The isolation and purification could be taken as synonyms, so to avoid misleading and confusion, these two terms have been discussed in detail separately for the convenience regarding readership. The separate addressing of isolation and purification of natural compounds have been discussed below.
1.1. Isolation of Natural Compounds
The most important step associated with natural compounds is the isolation of these medicinal agents or compounds. In general, the isolation process could be categorized as; (a) old method for isolation of natural compounds or drugs and (b) new methods for isolation of natural compounds
[1][2][3][4]. In old methods, limited studies were carried out including the; (i) selection of only those plant or plant parts which were known for their specific medicinal features, (ii) Identification of chemical nature of compounds and classification, identification or detection of compound(s) based on their chemical nature, (iii) Conventional method of isolation of natural compounds based on above-mentioned identification protocols, (iv) In vitro and in vivo characterization of these compounds, in which usually the in vivo characterization was carried out on human volunteers while the in vitro characterizations are carried out in laboratory set up, (v) Taxonomic classification of these compounds, (vi) Reporting of toxic characteristics of these compounds after experimentation/applications on humans
[4][5].
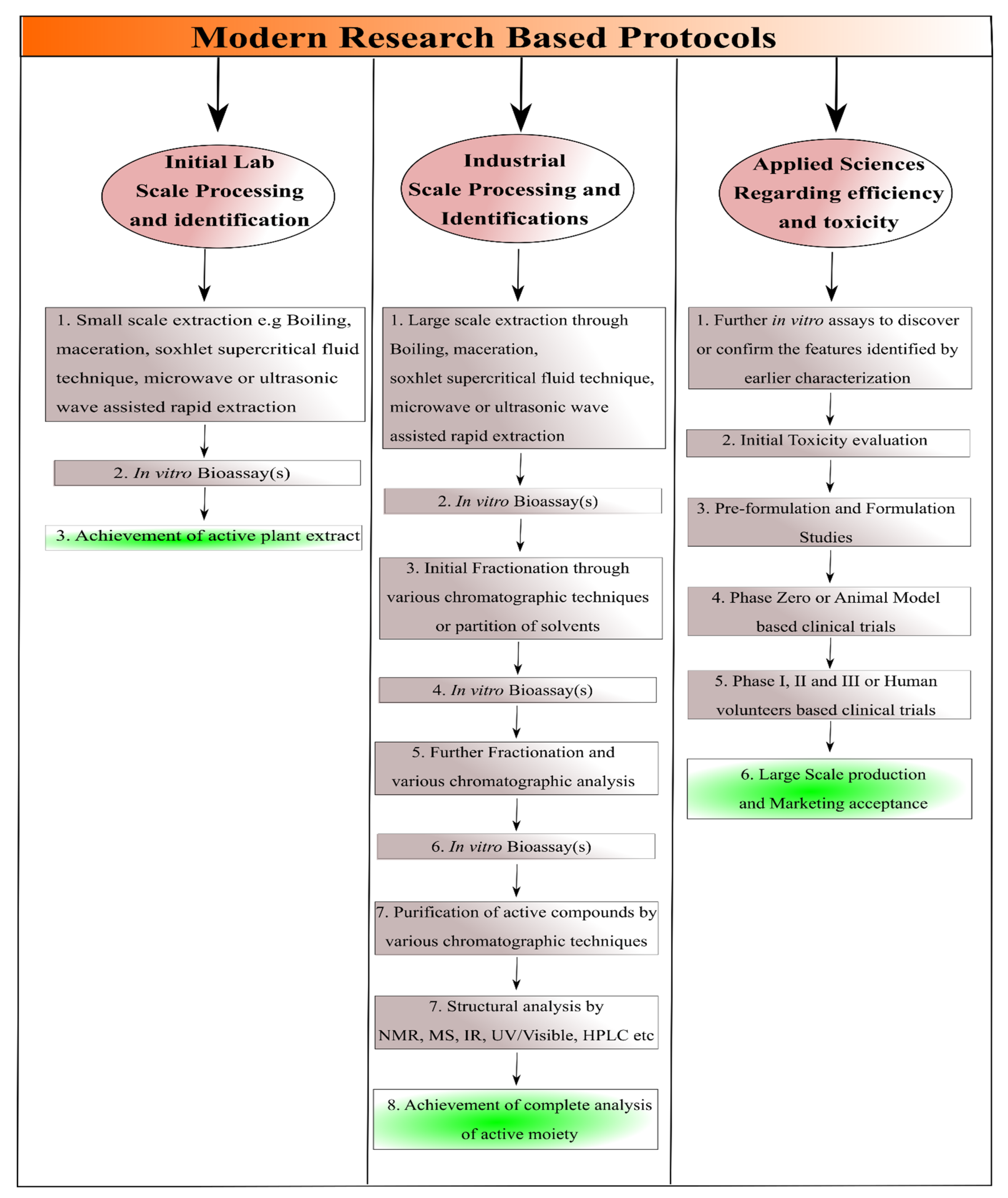
Before discussing the modern techniques for the isolation of natural compounds, it should be understood that in modern research, the protocols are carried out as reported by Sarker et al. (2005) and given in a
Scheme 1 [6]. The modern technique regarding isolation of natural compounds may include; (a) In vitro bioassay-based characterization for the confirmation or final identification of the natural compounds after the achievement of small scale and large scale processing/evaluation parameters as illustrated in
Scheme 1, (b) Achievement of such type of compounds in which further modification does not take place from the suitable evaluation parameters and comprehensive review of available literature, (c) Achievement of active moieties with medicinal effectiveness through advanced cell, tissue, genes or vector-based cultures either alone and/or via combinatorial strategy of these, (d) Achievement of sufficient data regarding the efficacy and toxicity profiling of natural compounds, (e) detection of their activity prior to the human-based clinical trials through various in vitro and in silico modeling approaches followed by ex vivo detection and subsequent in vivo animal-based clinical trials, (f) Combining the knowledge regarding selection of only those plant or plant parts which were known for their specific medicinal features (as mentioned earlier) and the modern techniques to achieve the best effective results, and (g) selection of other organisms or part of organism for the evaluation parameters such as marine algae, various bacteria and viruses, even in the modern scientific research cells, antigens or genes could be employed
[1][2][3][4][7].
Scheme 1. Dissolution and Permeation Enhancement of Isolated Purified Natural Compounds.
In addition, before initiation of isolation some of the features of natural compounds should be considered including water solubility, pH, size, charge and different types of stability profiles. Among these stability types, stability at different pH, temperatures, time periods and colloidal stability should be considered
[8]. Das et al. (2021) comprehensively discussed the phytoecdysteroids and gathered the literature from 1999 to 2019 regarding methodology associated with the history, search for available literature, chemistry, new and old compounds isolated from phytoecdysteroids, mechanism of actions and their biomedical applications. The author reported that more than 400 types of phytoecsteroids have been reported from since 1954 up until now. The author reported 212 types of phytoecsteroids from eighteen families including amaranthaceae, asteraceae, blechnaceae, caryophyllaceae, clavicipitaceae, commelinaceae, cioscoreaceae, gleicheniaceae, lamiaceae, liliaceae, limnanthaceae, lygodiaceae, malvaceae, menispermaceae, polypodiaceae, polyporaceae, rhodomelaceae, and taxaceae
[9]. The first phytoecsteroid called ecdysone was reported by Butenandt and Karlson in 1954
[10]. The common and most familiar isolated phytoecsteroids include 20-hydroxyecdysone, ponasterone A, ponasterone B, ponasterone C
[9]. Likewise, Lafont et al. (1994) discussed the isolation of Phytoecsteroids in detail and reported that phytoecsteroids could be isolated through variety of protocols and/or techniques. Likewise, the isolation could also be achieved through reverse phase thin layer chromatography (RP-TLC), high performance liquid chromatography coupled with mass spectrometry (HPLC-MS), gas chromatography (GC), mass spectrometry (MS), gas chromatography coupled with mass spectrometry GC-MS, supercritical fluid chromatography (SFC) and supercritical fluid chromatography coupled with mass spectrometry (SFC-MS)
[11]. For instance, Schooley et al. (1972) isolated phytoecdysone (phytoectosteroid) from
Podocarpus nakaii leaves, by initially concentrating the extract containing 900 g of leaves with 80% ethanol, followed by dissolving in methanol and subsequent diluting with equivalent amounts of distilled water (750 mL). After that the impurities were removed by using hexane thrice and after achieving precipitate and aqueous layer, the aqueous layer was diluted with a sufficient quantity of water (4L). After that the filtrate was passed through the column and by varying the amounts of ethanol, finally three different types of the phytoecdysones (3% in total), namely ponasterone A, ponasterone B and ponasterone C, were achieved
[12]. On the contrary a comparatively advanced type of isolation of phytoectosteroid, which is also an antimicrobial agent from
Nocardia levis has been reported by Kavitha et al. (2010). The author discussed the isolation by the protocol initiated with culturing in a suitable medium followed by fermentation of broth, filtration by centrifugation and subsequent withdrawal of supernatant as filtrate. After that, the extraction was done with ethyl acetate and then the extract was concentrated through rotary evaporation. After that, the achieved dark brown was concentrated through column chromatography and then one of the fractions was carefully selected followed by structure interpretation through ultra-violet (UV), infra-red (IR) spectroscopies, mass spectrometry and nuclear magnetic resonance (NMR) spectrometry. After that, the successful isolation of 1-phenylbut-3-ene-2-ol was achieved
[13]. In another study, Konishi et al. (1989) reported the isolation of antifungal cispentacin from bacillus cereus by a similar method with few modifications. In this method, after carrying out the initial three steps, column chromatography was achieved. The crude cispentacin was achieved, which was recrystallized with a mixture of acetone, ethanol and water and finally the pure form of cispentacin was achieved
[14]. Among the recent studies, Wang et al. (2017) reported the isolation of Phytoecdysteroids from
Ajuga iva for their anti-diabetic activity act. The isolation was carried out by drying 100 g of
Ajuga iva leaves followed by extraction through distilled water via refluxing for 36 h at 90 °C, and subsequently, the conversion into powder by vacuum evaporation
[15].
Guo et al. (2009) reported the isolation of two Bis(indolyl)methane alkaloids, namely streptindole and vibrindole A
[16], whereas Abe et al. (2013) isolated five different types of Bis(indolyl)methane alkaloids including streptindole, vibrindole A, arsindoline A, arsindoline B and arundine
[17]. Moreover, the author reported ten different types of isolation techniques associated with streptindole, two different types of isolation techniques associated with trisindoline, seven different techniques of isolation regarding vibrindole A, one isolation technique related to 2,2-bis(6-bromo-1 H-indol-3-yl)ethylamine and arundine and three isolation techniques linked with arsindoline A and arsindoline B. Regarding alkaloids, Praveen et al. (2015) gathered the data associated with the isolation of seven different types of Bis(indolyl)methane alkaloids in the review including streptindole, trisindoline, vibrindole A, 2,2-bis(6-bromo-1 H-indol-3-yl)ethylamine, arsindoline A and arsindoline B, arundine. Among these, most of the reported literature was based on the isolation of a single type of Bis (indolyl) methane alkaloids
[18].
Likewise, Soares et al. (2015) reported the isolation of
Bocageopsis pleiosperma-based alkaloids from the research-based study. In brief, initially the trunk bark, twigs and leaves were used and partitioned for the extraction of alkaloids for spectroscopic analysis (MS) and large-scale extraction, respectively. Regarding extraction, the samples associated with MS were dried, powdered, and stirred with aqueous solution of ammonium hydroxide and dichloromethane (DCM) followed by the transferring of organic phase in another container and subsequent stirring with acetic acid aqueous solution. On the contrary, the aqueous phase was separately treated with ammonium hydroxide to adjust the pH to 10, stirred with DCM and dried with anhydrous sodium sulfate and solvent evaporation in conjunction with nitrogen gas to achieve alkaloid. On the contrary, the large scale extraction was done by initially drying the leaves and conversion into powder followed by treating with ammonium hydroxide and DCM for 3 days. The organic and aqueous phase were separated such that the organic phase was agitated with aqueous acetic solution, whereas the aqueous phase was extracted with ammonium hydroxide, DCM and adjustment of pH 10 was carried out. Finally, the DCM-based phase was dried first with sodium sulfate and then evaporation to achieve the desired alkaloids. After that the fractionation and spectroscopic analysis were carried out for both samples i.e., sample associated with MS spectroscopy and large scale extraction
[19]. Kukula-Koch et al. (2016) reported isolation of eight phenolic acids and two alkaloids as flavonoids from herb of
Berberis sibirica by maceration and accelerated solvent extraction followed by identification and fractionation through various spectroscopic and chromatographic analysis. The spectroscopic analysis was carried out by Ultra High performance liquid chromatography (UHPLC), Hydrostatic counter-current partition chromatography (HCCC), High performance liquid chromatography with UV detector (RP-HPLC–DAD) and Ultra High performance liquid chromatography coupled with UV-detector, electrospray ionization and high resolution mass spectrometry UHPLC–DAD-ESI(±)HRMS techniques
[20]. Likewise, Rinaldi et al. (2017), reported the isolation of four aporphine alkaloids, namely actinodaphnine, anonaine, isoboldine and nornuciferine from the extract associated with stem of
Annona hypoglauca. The extraction was carried out by initial fractionation by column chromatography followed by elution through solvent mixture of DCM and methanol using different ratios to achieve 10 fractions. Among these three, fractions were again fractionated with preparative thin layer chromatography (PTLC) and after that actinodaphnine and nornuciferine were isolated by PTLC, whereas anonaine and isoboldine were isolated by HPLC. All these aforementioned compounds were then identified with HPLC
[21]. Regarding terpenoids Rodrigues et al. (2009) and Skalicka et al. (2013) both reported the isolation of terpenoids by high-performance counter-current chromatography (HPCCC). In both these studies, sample preparation, selection of suitable solvent system and appropriate spectroscopic analysis was carried out by NMR, MS and finally with HPCCC. The difference was associated with selection of plant and plant part as Rodrigues et al. (2009) selected the stem of
Trichiliaquadrijuga, whereas Skalicka et al. (2013) used Fruits of
Pimpinella anisum for the extraction of terpenoids
[22][23].
Among the recent studies, Kishore et al. (2018) reported the comprehensive study regarding isolation of a new compound called myricetin 3-O-(2″,4″-di-O-acetyl)-α-L-rhamnopyranoside and eleven known compounds from
Myrsine africana. The known compounds were associated with flavonoids and flavonoids glycoside class of natural compounds including Myrsinoside A and B, quercetin, quercetin 3-O-α-L-rhamnopyranoside, quercetin 3-(3″,4″-di-O-acetyl)-α-L-rhamnoside, mearnsitrin and mearnsetin 3-O-(4″-O-acetyl)-α-L-rhamnopyranoside, myricetin 3-O-(4″-O-acetyl)-α-L-rhamnopyranoside, myricetin-3-O-(2″,4″-di-O-acetyl)-α-L-rhamnopyranoside, myricetin 3-O-α-L-rhamnopyranoside and rutin
[24]. Likewise, based on the recent study associated with terpenoids, Zhang et al. (2021) reported the isolation of austrobuxusin-N, austrobuxusin A, austrobuxusin B, austrobuxusin C and austrobuxusin-D from the leaves of the
Austrobuxus swainii. The author reported that isolation and identification and elucidation of structure was carried out through one dimensional nuclear magnetic resonance spectrometry (1D-NMR), two dimensional nuclear magnetic resonance spectrometry (2D-NMR) and mass spectrometry (MS). The 1D-NMR may also detect the peaks associated with functional groups but to avoid misleading associated with overlapping of peaks if any 2D-NMR was also used in conjunction which has the ability to differentiate overlapping peaks of functional groups as well
[25].
1.2. Purification of Natural Compounds
As the isolated natural compounds may be associated with the presence of different types of impurities, so the natural compounds must be purified before carrying on pre-formulation and formulation processing and evaluations
[26]. Therefore, the purification methods should be addressed so that the researchers seeking expertise in the isolation and extraction of natural compounds for various biomedical application should benefit. In addition, such data may also be added in the existing scientific knowledge for the wide community of readers. In this regard, a study conducted by Otsuka (2006) reported in a book chapter that purification associated with the achievement of desired natural compounds could also be achieved by the phenomenon of partition co-efficient. The author reported that the achievement of desired natural compounds could be achieved by partitioning the desired natural compound in immiscible solvents as mentioned earlier by Schooley et al. (1972)
[12][27]. The author stated that it is comparatively easy compared to the achievement of desired natural compounds in the mixture of miscible solvents. In this technique the initial dissolving of extract in methanol followed by extraction with n-hexane are similar. However, after that, the dilution of methanol is carried with water to achieve different solutions (%
v/
v) i.e., 80% methanol-aqueous solution followed by extraction with CCl
4 and 65% methanol-aqueous solution followed by extraction with CHCl
3. By doing this, glycosides and hydrophilic polysaccharides could be achieved
[27]. Likewise, Sulkowski (1985), in review, reported the purification of several types of proteins including Human serum proteins, Lactoferrin, α
2-SH glycoprotein, Human fibroblast interferon, α
2-Macroglobulin, Plasminogen activator, Lysozyme, Nucleoside diphosphatase,
Dolichos biflorus lectin, Non-histone proteins, Human serum albumin, Human fibrinogen, Phosphotylrosyl-protein phosphatase Superoxide dismutase Human serum proteins, Bovine pancreatic ribonuclease, Cytochrome c, Calmodulin, Avidin, Myoglobins, Protein A, Albumins and Interferons by immobilized metal-affinity chromatography
[28]. Later on in 1994, Chase reported the purification of proteins by adsorption chromatography
[29]. Likewise, Dr. Granier reported the purification of proteins by electrophoresis
[30]. In addition, Safarik and Mirka Safarikova (2004) in their review reported several types of proteins and peptides comprehensively, that may be purified through magnetic techniques. These proteins include aminopeptidases, ACE, caspase chymotrypsin, nuclear inclusion-a-proteases, bacterial trypsin, urokinases, lysozymes, various hydrolytic enzymes of carbohydrates and some other types of enzymes, antibodies, nucleotides or aptamer binding proteins, albumin and Hb and various other proteins
[31]. In addition, similar to the polysaccharide purification the proteins may also be purified through a) denaturing of proteins followed by conversion of proteins to gel or jelly form, which can be purified or separated through centrifugation, and precipitation of proteins through Trichloroacetic acid solution or Trichloro ethane/Trifluoro ethane solution. The difference is that rather than using the supernatant, the supernatant will be used for the achievement or purification of proteins
[32][33]. Moreover, the proteins could also be purified by salting out, PEGylation, dialysis, centrifugation, Lyophilization and ultrafiltration as reported elsewhere
[33]. Recently, Eivazzadeh-Keihan et al. (2021), comprehensively described the purification of proteins and peptides by surface modification of nanoparticles with metals, polymers, biomolecules or antibodies
[34].
Among lipids purification techniques, lipids could be purified through chloroform-methanol solvent system
[35], by centrifugal partition chromatography
[36][37], through supercritical fluids
[38] and through membrane techniques
[39]. The recent study is based on the purification of lipids by various techniques associated with lipid-based drug delivery approaches
[40].
2. Physicochemical Characteristics Based Approaches for Solubility Enhancement
2.1. Particle Size Reduction
The particle sizes of natural compounds are reduced through various techniques, which may enhance the surface area. An increase in surface area may ultimately result in the improvement of aqueous solubility
[41]. In general, the particle size may be reduced by comminution through grinding and/or jet milling
[42], spray drying and recrystallization using anti-solvents through natural lipophilic compounds
[43]. Charoenchaitrakool et al. (2000) reported the solubility enhancement of pure racemic enantiomer of ibuprofen and stated that 60% improvement in solubility was achieved through micronization associated with quick Expansion of Supercritical Solutions
[44]. Likewise, Sievers et al. (2003) reported the solubility enhancement of natural and allopathic drugs and reported that micronization may enable the drugs suitable for pulmonary delivery
[45]. In another study Suo et al. (2005) revealed the solubility enhancement of natural pigment called Bixin by particle size reduction through Pre-filming Atomization process
[46]. In another study the author reported the solubility enhancement of salicylic acid and taxol by particle size reduction through supercritical solution expansion. The author also stated that the micronization and temperature both were inversely proportional to the particle size. In addition, it has also been reported that the method did not affect the chemical nature of compounds as well
[47]. Likewise, the recent study reported by karimi and Raofie is based on the improvement of dissolution and water solubility by particle size reduction
[48]. The authors reported the extraction of vincristine as vinca alkaloids which can be utilized for a variety of cancers from
Catharanthus roseus. In the study, the solubility was enhanced by supercritical fluid expansion-based particle size reduction.
2.2. Solid Dispersion
The solid dispersion is another auspicious strategy regarding the improvement of water solubility of drugs. It has been reported by Bikiaris in the comprehensive review that better enhancement of dissolution or solubility is achieved through solid dispersion compared to the micronization or particle size reduction. The author reported various preparation techniques of solid dispersions but primarily discussed the allopathic drugs as model drugs and marketed products associated with the solid dispersions of these allopathic drugs. The author discussed that possible techniques for the achievement of solid dispersion may include drug and carrier dissolution in organic solvent(s) followed by solvent evaporation, kneading technique, wet milling and spray drying
[49]. Likewise, Vasconcelos et al. (2007) categorized the solid dispersions associated with allopatic drugs in three different generations as first, second and third generation cyclodextrins in the review. Vasconcelos et al. (2007) also reported that solid dispersion could be preferred over various other dissolution enhancement techniques due to their improved features in conjunction with the merits of solid dispersion
[50]. Mishra et al. (2015) also highlighted the fourth generation of solid dispersions associated with allopathic drugs in addition to first, second and third
[51]. Moreover, the author also reported various other techniques in addition to those discussed by Bikiaris (Solvent evaporation through vacuum drying and rotavapour, cryogenic processing, supercritical fluid technology, electrospinning, lyophilization and microwave irradiation). In addition, the author also discussed some limitations associated with solid dispersions in juxtaposition to its merits. Regarding natural medicinal agents, Zhaojie et al. (2014) reported the achievement of solid dispersion of natural alkaloid berberin in conjunction with permeation enhancer by solvent evaporation technique
[52]. The author stated that the improved solubility and permeability were achieved which collectively enhanced the bioavailability of berberine. The author also publicized that it was the first study about berberine regarding its solubility and permeability enhancement and furthermore revealed that enhanced in vivo efficacy for the treatment of diabetes mellitus was achieved through the developed solid dispersion of berberine. Likewise, Zhang et al. (2014) also highlighted both in vitro and in vivo solubility enhancement associated with berberin-based solid dispersion. In the current above-mentioned study, both solid dispersion and complexation techniques were combined to achieve the synergistic solubility enhancement of berberin. The author reported that highest in vitro dissolution was achieved through the complexed solid dispersion followed by berberin solid dispersion and the least dissolution was observed through berberin
[53]. In another similar study, Shi et al. (2015) presented the in vitro and in vivo evaluation of solid dispersion of berberine used in conjunction with hydrogenated soy phosphatidylcholine through vacuum desiccation and solvent evaporation technique. The author stated that an enhanced in vitro dissolution and in vivo bioavailability were achieved in the formulation of solid dispersion containing equivalent amounts of berberine and hydrogenated soy phosphatidylcholine
[54]. Regarding glycosides, Pang et al. (2015) reported the solubility enhancement of rebaudioside D (belong to the class of steviol glycoside) and potassium sorbate (as a carrier)-based solid dispersion and stated that the solid dispersion was achieved through spray drying. The author revealed that the optimum enhancement in solubility was achieved through using rebaudioside D and potassium sorbate in equivalent quantities
[55]. Kanaze et al. (2006) reported the development of solid dispersions using five flavonoids as flavanone glycosides, naringin, hesperidin, naringenin and hesperetin by solvent evaporation method. The author stated that two different sets were developed based on the carrier using polyvinylpyrrolidone (PVP) and polyethylene glycol (PEG) as carriers and revealed that PVP-based solid dispersions were completely amorphous and represented higher dissolution compared to PEG-based solid dispersions
[56]. Likewise, Li et al. (2013) also reported the conversion of naringenin to amorphous form followed by solubility enhancement. The author used four different types of carrier including carboxymethylcellulose acetate butyrate (CMCAB), hydroxypropylmethylcellulose acetate succinate (HPMCAS), cellulose acetate adipate propionate (CAAP) and polyvinylpyrrolidone (PVP) and reported that the highest solubility was achieved through PVP-based solid dispersion and improved the water solubility of naringenin from 38 µg/mL to 8000 µg/mL
[57]. Similarly Khan et al. (2015) reported the use of five different carriers and stated that kneading and rotary evaporation was carried out for the achievement of solid dispersion. The author reported the highest in vitro dissolution, solubility and in vivo bioavailability through soluplus
®-based formulations. In addition, it was also highlighted that the solid dispersions achieved through solvent evaporation method were more soluble and with improved dissolution compared to the solid dispersions achieved through kneading technique
[58]. Among the recent studies, Thenmzohie and Yoo (2017) reported the solubility enhancement of piperin from
piper nigrum by solid dispersion through the use of hydrophilic carriers including sorbitol, PVP and PEG. The author stated the achievement of quick release of piperin through dissolution enhancement through these carriers. Less than 5% of pure piperine was released within two hours, whereas, more than 70% of piperine was released achieved through the aforementioned hydrophilic carriers-based solid dispersion of piperine
[59].
2.3. Complex Formation
Complex formation is another promising approach regarding solubility enhancement of natural compounds. Among different type of complexations, the cyclodextrins (α-cyclodextrins, β-cyclodextrins and γ-cyclodextrins) gain sufficient scientific interest for solubility enhancement of pharmaceutical and cosmetic ingredients
[60]. Uekama et al. (1983) initially diverted the research interest towards solubility enhancement of cardiac glycosides including digoxin, digitoxin and methyl digoxin through the aforementioned types of cyclodextrins (α-cyclodextrins, β-cyclodextrins and γ-cyclodextrins). The author mentioned the study outcomes and stated that the peak solubility enhancement was achieved through γ-cyclodextrins followed by solubility enhancement of β-cyclodextrins and the least solubility enhancement was achieved through α-cyclodextrins (improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation)
[60]. Likewise, in the last decade, Upreti et al. (2011) reported the complexation of α-cyclodextrins with steviol glycosides rebaudioside as rebaudioside A, rebaudioside B, rebaudioside C, rebaudioside D. rebaudioside E and rebaudioside F. The author stated that the solubility enhancement of the α-cyclodextrins complexed was achieved. In addition, the author reported that the effect was very prominent in rebaudioside A, rebaudioside B and rebaudioside D compounds as the solubility enhancement was directly proportional to the amounts of α-cyclodextrins as inclusion complexes
[61]. Likewise, Wan et al. (2013) reported the solubility enhancement of resveratrol (as natural antioxidant) by complexation with another type of hydrophilic Steviol Glycosides known as stevioside. The author revealed that by increasing the quantity of stevioside, the solubility of stevioside resveratrol complex increases
[62]. Zhang et al. (2016) reported the poor use of water soluble triterpenoid as a natural anticancer agents and reported the solubility enhancement through complexation with another type of highly water soluble steviol glycoside termed as rubusoside. The author reported that the optimum anticancer efficacy was not achieved from aforementioned triterpenoids in the previous studies due to its poor water solubility and contrarily improved and optimum anticancer efficacy against Caco-2 cells was achieved after complexation with rubusoside
[63]. Ping et al. (2017) reported the 4.3 times higher solubility of sanguinarine after complexation with highly water soluble carboxylatopillar-6-arene which is among the class of natural compounds called pillararenes. The sanguinarine was used as a model natural medicinal agent belong to the class of alkaloids and is indicated for various types of microbial infections, inflammations and cervical cancer
[64]. Nguyen et al. (2017) used four different types of steviol glycosides for complexation of epigallocatechin gallate namely rubusoside, stevioside, rebaudioside A and stevioside glucosides. The author reported improved antibacterial activity, antioxidant activity and 16- to 17.5-fold enhanced solubility achievement through the complexes. Furthermore, among the aforementioned four complexes highest water solubility was achieved through the epigallocatechin gallate-stevioside complex
[65]. The recent studies are associated with the in silico modeling of tropane alkaloids and beta cyclodextrins complex through Molecular dynamic simulation and docking study. The author stated that directly proportional behavior of solubility and beta cyclodextrins was observed. Analytical and in silico study was of the inclusion complexes between tropane alkaloids atropine and scopolamine with cyclodextrins
[66]. The most recent study by Ko et al. (2021) also highlighted the use of steviol glycosides for the conversion of poorly water soluble curcumin into water soluble curcuminoids (Curcumin-steviol glycosides complex) for antiviral theapy. Moreover, the author reported the novel method of Microwave-assisted extraction and stated that by using in conjunction with the Curcumin-steviol glycosides complex several folds enhanced solubility (11 mg/L to 1320 mg/L) was achieved
[67].