO involving oxidative and membrane stress that result in DNA, protein, and membrane damage.
Figure 2. Physical factors that, through graphene-derivative microorganism interaction, can affect microbial growth.
Moreover, GO can establish antimicrobial activity for pathogenic bacteria by the GO’s wrapping mechanism (
Figure 2). During this process, GO traps the bacterial cell by wrapping around it and stopping it from proliferation by disconnecting the cell from its environment, which results in cell distortion and affects cell metabolism
[5][10]. In some situations, the GO sheets can be removed by sonication, and bacteria can proliferate and become activated again
[5]. This effect is size-dependent: large GO sheets can completely wrap microorganisms, whereas smaller GO sheets leave the cell partially uncovered, which allows the uptake of nutrients and survival
[8]. In the trapping effect, microorganisms face aggregated GO materials and are trapped by them, consequently inhibiting their growth
[7].
On the other hand, the chemical factor involves the excess production of reactive oxygen species (ROS) that causes oxidative stress, leading to lipid, protein, and DNA damage and, thereby, causing cell death
[7]. By Chong et al. reported that the exposure of GO to simulated sunlight increased the antibacterial activity. The measurements of ROS indicated that only singlet oxygen (
1O
2) was generated by GO’s exposure to simulated sunlight, which contributed to some extent to oxidative stress, causing antibacterial activity. However, the key cause of antibacterial activity was light-induced electron-hole pairs produced on the GO surface, which encouraged the reduction of GO, introducing additional carbon-centered free radicals that also increased the antibacterial activity of GO. Therefore, it can be concluded that oxidative stress caused by GO is mainly ROS-independent, and simulated sunlight speeds up the electron transfer from the bacterial membrane to GO, causing ROS-independent oxidative stress
[11].
1.2. Factors Affecting the Antimicrobial Nature of GO
Lateral Size
The size of GO nanosheets influences properties such as adsorption, dispersion, and the sharp edges of GO. These are important for GO’s interactions with microorganisms. Lateral size is a key factor in determining the effectiveness of the antimicrobial action of GO
[2]. Perreault et al. investigated the effect of GO sheets’ size areas (ranging from 0.01 to 0.65 μm
2) on antimicrobial activity by using
E. coli as a model microorganism. The results indicated that, as the size area of the GO sheets decreased, the antimicrobial activity of GO increased four-fold because smaller-sized GO sheets exhibited greater defects
[12]. The defects existing on GO sheets allowed more oxygen absorption and higher defect density in smaller GO sheets, which explained their higher oxidative potential
[11]. However, larger GO sheets demonstrated good antimicrobial activity when GO interacted with bacterial cells using a mechanical wrapping mechanism. In this situation, larger GO sheets trapped or covered the cell fully and more easily to stop the cell from proliferation, which resulted in loss of cell viability
[13]. However, the cell inactivation by the wrapping mechanism was reversible upon removing the GO sheets with sonication
[11].
In another report demonstrated the relationship between GO size and its antibacterial activity against the Gram-positive bacteria
Streptococcus mutans. Increasing the size reduced the cutting effect but enhanced the cell entrapment effect, and vice versa. The smallest GO (1295 nm) caused a significant cutting effect and cell breakage, but the cell entrapment effect could hardly be found. However, larger GO sheets (2015 nm) had a stronger cell entrapment effect, but the cutting effect was weaker. The largest GO sheets (4544 nm) had almost no cutting effect and only showed a strong cell entrapment effect. GO with a smaller size had a higher edge density and, thus, had a stronger cutting effect; in contrast, the GO with a larger size had a wider lateral dimension and, thus, had a higher potential to entrap bacterial cells
[14]. In another one, Liu et al. also evaluated the role of lateral size on antimicrobial activity in
E. coli bacteria. They observed a size-dependent trend in which larger GO sheets showed stronger antibacterial activity than smaller ones. The large GO sheets covered cells more efficiently, hence, the cells could not proliferate, resulting in cell viability loss
[15].
Morphology
It was reported that a wrinkled surface is a typical feature of GO films that possesses great antibacterial properties due to its corrugated nature
[16]. It was also observed that wrinkled GO surfaces could interact with the cell membrane more efficiently than planer GO surfaces to reduce cell viability
[9]. Recently
[16], many types of wrinkled-surface GOs with various levels of roughness were prepared to observe their effects on various bacterial species, such as
E. coli, Mycobacterium smegmatis, and
S. aureus. The results showed that the antibacterial action of GO was influenced by the ratio of GO surface roughness to bacterial size. The GO with ~500 nm surface roughness exhibited the most effective antibacterial activity against
E. coli and
S. aureus due to the matching of the wrinkle size to the bacterial size, whereas GO film with a much higher surface roughness of ~845 nm exhibited the greatest antibacterial activity against
M. smegmatis. Herein, it is important to highlight that the production of wrinkled-surface GO sheets is a simple and cheap process. Additionally, a wrinkled-surface GO sheet has a more effective ability to entrap the bacterial cell, resulting in tight interaction between them, which leads to membrane stress, causing disruption and intracellular leakage and leading to cell lysis
[16].
Zou et al. observed that smooth top-side GO film possessed effective antibacterial activity toward round-shaped
S. aureus and
P. aeruginosa, whereas rough bottom-side GO film possessed effective antibacterial activity toward only rod-shaped
P. aeruginosa. Therefore, both the shape and morphology of bacteria also can contribute to the effectiveness of GO’s antibacterial activity
[2].
Aggregation
Abiotic external factors may affect antimicrobial activity in two ways: (i) by influencing the aggregation process and bioavailability of GO and (ii) by modifying microorganisms’ behavior. The most-recognized abiotic factor is ionic strength. The reduction in antimicrobial activity that is caused by hindered interaction between GO and microorganisms is due to cations that induce the aggregation of GO
[17]. It was reported that GO’s interactions with bacteria were influenced by GO’s concentration, dispersibility charge, and aggregative state. When tested in different solutions, GO could easily aggregate in electrolyte-containing solutions and demonstrated efficient antibacterial action in all solutions at concentrations below 6 μg/mL. In water, as the GO concentration was increased, the GO antibacterial activity also increased. However, in other solutions (NaCl, CaCl
2, MgCl
2, and phosphate-buffered saline (PBS)), GO exhibited no influence on microbial growth due to aggregates that covered the GO edges. Meanwhile, for GO at concentrations above 100 μg/mL in NaCl, CaCl
2, and MgCl
2 solutions, large-sized aggregates were formed that mechanically trapped the bacterial cell and, thereby, inhibited their growth. In a PBS solution, the GO aggregates promoted bacterial proliferation. It was concluded that GO’s antimicrobial impact could be adjusted by changing the surrounding solutions
[18].
Basal Plane
The availability of the GO basal plane facilitates interactions with bacterial cells and has a direct influence on the antibacterial activity of GO
[9]. Hui et al. investigated the antibacterial properties of GO in Luria-Bertani (LB) nutrient broth. The results indicated that there was an increase in bacterial cell proliferation due to the inactivation of the antibacterial properties of GO. The reason behind inactivation was the noncovalent adsorption of LB components onto GO basal planes
[19]. In LB medium, the GO entraps bacterial cells, but once the availability of the GO basal plane is saturated by the growing bacteria, the antibacterial ability is deactivated. However, this deactivation is temporary and can be activated again by increasing the concentration of GO
[18]. In another one, a multilayered GO film made by the immobilization of GO sheets on polyethylene terephthalate (PET) showed antibacterial activity as the number of layers increased, where GO positioned itself flat on the substrate, providing more basal plane than GO sharp edges. It can be concluded that the antimicrobial properties of GO depend on its basal plane, where various modes of bacterial deactivation may occur
[7].
Purity
Barbolina et al. observed that highly purified and diligently washed GO exhibited neutral behavior, which did not motivate nor suppress bacterial growth, whereas poorly purified GO possessed antibacterial properties due to the presence of soluble acidic impurities that could be eliminated by further purification through neutralization with alkaline substrates. Therefore, the purification status of GO is important when dealing with biological systems because the effect of the material can be concealed by the influence of contaminants
[20].
Composites
Carpio et al. reported that a composition of poly(vinyl-N-carbazole) and GO (PVK-GO) possessed higher antimicrobial activity against planktonic microbial cells,
E. coli, Cupriavidus metallidurans, B. subtilis, and
Rhodococcus opacus biofilms than GO alone
[21]. GO–metal composites possessed enhanced antibacterial natures and distinctive molecular affinities or selectivities
[9]. Whitehead et al. detected the effect of GO–metal hybrids (AgGO and ZnGO) against four types of bacteria (
E. coli, S. aureus, Enterococcus faecium, and
K. pneumonia) and showed that AgGO had the most successful antimicrobial activity since the insertion of Ag into GO increased the antimicrobial activity of GO. GO–metal hybrids could be used as advanced antimicrobial agents
[22]. Li et al. observed that carbon nano-scrolls composed of graphene oxide-silver nanocomposites possessed ideal, broad antifungal activity against
Candida albicans and
Candida tropical [23]. Moreover, Ag nanoparticles (AgNPs) coated with aminoglycoside antibiotic tobramycin were associated to GO. This composite showed antibacterial mechanisms toward multidrug-resistant
E. coli [24]. GO contributed to cell wall disruption, while AgNPs led to intracellular oxidative stress, and tobramycin obstructed protein production in the bacteria
[24]. GO-chitosan nanocomposite films were produced by crosslinking GO with chitosan at a high temperature (120 °C). The antibacterial properties of the nanocomposite films were investigated, and the obtained results showed that these films possessed bacterial inactivation behavior against
E. coli (Gram-negative) and
B. subtillis (Gram-positive). The effectiveness of the antibacterial action of these films made them suitable for application in food packaging
[25]. GO coatings on metal films such as Zn, Ni, Sn, and steel could improve electrical conductivity. This happens because GO coated on a metal substrate pumps the electrons to fasten the electron transfer, which improves oxygen-containing functional groups on the GO surfaces to form ROS, causing oxidative stress and resulting in efficient antibacterial activity
[26]. Stabilizing agents and polymeric coagents can improve the antimicrobial action of GO. In a recent work, pluronic, a bioinert copolymer, improved the dispersion stability of GO and applied osmotic pressure on the cell membrane, consequently enhancing the antimicrobial activity on hypoosmotically challenged cells
[17].
2. Water Treatment (Purification)
The incorrect disposal of many human activities causes elevated contamination levels, affecting the environment, plants, and animals. Even at lower concentrations, some pollutants are aggressive and can accumulate through the food chain. For this reason, it is essential to treat contaminated waters before disposal into the environment [27]. GO has attracted much attention as a nanosized adsorbent because it can interact with different pollutants in molecular or ionic forms through electrostatic interaction, π–π interaction, and hydrophobic interaction mechanisms. GO directly interacts and then adsorbs at an excellent level towards ions or molecules [28]. Adsorption is a mass transfer mechanism that transfers a substance in a liquid phase to the surface of a solid and connects it to the surface by physical or chemical interaction [29]. GO nanosheets can be used as nanosorbent material; they possess an excellent adsorption property to efficiently eliminate heavy metals such as Pb, Ni, Cr, Zn, Cd, and Cu that originate from pharmaceutical effluents. Adsorption is an efficient and inexpensive method for eliminating heavy metal ions and organic impurities from wastewaters. GO can fully eliminate Cr and Pb ions, but Ni ions can be eliminated gradually by increasing the GO concentration. It was observed that all heavy metals ions were eliminated successfully at a GO concentration of 70 mg at pH 8 [27]. It was reported that poly(amidoamine)-modified GO had the adsorption ability to eliminate Fe(III), Cr(III), Zn(II), Pb(II), and Cu(II) from water or aqueous solutions at room temperature [30]. A GO–ZrO(OH)2 composite eliminated As(III) and As(V) from water or aqueous solutions. An rGO–Fe(0)/Fe3O4 composite removed As(III), Cr(VI), Hg(II), Pb(II), and Cd(II) from water at pH 7.00 and a temperature of 298 K [31]. Rare earth metal ions are poisonous and present in wastewater, and GO was utilized for the adsorption of Gd(III) La(III), Y(III), and Nd(III) for elimination from water [32].
Kovton et al. reported that GO was a promising nanoporous adsorbent or filter for water purification. The combination of GO sheets with commercial polysulfone (PSU) membranes was utilized to effectively eliminate organic impurities from tap water. The results showed that PSU-GO composites demonstrated a more efficient purification ability than benchmark commercial PSU membranes in the elimination of impurities such as rhodamine B and ofloxacin. The adsorption process of impurities on PSU-GO composites obeyed the Langmuir and Brunauer–Emmett–Teller models with unfinished swelling and the intercalation of molecules between GO layers. This approach was simple, and just the least amount of GO was needed, which was directly deposited on the surface of the polymer and then stabilized using microwaves or heat. PSU-GO composites had great stability, demonstrated after 100 h of tests in commercial water cartridges
[33].
The main cause of dye pollutants is the textile industry. The dyes and pigments are harmful pollutants of water and are toxic for aquatic species
[32]. GO-TiO
2 composite films were used as filtration membranes to eliminate dye molecules such as methyl orange and rhodamine B from water
[34]. Additionally, GO could remove methylene blue, methyl violet, acridine orange, and methyl green dyes from water. The use of graphene-based adsorbents for efficient dye removal was found to be directly proportional to the ionic strength and pH. Due to good electrostatic interactions between GO and dye molecules, the removal process was efficient
[32]. Anionic dyes such as AO8 and DR23 were found to be easily adsorbed by GO since both are nonplanar molecules that attach to the skeleton of GO through the formation of π–π stacking interactions due to spatial restriction, whereas electrostatic attraction was the main contribution to the mechanism. This was because the adsorption followed more of a Langmuir model
[28]. More interestingly, chitosan was used with GO to synthesize adsorbent hydrogel materials, which have affinities for both cationic and anionic dyes and for heavy metals
[35]. Due to the antimicrobial properties of GO, it decreased the GO membrane biofouling while increasing its lifetime and enhancing the energy consumption of water purification
[36]. The fabrication of a graphene oxide–silver nanoparticles (GO–AgNPs) composite on a cellulose acetate membrane generated an antibiofouling membrane. It was observed that the GO–AgNPs composite on the membrane possessed a strong antibacterial activity to remove bacteria from water
[37].
3. Water Desalination Membranes
There is a shortage of fresh water in some regions of the world. The desalination of seawater is one of the solutions to this problem. Layered GO membranes (GOMs) have a controlled, subnanometer-wide interlayer distance and versatile surface chemistry, which gives GOMs the ability to accurately sieve small ions and molecules. Pristine and chemically improved GOMs efficiently blocked organic dyes and nanoparticles but were unsuccessful in blocking smaller ions with hydrated diameters
[38]. To overcome this, it is possible to reduce the interlayer spacing down to only several angstroms to block small inorganic salt ions. However, compressed GOMs greatly decrease the water flux, limiting practical applications. Planar heterogeneous graphene oxide membranes (PHGOMs) are the latest approach to have been tested as an efficient water desalination process. The results have shown that PHGOMs possessed excellent salt elimination ability and high water flux. PHGOMs are composed of pristine, negatively charged GOs (n-GOs) and polyethyleneimine-conjugated, positively charged GOs (p-GOs). Horizontal ion transport through oppositely charged GO multilayer lateral hetero-junctions exhibits a bi-unipolar transport manner, which stops the conduction of both cations and anions. The salt concentration is depleted in the near-neutral transition area of the PHGOM with the help of a forward electric field, and deionized water can be extracted from the depletion zone. This approach gave a great NaCl rejection rate, reaching 97.0%, and very high water flux through an inverted T-shaped water extraction mode
[38]. It was reported that the GO membrane alone was permeable to water but impermeable to other molecules and impurities, such as bacteria, gases, vapors, and metal ions, but had poor salt elimination and water flux. In addition, another obstacle was observed: GO membranes swelled when immersed in water. An rGO membrane with the same laminated structure and high stability in water was found to resolve this obstacle by decreasing the rGO membrane thickness, which in turn increased the permeability of the rGO to impurities. Liu et al. observed that freestanding, ultrathin rGO membranes (thickness range of 200–20 nm) treated with hydriodic acid exhibited high salt rejection with fast water flux compared to GO membranes
[39], but the latest and most effective water desalination approach consists of using a PHGO membrane
[38].
4. Removal of Oil Pollution
Crude oil pollution (oil spill) is a serious worldwide problem that causes disastrous effects on the environment and ecosystem. Standard treatment methods such as in situ burning, manual skimming, and bioremediation require a great workforce and a long duration. In addition, crude oil possesses a high viscosity, which forms another obstacle for standard adsorbents
[40]. One solution is a floating adsorbent composed of rGO produced by a facile, one-pot hydrothermal method, a melamine sponge (MS), and a 3D-printed mounting platform. rGO-MS composites have appealing hydrophobicityand oleophilicity for oil absorption in water at a contact angle of 122°. rGO-MS composites can absorb nearly 95 times their weight in crude oil in 12 min under light irradiation because of efficient light-to-heat conversion. However, a 3D-printed mounting platform for rGO-MS composites has been fabricated to enhance their performance for enhanced extraction. rGO-MS composites were successful for in situ crude oil removal due to rGO’s hydrophobicity, oleophilicity, and photothermal characteristics, as well as the MS’s floating capability
[40].
The green, absorbent material created by the inclusion of rGO in natural rubber (NR) latex to synthesize an (NR/rGO) composite improved the petroleum oil (gasoline and crude AXL oil) adsorption capacity of the composite compared to pure NR foam and a cost-effective adsorbent. The NR/rGO composite had high elasticity and enhanced oil adsorption capacity. Additionally, the reusability of the adsorbent material for oil removal ability was greater than 70% after 30 uses. Moreover, environmental conditions such as temperature and ocean waves could affect the oil adsorption capacity of the NR/rGO adsorbent composite. It was observed that, as the temperature increased up to 45 °C or as an external force such as waves increased, the oil adsorption capacity of the adsorbent composite increased. It was concluded that the (NR/rGO) composite was a reassuring substitute for oil adsorbent in oil spill purification under serious field conditions in the ocean
[41].
5. Cancer Treatment
Recently, aminated GO (GO-NH
2) could activate powerful cytotoxic and genotoxic effects in colorectal cancer cells when compared to pristine (pure) GOs. The cytotoxicity of GO-NH
2 in colon cancer cells consisted of various mechanisms, such as the induction of ROS production, the blockage of cell proliferation, increased cytotoxicity, the induction of DNA damage, and the initiation of apoptosis. These parameters were more efficient at the highest concentrations of GO, which resulted in higher ROS production. It was concluded that GO-NH
2 nanoparticles were promising for the treatment of colon cancer
[42]. In another one, GO was used as a multifunctional platform for therapeutic delivery, biological imaging, and cancer sensing due to its pH-influenced fluorescence emission in the visible and near-infrared spectra, which yielded possibilities for molecular imaging and pH sensing
[43]. Additionally, GO is water-soluble and can be a platform for functionalization, allowing its use for drug delivery. Furthermore, GO showed a great cellular internalization capacity with lower depuration after 24 h, making it a good delivery agent. Being a pH-sensitive, fluorescent nanomaterial, GO can be utilized for the identification of the pH levels of the cellular environments. Thus, it can be potentially used for sensing the acidic extracellular environments of cancer cells
[43]. It was reported that GO confined the growth of tumors in different cell lines, including ovarian, pancreatic, breast, and lung cancers, as well as glioblastoma. GO could promote toll-like receptor response and cause autophagy and antitumor effects
[44].
Phenolic compound resveratrol was used for the green process of reducing GO to rGO, and its antitumor potential was tested against ovarian cancer cells. Dose-dependent effects were observed, including membrane leakage and oxidative stress. rGO was significantly more cytotoxic compared to GO, which could induce cell death in less than 60% of the A2780 cells even at the highest tested concentration, whereas rGO already caused significant cytotoxicity at 20 μg/mL; at 80 μg/mL, 90% of the cells were dead
[45].
Graphene nanocomposites have also been developed for this purpose. Composite rGO–AgNPs showed stronger antitumoral effects when compared to other tested nanomaterials such as graphene oxide, rGO, and AgNPs. This composite inhibited cell viability in A2780 ovarian cancer cells and increased lactate dehydrogenase leakage, reactive oxygen species generation, caspase-3 activity, and DNA fragmentation
[46]. The inhibition of the cell viability was dose-dependent. While the IC
50 for GO, rGO, and AgNPs were ~60 μg/mL, ~25 μg/mL, and ~20 μg/mL, respectively, for the nanocomposite (rGO–AgNPs), the IC
50 was only ~12.5 μg/mL, showing that the association of this nanomaterial could increase antitumor activity
[46]. In another one, similar nanocomposite rGO–AgNPs were tested against human lung cancer A549 cells, and the IC
50 was only 30 μg/mL
[47]. A GO-CuO nanocomposite demonstrated activity against HCT-116 human colon cancer lineage, leading to a 70% reduction in cell viability at the concentration of 100 μg/mL
[48].
Additionally, graphene-based materials can be used as drug carriers or antitumor agents for photothermal therapy. Photodynamic therapy is a noninvasive treatment methodology to treat diseases such as cancer. In this process, the photosensitizer molecule transfers the photon energy to surrounding oxygen molecules for ROS production and is heated under the irradiation of light with appropriate wavelengths
[49][50]. This approach allows a selective effect since only the lesion exposed to the light and to graphene-based nanomaterials is treated. GO-PEG-Ce6 showed excellent water solubility and caused cytotoxic effects on human nasopharyngeal epidermal carcinoma KB cells under light excitation. This complex enhanced the antitumoral potential when compared to free Ce6 photosensitizers due to the cell uptake of Ce6 delivered by graphene
[49]. rGO with a noncovalent PEG coating could eliminate 4T1 tumors in mice after an intravenous injection of rGO-PEG with a dose of 20 mg/kg and under 808 nm laser irradiation (0.15 W/cm
2). However, under the same conditions, GO-PEG could not inhibit tumor growth. All the mice treated with rGO-PEG survived over 100 days without a single death, side effect, or tumor regrowth
[50]. In addition, the GO-Fe
3O
4 composite was modified with PEG and cetuximab, an antibody for the epidermal growth factor receptor (EGFR). The composite could carry the anticancer drug doxorubicin (DOX) for a pH-dependent release. Additionally, the composite could inhibit the CT-26 murine colorectal cell growth in both in vitro and in vivo assays in photothermal therapy
[51]. GO modified with hyaluronic acid (HA) was developed for photothermal therapy against cancer cells. HA specifically binds to the CD44 receptor, which is abundantly overexpressed on the surface of various cancer cells, allowing more specific activity against tumor cells. After laser irradiation treatment (808 nm), GO-HA at a concentration of 50 μg/mL for 24 h was able to inhibit 48.04% of human breast cancer MCF-7 cell lines
[52].
6. Bone and Teeth Implantation
Titanium (Ti) surface improvement was achieved using GO coating and aspirin (A) loading (A/Ti-GO). Ti-GO was synthesized using an alkali-hydrothermal reaction and a coupling agent. The torsion test revealed that there was stable bonding between the GO coating and Ti under a torsional shear force in clinical settings, and there was no falling off of the GO coating from the sample surface (good adherence). In addition, the release of aspirin loaded on the Ti-GO surface was retained for 3 days due to π–π stacking interactions. In vitro cell one, it was showed that A/Ti-GO facilitated the proliferation of MC3T3-E1 cells and osteogenic distinction. The A/Ti-GO surface can be beneficial in enhancing the success rate of Ti implants in patients with bone conditions such as diabetes and osteoporosis
[53]. Recently, GO, lysozyme (Lys), and tannic acid (TA) have been combined using an easy and controlled layer-by-layer technique to produce a powerful antibacterial and modified osteogenic multilayer coating. Coatings with antibacterial and osteogenic agents can be useful in dental implants. GO, Lys, and TA coatings possess physical characteristics such as wettability, roughness, stiffness, and continual growth with the deposited process. Additionally, the obtained coatings showed improved osteogenesis of dental pulp stem cells (hDPSCs), which determined the potential application of coatings for dental implants
[54].
7. Scaffolds for Mammalian Cell Culture
Due to GO properties that advance cell adhesion and growth, graphene-based material substrates are a good prospect for tissue engineering and cell scaffolds. Scaffolds are structures able to support living cells that create a suitable microenvironment, which enables cells to grow and maintain themselves. Scaffolds need a high level of porosity, allowing the passage of nutrients and oxygen into the system for maintaining the cells, as well as the possibility to release the waste products and therapeutic products secreted by the cells
[55].
Various oxidized graphene-based papers can be used as substrates for cell culture by using starting materials with various thicknesses and lateral dimensions. Graphite oxide has thicker sheets compared to the thinner, large graphene oxide (l-GO) and small graphene oxide (s-GO) sheets. These substrates were tested for their cellular adhesion and proliferation ability with two epithelial cell lines, human lung cell culture (A549) and human neural cell culture (SH-SY5Y); the variances in their morphologies were observed using microscopic analysis. These GO sheets promoted cell growth with no impact on cell adhesion, proliferation, and morphology. In addition, although these three GO materials exhibited different topographies, they had similar structural and physicochemical characteristics. This concluded that paper-based GO substrates were successful biocompatible cellular materials that promoted anchorage-influenced cell growth and had the potential to be researched further for utilization in tissue engineering, regenerative medicine, substrates for cell growth, and bionic applications
[56]. Another one was indicated that GO film was effective for regulating the structure and function of human adipose-derived stem cells (hASCs). GO films fabricated through the utilization of a self-assembly method presented appropriate conditions for the adhesion, affinity, proliferation, and distinction of hASCs. Good attachment and higher affinity of the hASCS were observed on GO film compared to an uncoated substrate. Moreover, time-dependent cell viability of the hASCs occurred on the GO film. The GO films improved the differentiation of hASCs such as osteogenesis, adipogenesis, and epithelial genesis, but they reduced the chondrogenic differentiation of the hASCs. The GO films were a successful substrate for hASC cultures and could be used in designing and manipulating scaffolds for biological, stem cell, and tissue-engineering applications
[57].
To use graphene, it is necessary to evaluate its cytotoxic potential, which can alter metabolic functions and proliferative capacity and can induce apoptosis. In addition, it is necessary to evaluate the possible transformations after the interaction with cells, such as aggregation and changes in lateral size. In addition, it is also important to assess the potential to induce inflammatory responses when administered in vivo
[55].
8. Biofunctionalization with Proteins and DNA
GO has a larger surface area and is rich in oxygen content, which enhances the immobilization process and detection sensitivity. A GO-based sensor can detect many targets, such as single-stranded DNA (ssDNA), living cells, and metal ions
[44]. Proteins can be immobilized onto GO without the need for surface modifications or coupling reagents and can be applied to the detection process
[58]. The amphiphilic protein hydrophobin was successfully immobilized on GO sheets at the hydrophobic surface
[59]. The attachment of amino Fe
3O
4 onto GO through covalent bonds produced magnetic GO, which was used in the immobilization of laccase, resulting in higher thermal stability and different pH values
[60]. The immobilization of naringinase with graphene resulted in high isoquercitrin production. In addition, it was observed that, when graphene sheets were immobilized with an enzyme, it increased the specificity, and a moderate catalytic characteristic was observed so that the enzyme could be reused
[61].
Green fluorescent protein (GFP) is biologically inert and releases bright green, fluorescent radiation upon exposure to blue ultraviolet light
[44]. It is utilized in the identification of cells and tissues that have targeted gene expression
[62]. The incubation of cells with GFP-rGO did not impact cell morphology, indicating that the composite was nontoxic for the cells and had the potential to be utilized as a biomarker to investigate the cytotoxicity and identification of cells or areas of tissues possessing an expression of target genes
[63]. The ssDNA could be effectively adsorbed on the graphene surface due to the big, 2-D, aromatic surface of graphene. The ssDNA–graphene biointerface was utilized in a field-effect transistor for the label-free and reversible identification of complementary ssDNA. Additionally, ssDNA adsorbed onto GO was used for learning the surface-enhanced laser desorption ionization time-of-flight mass spectrometry
[58].
9. Biosensing and Bioimaging
Graphene-based materials, due to their high sensitivity, inexpensive, fast response and simple operation, are utilized in the fabrication of biosensors based on various sensing methods, such as optical and electrochemical signaling. These materials are successful electrode materials due to their electrochemical characteristics, which can enhance the detection of biomolecules
[64] such as thrombin, oligonucleotides, ATP, amino corrosives, and dopamine
[44]. Biomolecules have an essential duty in all life activities, such as disease development, so the precise identification of biomolecules is necessary for disease diagnosis and therapy. GO is incorporated in the fabrication of biosensors due to its excellent optical properties, such as its ability to fluoresce over a broad range of wavelengths (from near-infrared to ultraviolet) and efficiently quench the fluorescence of other fluorescent dyes. FRET is a well-developed technology for DNA identification and various atoms
[64] and for measuring nanometer-scale distance and changes, both in vivo and in vitro
[58]. GO is used as an energy acceptor in FRET biosensors, but it can be used as the energy donor or acceptor in various immunosensors
[64].
Recently, an aptamer-carboxyfluorescein (FAM)/GO nanosheet complex could detect ATP and GTP in functional cells. The aptamer was shielded from enzymatic digestion, and the FAM fluorescence was extinguished through absorbing onto the GO surface. After uptake by JB6 cells or a human breast cancer MCF-7 cell, the identification between the aptamer and the intracellular ATP or GTP caused conformation changes in the aptamer structure, resulting in the restoration of FAM fluorescence
[65]. Magnetic resonance imaging (MRI) is an important in vivo and noninvasive imaging technique that is employed in clinical practice. Aminodextran-coated Fe
3O
4 nanoparticles were immobilized onto GO to improve biocompatibility and cellular MRI signals. Additionally, it had excellent physiological stability, low cytotoxicity, and could be internalized by HeLa cells
[66]. Nuclear medical imaging positron emission tomography is capable of quantifying radioisotope concentrations in vivo with great tissue penetration. GO united with a TRC105 antibody has been utilized as an in vivo tomography imaging agent for CD105 (a biomarker for tumor angiogenesis) targeting
[64][67].
10. Gene Delivery and Drug Delivery
Gene therapy is an assuring approach for the treatment of different diseases caused by genetic disorders. It has been determined that graphene and GO have great potential to be utilized as gene carriers because ssDNA and RNA can immobilize onto graphene and GO with noncovalent adsorption through stacking, electrostatic, and other molecular interactions. GO–PEI (polyethylenimine) is an ideal gene vector due to its lower cytotoxicity and advanced transfection efficiency at an optimal mass ratio. PEI–GO possesses the ability to condense DNA at a low mass ratio with a positive potential and to transport plasmid DNA into cells and be localized in the nucleus efficiently
[68][69].
Graphene nanomaterials have an ultrahigh surface area and sp
2-hybridized carbon area, making them effective drug carriers to load a high amount of drug molecules on both sides of a single atom-layer sheet. They have many chemically reactive oxygen-containing groups on their surfaces that can be used for the functionalization of diverse compounds via covalent bonding from carboxylic acid, epoxy, and hydroxyl groups. Beyond that, GO sheets also exhibit noncovalent binding with some molecules via hydrophobic interaction, π–π interaction, or van der Waals interaction on sp
2 networks that are not oxidized
[70]. Functionalization can improve different properties, such as solubility, stability, and specificity, as well as cellular uptake, by enhancing its ability to go across the target cell membrane
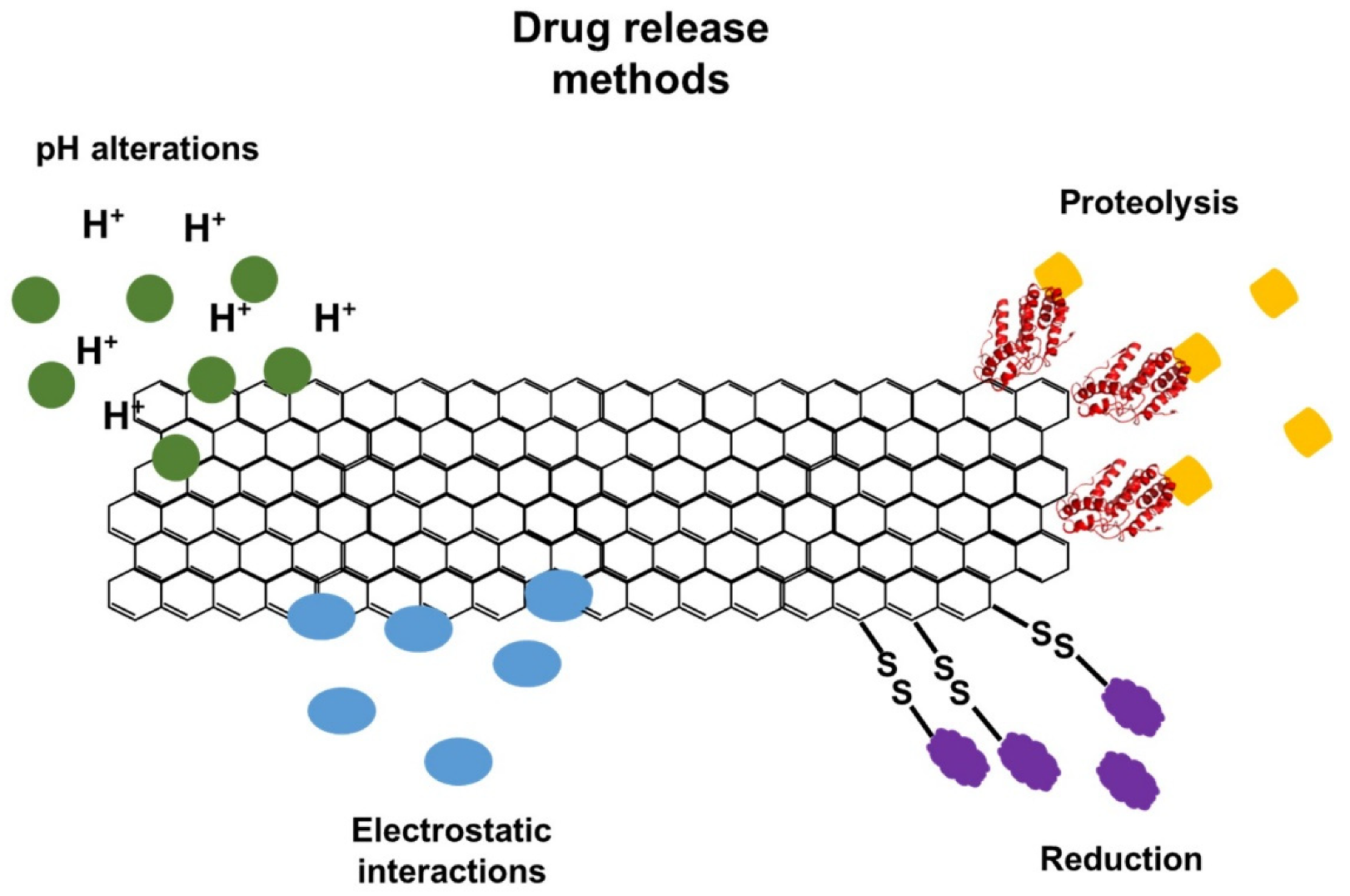
[70]. In addition, it is possible to adopt different approaches for the controlled release of drugs, such as changes in pH, enzymatic action, reducing environment, or electrostatic interactions (
Figure 3)
[70][71][72].
Figure 3. Different methods that can be applied for the controlled release of drugs functionalized in graphene and derivative sheets.
The anticancer drugs SN38 and DOX can be loaded onto GO by simple physisorption through π–π stacking to target cancer cells. PEI is covalently bonded to GO through a simple amidation process. PEI-GO assists in loading siRNA (small interference RNA), which restricts protein expression by targeted the cleavage of mRNA through electrostatic adsorption and aromatic anticancer drugs (such as DOX and camptothecin) through π–π stacking, resulting in improved anticancer efficiency
[64].
GO was functionalized with methotrexate, an anticancer drug, to assess its potential as a carrier for the delivery of anticancer drugs. GO did not cause any considerable cytotoxic effect to any of the cells tested: hepatocellular carcinoma cells (HepG2 cells), human embryonic kidney cells (HEK293A cells), and porcine skin fibroblasts (PEF). On the other hand, methotrexate was toxic to all the cells without any apparent selectivity. However, GO-methotrexate displayed significant, specific cytotoxicity to the tumor cell line (HepG2 cells) when compared to normal cells
[73]. Furthermore, changes in the pH of the tumor environment can be used for a drug delivery approach. In this regard, GO was functionalized with carboxymethyl cellulose (CMC) and then DOX to form GO-CMC/DOX. The release rate of the drugs reached an optimum value of 65.2% at pH 5. The DOX and GO-CMC drug carriers were bound with π–π stacking action and a hydrogen bonding interaction. The π–π bond at the lower pH environment was broken, which led to the slow release of the drug
[74].