Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hao Xu | -- | 2411 | 2022-06-15 04:25:52 | | | |
| 2 | Lindsay Dong | -5 word(s) | 2406 | 2022-06-15 11:21:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xu, H.; , .; Yan, W. Preparation Methods of Titanium Sub-Oxides Electrode. Encyclopedia. Available online: https://encyclopedia.pub/entry/24041 (accessed on 05 February 2026).
Xu H, , Yan W. Preparation Methods of Titanium Sub-Oxides Electrode. Encyclopedia. Available at: https://encyclopedia.pub/entry/24041. Accessed February 05, 2026.
Xu, Hao, , Wei Yan. "Preparation Methods of Titanium Sub-Oxides Electrode" Encyclopedia, https://encyclopedia.pub/entry/24041 (accessed February 05, 2026).
Xu, H., , ., & Yan, W. (2022, June 15). Preparation Methods of Titanium Sub-Oxides Electrode. In Encyclopedia. https://encyclopedia.pub/entry/24041
Xu, Hao, et al. "Preparation Methods of Titanium Sub-Oxides Electrode." Encyclopedia. Web. 15 June, 2022.
Copy Citation
Compared with the instability of graphite electrodes, the high expenditure of noble metal electrodes and boron-doped diamond electrodes, and the hidden dangers of titanium-based metal oxide electrodes, a titanium sub-oxide material has been characterized as an ideal choice of anode material due to its unique crystal and electronic structure, including high conductivity, decent catalytic activity, intense physical and chemical stability, corrosion resistance, low cost, and long service life, etc.
electrocatalytic oxidation
anode materials
Magnéli phase
titanium oxide
1. Electrocatalytic Oxidation Technologies
The rapid development of modern industries in various fields directly brings about growing pollution and ever-increasing refractory and toxic substances in the different liquid effluent. Traditional wastewater treatment methods have difficulty meeting the environmental requirements for such organic wastewater [1]. Advanced oxidation technology has developed rapidly in recent years as a practical approach to constructing a carbon-neutral treatment mode and realizing the green development of wastewater treatment technologies. Electrocatalytic oxidation technology is a promising way of removing organic pollutants. It is a green chemical technology that adopts electrodes with favorable catalytic performance to generate hydroxyl radicals (or other radicals and groups) with strong oxidation ability and react with the organic pollutants in the solution to decompose them into H2O and CO2 [2][3]. Compared with traditional water purification technologies, it has drawn significant interest thanks to its advantages, such as strong oxidation capacity, with no chemical agents added, and clean and mild conditions [4][5][6].
At present, there are three types of titanium sub-oxide materials used in the electrocatalytic oxidation wastewater systems, including a coated electrode, integrated electrodes, and composite electrodes. Different kinds of electrodes have their own advantages and disadvantages. Many researchers have made many contributions in optimizing the preparation process and improving the performance of electrodes, which is conducive to broadening the application prospect of titanium sub-oxides electrodes.
2. Preparation Methods of Titanium Sub-Oxides Electrode
2.1. Preparation of Titanium Sub-Oxides Powder
As the primary raw material for electrode preparation, titanium sub-oxides powder has been used in many processes. Current synthesis methods of titanium sub-oxides powder focus on reducing of titanium dioxide at a high temperature between 600 and 1000 °C [7][8][9]. In addition, there are also studies on the preparation of titanium sub-oxides powder using metallic titanium, organic titanium, inorganic titanium, and other raw materials as precursors [10][11][12]. Briefly, titanium dioxide is used as a prevalent raw material due to its abundant reserves and relatively low cost [13]. Table 1 lists the principles and process comparisons of the three methods of carbothermal reduction, hydrogen reduction, and metallothermic reduction using titanium dioxide as the precursor.
Table 1. Synthesis methods of titanium sub-oxides powder with TiO2 as precursor.
| Synthesis Method | Principle | Process Condition and Results | References |
|---|---|---|---|
| Carbothermal reduction | nTiO2(s) + C(s) = TinO2n−1(s) + CO(g) | C-Ti4O7 was obtained at 1025 °C in N2 gas flow. | [14] |
| Hydrogen reduction | nTiO2(s) + H2(g) = TinO2n−1(s) + H2O(g) | Ti4O7 was obtained at 1050 °C in H2 gas flow. | [15] |
| Metallothermic reduction | nTiO2(s) + Me(s) = TinO2n−1(s) + MeO(s) | Ti4O7and Ti6O11 were obtained by mechanical activation of Ti and TiO2 powder and annealing at 1333–1353 K in Ar gas flow for 4 h. | [16] |
| (2n−1) TiO2(s) + Ti(s) = 2TinO2n−1(s)(n = 1,2,3…) | With silicon as reducing agent and calcium chloride as additive, TiO2 powder was reduced to various mixed phases of titanium sub-oxide under different experimental conditions. | [17] |
2.2. Titanium Sub-Oxides-Coated Electrode
Titanium sub-oxides-coated electrode refers to the preparation of titanium sub-oxides powder by reduction and other methods, which is then coated or deposited on the anode substrate, which is adopted as a catalytic electrode. The preparation methods of coating electrodes mainly include the coating methods, magnetron sputtering methods, the electrodeposition method, and the sol–gel method, etc.
2.2.1. Coating Method
The coating method includes coating, spraying, and powder curing technology, among which plasma spraying is a new kind of multipurpose spraying method with relative maturity and precision. Thanks to its characteristics of ultra-high temperature, fast jet particles, compact coating density, high bond strength, inert gas as the working gas, and no easy oxidation of spraying material, etc., it is widely used in the preparation of titanium sub-oxides electrodes. Teng et al. [18] coated titanium sub-oxides powder on titanium mesh and titanium plate by plasma spraying technology, which successfully prepared a Ti/Ti4O7 electrode. Ti4O7 powder was approximately spherical and uniformly and compactly covered on a Ti matrix, providing a large surface area and more active sites for the electrochemical reaction. The electrode had high conductivity and low oxygen evolution potential, presenting fair oxidation activity to sulfadiazine. Soliu et al. [19] also prepared a Ti/Ti4O7 electrode by plasma spraying technology, which was employed for the electrocatalytic degradation of amoxicillin. In the plasma spraying system, 20–60 μm of Ti4O7 particles were sprayed on the surface of the pretreated titanium plate under the conditions of a 700 A current, argon, and with hydrogen-mixed gas (19.6% H2) as the carrier at 2 rpm.
Al, carbon cloth, and other conductive substrates are also alternatives for the matrix and the coating on a titanium base. Han et al. [20] successfully coated Ti4O7 ceramic particles on an Al substrate by plasma spraying. The system was operated under the condition of a 10 V spray voltage in a nitrogen atmosphere, and the accelerated life of the prepared electrode was 69.6% longer than that of the commonly used titanium-based SnO2 electrode. The current efficiency and energy consumption were increased by 5.59% and 16.2%, respectively, with electrochemical performance greatly improved. Geng et al. [21] impregnated a layer of TiO2 on a porous Al2O3 matrix by the coating reduction method. The as-prepared electrode was aged at room temperature for 2 h, dried at 100 °C for 1 h, and sintered in air at 800 °C, which was all repeated two to eight times, and it was finally reduced in the H2 atmosphere at 1050 °C to obtain the Ti4O7 layer. The conductivity of the prepared electrode is more than 200 S cm−1, with the particle size of the coating in the 200–300 nm range and the average pore size being 350 nm, exhibiting superior electrochemical properties.
This technology pretreated the substrate and coated the anode surface with a dense and uniform Ti4O7 particle coating, which has the advantages of simple operation, low equipment maintenance cost, and flexible regulation performance. However, there are still technical problems, such as the weak adhesion between the substrate and the coating, which impairs the stability of the electrode.
2.2.2. Magnetron Sputtering Method
Magnetron sputtering is a physical vapor deposition method with the advantages of easy control, a large coating area, and strong adhesion. In Ar + O2 plasma, Wong et al. [22] deposited a series of TiOx(0 < x ≤ 2) films with different O/Ti ratios on silicon substrates by reactive magnetron sputtering and discussed the effects of the oxygen flow rate and substrate temperature on the structural properties, mechanical properties, and electrical properties of the films, respectively. The results showed that the titanium, Ti(-O) solid solution, TiO, Ti2O3, Magnéli, rutile, and anatase TiO2 phases appeared successively with the increase of the oxygen flow rate. The conductivity decreased with the rise of oxygen content but increased with the elevation of the deposition temperature. The hardness of the films changed in the order of TiO > Ti2O3 > Ti4O7. Titanium sub-oxides films with high performance can be obtained at the deposition temperature of 500 °C. These thin films possessed properties in terms of a small crystal diameter, the proper crystal structure of defects and grain boundaries, and good electrocatalytic oxidation performance.
This technology is easy to control, with a stable prepared electrode morphology and a strong coating adhesion. In contrast, the composition is relatively impure, and is greatly affected by various preparation factors.
2.2.3. Electrodeposition Method
Ertekin et al. [23] successfully prepared titanium sub-oxide thin-film electrodes by the electrochemical deposition of titanium peroxide solution on indium tin oxide-coated glass with acetonitrile and hydrogen peroxide as supporting electrolytes. Ertekin et al. confirmed the feasibility of the electrochemical deposition of crystalline TinO2n−1 film, a preparation method with an electrochemical application prospect. SEM characterization in At different electrochemical deposition potentials and temperatures, TinO2n−1 was Ti3O5, Ti4O7, and Ti5O9, among which there was a low content of the Ti4O7 phase with the best performance.
This method is simple to operate with a relatively low cost. However, much the same as magnetron sputtering, the biggest problem of this technology is that the coating composition is not easy to control, which will have a significant influence on the performance of the electrode.
2.2.4. Sol–Gel Method
The sol–gel method can reduce the temperature required for titanium sub-oxide electrode preparation. Sasmita et al. [24] used synthetic titanium dioxide powder dispersed in an aqueous solution of polyacrylic acid as a raw material, with the addition of monomer (MAM) and a crosslinking agent (MBAM) to the slurry in a ratio of 4:1. Next, 0.01 wt% APS was added to the mix for 6 h. The slurry was injected into a Teflon mold by adding 10 mL of TEMED to the slurry for catalytic polymerization. Polymerization took place within 5 min, and the pellets were cast. After drying at 50 °C, gel-cast electrodes were prepared by sintering for 6 h in a tubular furnace at 1050 °C.
2.3. Titanium Sub-Oxides-Integrated Electrode
The methods of preparing integrated electrodes mainly include compression reduction, powder sintering, and membrane preparation by hydrothermal reduction, etc. The preparation diagrams of different processes are shown in Figure 1.
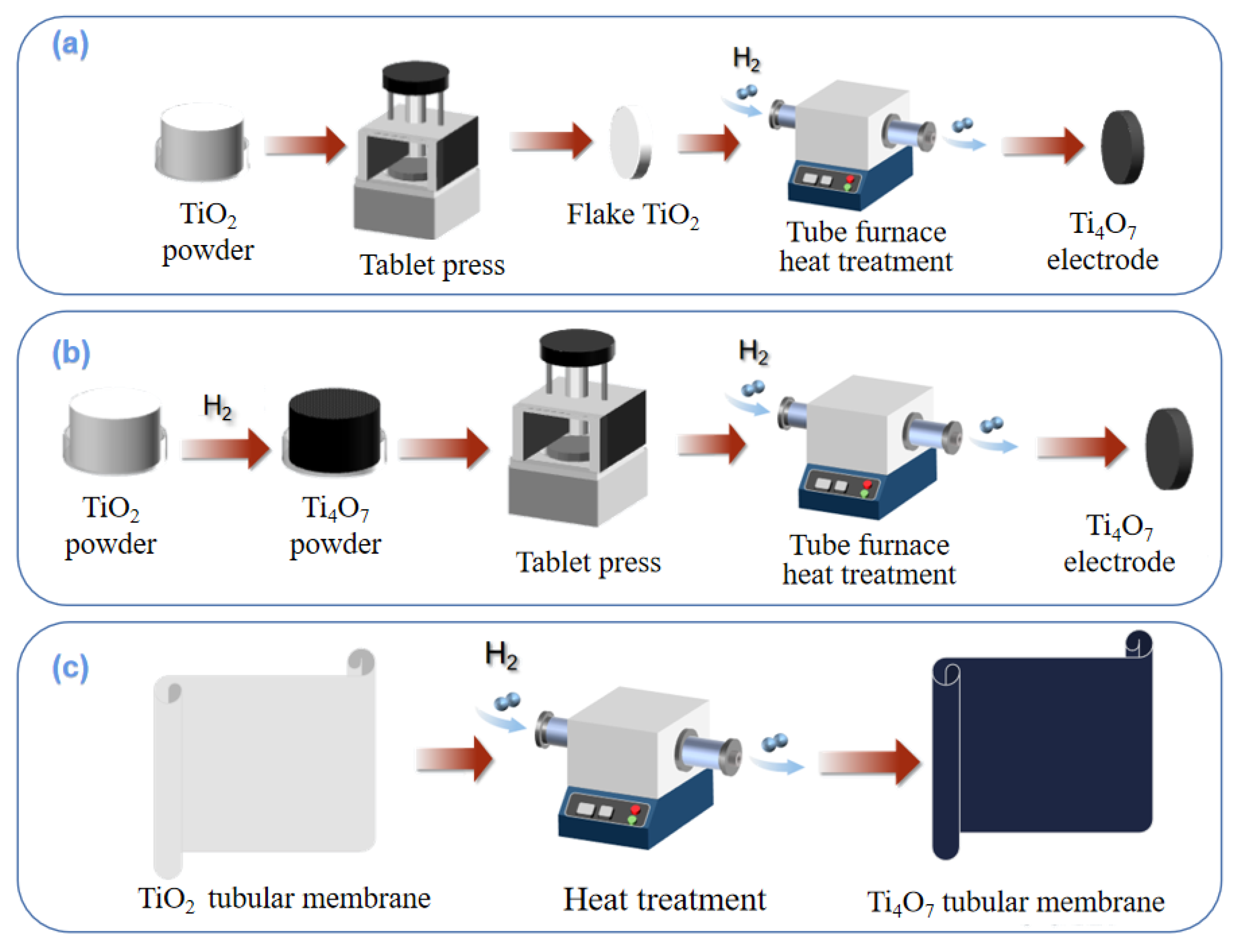
Figure 1. Preparation processes of compression reduction method (a); powder sintering method (b); membrane preparation by hydrothermal reduction method (c).
2.3.1. Compression Reduction Method
As shown in Figure 1a, TiO2 powder is first pressed, molded, and then calcined in a reducing atmosphere to produce a titanium sub-oxides-integrated electrode. Regonini et al. [25] mixed titanium dioxide particles with polyethylene glycol as binder under pressure, sintered them into spheres, and then reduced them into titanium sub-oxide electrodes under the protection of Ar gas at 1300 °C.
2.3.2. Powder Sintering Method
The powder sintering method can effectively avoid the disadvantages of the compression reduction method. The technical route is shown in Figure 1b, where titanium sub-oxides powder is prepared before compression sintering. Lin et al. [26] first synthesized Ti4O7 nanopowder by reducing titanium dioxide nanopowder at 950 °C in a H2 atmosphere. Then, the prefabricated Ti4O7 nanopowder was mixed with polyacrylamide/polyvinyl alcohol binder to form slurry, and spray dried to small ceramic particles (40–80 mesh, water volume 5%). After the ceramic particles were loaded into the mold and fully vibrated, the prefabricated ceramic parts were pressed for 5 min by a 60 MPa static press. The macroporous Ti4O7 ceramic-integrated electrode was successfully prepared by drying the ceramic preform and sintering it for 11 h in a vacuum at 1350 °C. The average pore size of the electrode surface was 2.6 μm, and the porosity was 21.6%. The electrode had excellent electrochemical performance, including ultra-high oxygen evolution potential, decent electrochemical stability, high electroactive surface, and weak adsorption performance.
2.3.3. Membrane Preparation by Hydrothermal Reduction Method
A tubular titanium dioxide membrane can also be used to synthesize a titanium sub-oxides electrode, where titanium dioxide will be reduced to Ti4O7 after hydrothermal treatment. The specific path is shown in Figure 1c. Guo et al. [26] used a tubular titanium dioxide ultrafiltration membrane and reduced it to Ti4O7 by a hydrothermal method in a tubular furnace at 1050 °C in a H2 atmosphere. With different treatment times, the main composition of the membrane also changed, and the phase change from Ti6O11 to Ti4O7 occurred from 30 h to 50 h. Based on this, Liang et al. [27] designed a set of tubular electrode assembly reactors using titanium oxide thin film, in which stainless steel tubes (SSP) were used as cathodes, and tubular titanium oxide film was placed in the center of the tubes as anodes, making full use of advantages such as the good stability and low internal resistance of thin-film electrodes. The optimal conditions for the catalytic degradation of methylene blue are as follows: the removal rate was close to 100% when the current density was 9 mA·cm−2 for 90 min.
2.4. Titanium Sub-Oxides Composite Electrode
The composite electrode is also a recent research hotspot for electrode performance improvement. It can be mainly divided into titanium sub-oxides modified by other kinds of electrodes and titanium sub-oxides doped with other metals.
2.4.1. Titanium Oxide-Doped Composite Electrode
By doping Magnéli phase Ti4O7, the electrocatalytic activity and the stability and conductivity of specific electrode materials can be increased to some extent. Zhang et al. [28] prepared a Ru@Pt-type core–shell catalyst containing Ti4O7 (Ru@Pt/Ti4O7) by microwave pyrolysis. In this method, the mixture of the ruthenium precursor and TiO2 was heat-treated in a H2 atmosphere, Ru nuclei were obtained on the Ti4O7 carrier, and platinum shells were generated by microwave radiation. The characterization results showed that the catalytic material had a core–shell structure of Ru core and Pt shell, which significantly improved PT-Ru’s durability. Zhao et al. [29] synthesized a MoS2/Ti4O7 composite HER electrocatalyst by the hydrothermal method. Under the condition of 0.5 M H2SO4, the hydrogen evolution activity of the MoS2/Ti4O7 composite catalyst was significantly improved. The results presented that, with the addition of conductive carrier Ti4O7, the HER activity of MoS2 could be significantly enhanced by the formation of an interface SeO-Ti bond, thus improving the electrochemically active surface area fast charge transferability.
2.4.2. Metals-Doped Composite Titanium Sub-Oxides Electrode
In addition, there are many types of research on the modification of titanium sub-oxides electrodes doped with other metals. For example, Linet et al. [27] doped a Ce3+ electrode preparation by the powder sintering method. Compared with the electrode before doping, the increased oxygen evolution potential and the reduced internal resistance of the charge transfer of the prepared electrode could be obtained, and the degradation effect of perfluorooctane sulfonic acid (PFOS) was further improved because the doping of Ce3+ resulted in more active sites on the electrode surface, which improved the rapid transfer of charge. Huang et al. [30] studied the effects of loaded crystalline and amorphous Pd on titanium sub-oxides electrodes prepared by plasma spraying technology. The results showed that the performance of electrodes doped with Pd was greatly improved. The effect of amorphous Pd was more obvious due to the disordered atomic arrangement of amorphous metals and the unsaturated coordination of amorphous metals. The amorphous metal formed a stronger interaction with the substrate, and this electron-metal interaction support (EMSI) enhanced electron transfer, which ultimately promoted the oxidation kinetics of the electrode. Doping Pt, Ce, Pd, and other active substances positively affects the performance of the titanium sub-oxides electrode, which means it is of further research interest.
References
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261.
- Panizza, M.; Michaud, P.-A.; Iniesta, J.; Comninellis, C.; Cerisola, G. Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim. Acta 2001, 46, 3573–3578.
- Lei, J.; Xu, Z.; Xu, H.; Qiao, D.; Liao, Z.; Yan, W.; Wang, Y. Pulsed electrochemical oxidation of acid Red G and crystal violet by PbO2 anode. J. Environ. Chem. Eng. 2020, 8, 103773.
- Zhang, Y.; Zhang, C.; Shao, D.; Xu, H.; Rao, Y.; Tan, G.; Yan, W. Magnetically assembled electrodes based on Pt, RuO2-IrO2-TiO2 and Sb-SnO2 for electrochemical oxidation of wastewater featured by fluctuant Cl− concentration. J. Hazard. Mater. 2022, 421, 126803.
- Kobya, M.; Hiz, H.; Senturk, E.; Aydiner, C.; Demirbas, E. Treatment of potato chips manufacturing wastewater by electrocoagulation. Desalination 2006, 190, 201–211.
- Dargahi, A.; Shokoohi, R.; Asgari, G.; Ansari, A.; Nematollahi, D.; Samarghandi, M.R. Moving-bed biofilm reactor combined with three-dimensional electrochemical pretreatment (MBBR-3DE) for 2,4-D herbicide treatment: Application for real wastewater, improvement of biodegradability. RSC Adv. 2021, 11, 9608–9620.
- Xu, B.; Sohn, H.Y.; Mohassab, Y.; Lan, Y. Structures, preparation and applications of titanium suboxides. RSC Adv. 2016, 6, 79706–79722.
- Malik, H.; Sarkar, S.; Mohanty, S.; Carlson, K. Modelling and synthesis of Magnéli Phases in ordered titanium oxide nanotubes with preserved morphology. Sci. Rep. 2020, 10, 8050.
- Zhang, X.; Liu, Y.; Ye, J.; Zhu, R. Fabrication and characterisation of Magnéli phase Ti4O7 nanoparticles. Micro Nano Lett. 2013, 8, 251–253.
- Xu, B.; Zhao, D.; Sohn, H.Y.; Mohassab, Y.; Yang, B.; Lan, Y.; Yang, J. Flash synthesis of Magnéli phase (TinO2n−1) nanoparticles by thermal plasma treatment of H2TiO3. Ceram. Int. 2018, 44, 3929–3936.
- Han, W.-Q.; Wang, X.-L. Carbon-coated Magnéli-phase TinO2n-1 nanobelts as anodes for Li-ion batteries and hybrid electrochemical cells. Appl. Phys. Lett. 2010, 97, 243104.
- Conze, S.; Veremchuk, I.; Reibold, M.; Matthey, B.; Michaelis, A.; Grin, Y.; Kinski, I. Magnéli phases Ti4O7 and Ti8O15 and their carbon nanocomposites via the thermal decomposition-precursor route. J. Solid State Chem. 2015, 229, 235–242.
- Wang, G.; Liu, Y.; Ye, J.; Qiu, W. Synthesis, microstructural characterization, and electrochemical performance of novel rod-like Ti4O7 powders. J. Alloy. Compd. 2017, 704, 18–25.
- Toyoda, M.; Yano, T.; Tryba, B.; Mozia, S.; Tsumura, T.; Inagaki, M. Preparation of carbon-coated Magnéli phases TinO2n−1 and their photocatalytic activity under visible light. Appl. Catal. B-Environ. 2009, 88, 160–164.
- You, S.; Liu, B.; Gao, Y.; Wang, Y.; Tang, C.Y.; Huang, Y.; Ren, N. Monolithic Porous Magnéli-phase Ti4O7 for Electro-oxidation Treatment of Industrial Wastewater. Electrochim. Acta 2016, 214, 326–335.
- Gusev, A.; Avvakumov, E.; Medvedev, A.; Masliy, A. Ceramic electrodes based on Magnéli phases of titanium oxides. Sci. Sinter. 2007, 39, 51–57.
- Hauf, C.; Kniep, R.; Pfaff, G. Preparation of various titanium suboxide powders by reduction of TiO2 with silicon. J. Mater. Sci. 1999, 34, 1287–1292.
- Teng, J.; Liu, G.; Liang, J.; You, S. Electrochemical oxidation of sulfadiazine with titanium suboxide mesh anode. Electrochim. Acta 2019, 331, 135441.
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Esmilaire, R.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electrooxidation of organic pollutants: A case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res. 2016, 106, 171–182.
- Han, Z.; Xu, Y.; Zhou, S.; Zhu, P. Preparation and electrochemical properties of Al-based composite coating electrode with Ti4O7 ceramic interlayer for electrowinning of nonferrous metals. Electrochimica Acta 2019, 325, 134940.
- Geng, P.; Chen, G. Magnéli Ti4O7 modified ceramic membrane for electrically-assisted filtration with antifouling property. J. Membr. Sci. 2016, 498, 302–314.
- Wong, M.-S.; Lin, Y.-J.; Pylnev, M.; Kang, W.-Z. Processing, structure and properties of reactively sputtered films of titanium dioxide and suboxides. Thin Solid Films 2019, 688, 137351.
- Ertekin, Z.; Tamer, K.; Pekmez, U. Cathodic electrochemical deposition of Magnéli phases TinO2n-1 thin films at different temperatures in acetonitrile solution. Electrochim. Acta 2015, 163, 77–81.
- Nayak, S.; Chaplin, B.P. Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes. Electrochim. Acta 2018, 263, 299–310.
- Regonini, D.; Adamaki, V.; Bowen, C.R.; Pennock, S.R.; Taylor, J.; Dent, A.C.E. AC electrical properties of TiO2 and Magnéli phases, TinO2n−1. Solid State Ion. 2012, 229, 38–44.
- Guo, L.; Jing, Y.; Chaplin, B.P. Development and Characterization of Ultrafiltration TiO2 Magnéli Phase Reactive Electrochemical Membranes. Environ. Sci. Technol. 2016, 50, 1428–1436.
- Liang, J.; You, S.; Yuan, Y.; Yuan, Y. A tubular electrode assembly reactor for enhanced electrochemical wastewater treatment with a Magnéli-phase titanium suboxide (M-TiSO) anode and in situ utilization. RSC Adv. 2021, 11, 24976–24984.
- Zhang, L.; Kim, J.; Zhang, J.; Nan, F.; Gauquelin, N.; Botton, G.A.; He, P.; Bashyam, R.; Knights, S. Ti4O7 supported core-shell catalyst for CO-tolerance in PEM fuel cell hydrogen oxidation reaction. Appl. Energy 2013, 103, 507–513.
- Zhao, J.; Li, W.; Wu, S.; Xu, F.; Du, J.; Li, J.; Li, K.; Ren, J.; Zhao, Y. Strong interfacial interaction significantly improving hydrogen evolution reaction performances of MoS2/Ti4O7 composite catalysts. Electrochim. Acta 2020, 337, 135850.
- Huang, D.; Wang, K.; Niu, J.; Chu, C.; Weon, S.; Zhu, Q.; Lu, J.; Stavitski, E.; Kim, J.-H. Amorphous Pd-Loaded Ti4O7 Electrode for Direct Anodic Destruction of Perfluorooctanoic Acid. Environ. Sci. Technol. 2020, 54, 10954–10963.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
17 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




