Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chiao-Ping Chen | -- | 1996 | 2022-06-14 06:10:55 | | | |
| 2 | Conner Chen | Meta information modification | 1996 | 2022-06-14 08:13:51 | | | | |
| 3 | Chiao-Ping Chen | Meta information modification | 1996 | 2022-06-14 11:36:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, C.; Cheng, C.; Wu, C. Precision Medicine in Cholangiocarcinoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/23995 (accessed on 07 February 2026).
Chen C, Cheng C, Wu C. Precision Medicine in Cholangiocarcinoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/23995. Accessed February 07, 2026.
Chen, Chiao-Ping, Chi-Yuan Cheng, Chiao-En Wu. "Precision Medicine in Cholangiocarcinoma" Encyclopedia, https://encyclopedia.pub/entry/23995 (accessed February 07, 2026).
Chen, C., Cheng, C., & Wu, C. (2022, June 14). Precision Medicine in Cholangiocarcinoma. In Encyclopedia. https://encyclopedia.pub/entry/23995
Chen, Chiao-Ping, et al. "Precision Medicine in Cholangiocarcinoma." Encyclopedia. Web. 14 June, 2022.
Copy Citation
Cholangiocarcinoma (CCA), or biliary tract cancer, has a poor prognosis. The median survival time among patients with CCA is under 2 years from diagnosis, and the global 5-year survival rate is only 10%. First-line therapy with chemotherapeutic agents, gemcitabine plus cisplatin, has traditionally been used to treat unresectable advanced CCA. Precision medicine has become a mainstream cancer treatment due to innovative next-generation sequencing technology.
cholangiocarcinoma (CCA)
targeted therapy
1. Introduction
Cholangiocarcinoma (CCA), or biliary tract cancer (BTC), is a malignancy arising from the epithelium of the bile ducts [1]. Chronic inflammation in parasitic infections, primary sclerosing cholangitis, congenital fibropolycystic liver, hepatitis B and C viruses, and Caroli’s diseases are major causes of CCA [2][3]. Based on its anatomical origin, CCA can be either intrahepatic (iCCA) or extrahepatic (eCCA). The latter can be subdivided into perihilar (pCCA) and distal (dCCA). iCCA, pCCA, and dCCA account for 5–10%, 60–70%, and 20–30% of CCA cases, respectively [4]. In addition to CCA, both gallbladder and ampullary cancers are considered subtypes of BTC. The following content focuses on CCA as a distinct genetic alteration between CCA and other malignancies.
CCA has a poor prognosis, as most cases are diagnosed at an advanced stage and respond poorly to current systemic treatment [1]. The CCA patients have medium survival of fewer than two years. In total, 90% of patients do not live more than five years after the first diagnosis [4]. Moreover, although the early stage of CCA can be completely removed by surgical resection, most patients experience recurrence within 2 years [5][6]. In terms of advanced or recurrent CCA, the median survival time is under 1 year [4][7][8], showing that it is a highly aggressive cancer that requires urgent attention.
First-line therapy with gemcitabine plus cisplatin, two chemotherapeutic agents, has traditionally been used for unresectable advanced CCA, despite the low efficacy of this approach based on the ABC-02 trial in 2010 [4][7][8]. Titanium silicate (TS-1) is another commonly used cytotoxic agent in Asia [9] and TS-1-based combinations, such as gemcitabine plus TS-1, have been widely studied [10][11]. Nevertheless, the lack of breakthroughs using these agents has given rise to the era of targeted therapy and immunotherapy. In recent years, the application of next-generation sequencing (NGS) technology has improved the knowledge of the molecular biology of cancer, as well as genetic alterations, and has expedited developments in targeted therapy for these alterations. Precision medicine involves the selection of suitable drugs, therapies, or preventive strategies based on an individual’s genotype or gene expression profile and clinical data, thereby achieving maximum therapeutic efficacy and minimal side effects [12][13][14].
2. Molecular Biological Classification of CCA
CCA can be divided into four clusters based on the genetic alterations and clinical features, in particular, whether it is associated with flukes and whether it is internal or external (Figure 1) [7]. Mutations in IDH1, IDH2, BAP1, and rearrangements in FGFR2 are often present in iCCA, whereas HER2 and TP53 mutations are frequent in eCCA. Among the above genetic alterations, FGFR2 fusions may be associated with the best prognosis [7]. Although both iCCA and eCCA originate from the bile ducts, the distinct etiology may contribute to the different genetic alterations in CCA. The acronyms and full names of the genes are listed in Table 1.
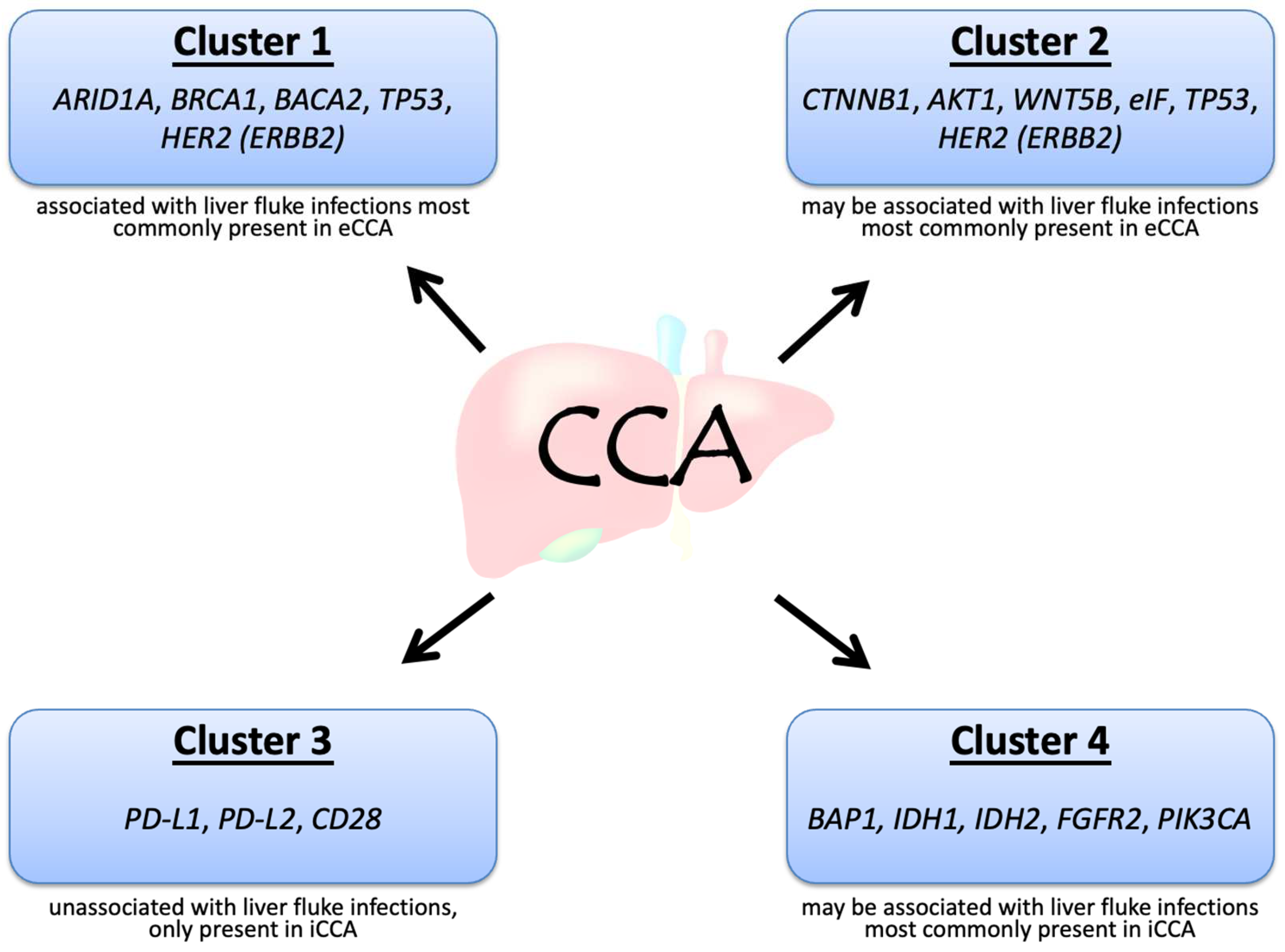
Figure 1. Molecular biological clusters of cholangiocarcinoma.
Table 1. Acronyms and full names of genes involved in cholangiocarcinoma.
| ARID1A | AT-rich interaction domain 1A |
| AKT1 | Protein Kinase B |
| BAP1 | BRCA associated protein 1 |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| BRCA1/2 | Breast cancer gene 1/2 |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| CTNNB1 | Catenin beta 1 |
| EGFR | Epidermal growth factor receptor |
| eIF | Eukaryotic initiation factor |
| FGFR2 | Fibroblast growth factor receptor 2 |
| HER2 | Human epidermal growth factor receptor 2 |
| IDH1/2 | Isocitrate dehydrogenase ½ |
| JAK/STAT | Janus Kinase/Signal transducer and activator of transcription |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| MEK | Mitogen-activated protein kinase |
| MET | MET proto-oncogene, receptor tyrosine kinase |
| mTOR | Mammalian target of rapamycin |
| NRAS | Neuroblastoma ras viral oncogene homolog |
| NTRK | Neurotrophic tyrosine receptor kinase |
| PBRM1 | Poly-bromo1 |
| PIK3CA | Phosphatidylinotitol 3-kinase catalytic subunit alpha |
| PTEN | Phosphatase and tensin homolog |
| ROS1 | ROS proto-oncogene 1 |
| TP53 | Tumor protein 53 |
| VEGF | Vascular endothelial growth factor |
| WNT5B | Wnt family 5B gene |
In addition, the distinct genetic landscape of iCCA was found in Western and Asian populations [15]. Patients with a higher burden of DNA repair mutations and frequency consistent with high tumor mutational burden (TMB-H) have been reported in the Asian population than among Western patients [15]. This finding suggests the need for different therapeutic strategies, such as immunotherapy, in different populations.
3. Genetic Alterations in CCA and Clinical Trials of Targeted Therapies
Genetic alterations in CCA, including mutations, gene fusions, and copy number variations, can disrupt DNA repair, cell cycle regulation, signal transduction of receptor tyrosine kinases, and epigenetic regulation. Table 2 shows a list of genetic alterations in CCA and corresponding clinical trials [16][17][18]. Some of the genetic alterations currently have no clinical features; therefore, these potential targeted agents based on preclinical studies that are yet to be validated in human studies (Figure 2).
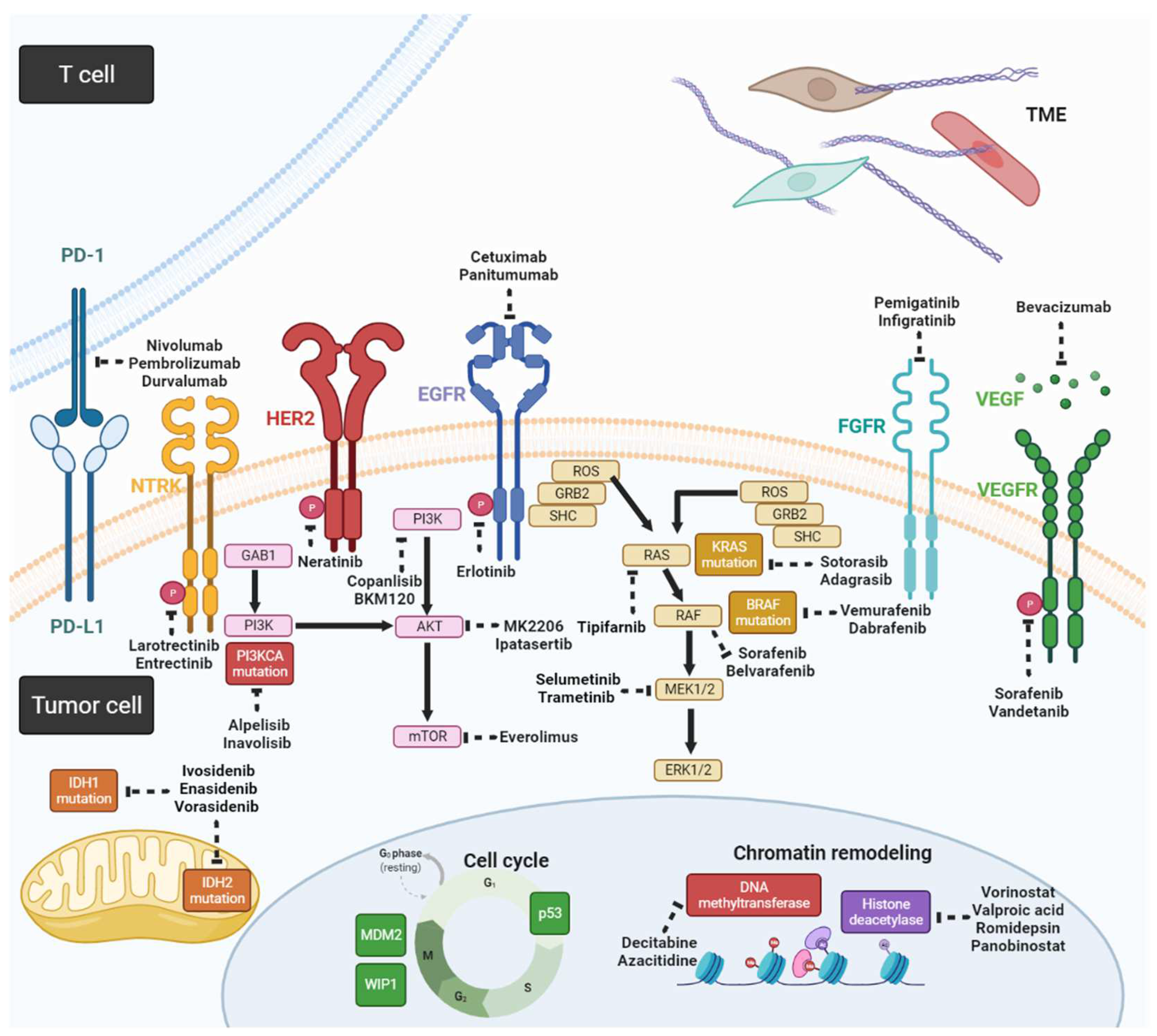
Figure 2. Schema for potential targeted agents and related signaling pathways in CCA (created with https://biorender.com/ (accessed on 25 May 2022)).
Table 2. Potential candidates in cholangiocarcinoma treatment by target gene alterations.
| Genetic Alteration | Targeted Therapies |
|---|---|
| ARID1A | HDAC inhibitors, EZH2 inhibitors, PARP inhibitors, mTOR inhibitors |
| BAP1 | HDAC inhibitors, EZH2 inhibitors, PARP inhibitors |
| BRAF | Dabrafenib, Vemurafenib, Trametinib, Selumetinib |
| CDKN2A | CDK4/6 inhibitors |
| EGFR | Cetuximab |
| FGFR2 | Infigratinib, Derazantinib, Erdafitinib, Futibatinib, Pemigatinib, Ponatinib, Debio 1347, FRA144, INCB054828, NVP-BGJ398, INCB054828 |
| HER2 | Trastuzumab |
| IDH1 | Ivosidenib (AG-120), Vorasidenib (AG-881) |
| IDH2 | Enasidenib (AG-221), Vorasidenib (AG-881) |
| JAK/STAT | Tofacitinib, Baricitinib, Peficitinib, Upadacitinib, Filgotinib |
| KRAS | MEK inhibitors: Trametinib, Selumetinib |
| MET | Capmatinib, Tepotinib |
| NRAS | MEK inhibitors: Trametinib, Selumetinib |
| NTRK | larotrectinib or entrectinib |
| PBRM1 | PARP inhibitors, Immume checkpoint inhibitors |
| PIK3CA | PIK3CA/AKT/mTOR inhibitors, Copanlisib, BKM12, MK2206, everolimus |
| PTEN | PIK3CA/AKT/mTOR inhibitors |
| ROS1 | Crizotinib, Ceritinib |
| TP53 | Wee1 inhibitors: Adavosertib (AZD1775), MDM2 inhibitors: idasanutin |
| VEGF | Bevacizumab, Sorafenib, Vandetanib, Regorafenib, Ramucirumab |
3.1. Fibroblast Growth Factor Receptor 2 Gene Fusions
Fibroblast growth factor receptors (FGFR, including FGFR1-5) comprise a family of receptor tyrosine kinases that regulate cell proliferation, differentiation, and migration upon the stimulation of fibroblast growth factor (FGF) [19]. FGFR gene alterations include indel mutations, amplifications, and fusions, leading to gain-of-function, which may drive CCA progression. Several small-molecule targeted therapies that block the FGF/FGFR signaling axis by tyrosine kinase inhibitors (TKIs) have been studied in CCA with FGFR alterations [20] and only CCA with FGFR2 fusion can benefit from FGFR inhibitors. FGFR2 gene fusions, such as FGFR2–BICCI, FGFR2–AHCYL1, FGFR2–TACC3, and FGFR2–KIAA1598, are closely associated with tumor progression in iCCA [7]. The outcomes of clinical trials of small-molecule targeted therapies for these genetic alterations are presented in Table 3 [7][13]. Previous results indicated a maximum disease control rate (DCR) of 80% and progression-free survival (PFS) of approximately 6 months [14][20][21]; therefore, FGFR inhibitors are promising drugs for targeted therapies for CCA with FGFR2 fusions. The U.S. Food and Drug Administration (FDA) approved the use of pemigatinib and infigratinib for treating CCA patients with FGFR2 gene fusions [22]. In addition, other FGFR inhibitors are under investigation in clinical trials.
Table 3. Outcomes of clinical trials of small-molecule targeted therapies for fibroblast growth factor receptor (FGFR) pathways.
| Targeted Therapy | FGFR | N | CR/PR (%) | SD (%) | DCR (%) | PFS (Months) |
|---|---|---|---|---|---|---|
| Pemigatinib | FGFR 1–3 | 107 | 35.5 | 46.7 | 82.5 | 6.9 |
| Infigratinib | FGFR 1–3 | 108 | 23.1 | NR | NR | 7.3 |
| Derazantinib | FGFR 1–3 | 29 | 20.7 | 62.1 | 82.8 | 5.7 |
| Erdafitinib | FGFR 1–4 | 11 | 27 | 27 | 55 | 5.1 |
| Futibatinib | FGFR 1–4 | 45 | 25 | 53 | 79 | NR |
3.2. Isocitrate Dehydrogenase 1 and 2 Mutations
Isocitrate dehydrogenase (IDH) is an enzyme involved in intracellular glucose metabolism. IDH1 and IDH2 mutations frequently occur in various myeloid malignancies and solid tumors [21]. Neomorphic mutation of IDH proteins produces the oncometabolite D-2-hydroxyglutarate, which blocks cellular differentiation via the inhibition of α-ketoglutarate-dependent dioxygenases involved in histone and DNA demethylation [23]; therefore, IDH mutations can result in abnormal cellular glucose metabolism, leading to DNA hypermethylation, abnormal cell proliferation, and angiogenesis [24]. IDH mutations are seemingly exclusive to iCAA rather than to eCCA. The results of a phase I study on CCA patients with IDH1 mutations treated with the IDH1 inhibitor ivosidenib (AG-120) revealed that the objective response rate (ORR) was 5%, median PFS was 3.8 months, and median overall survival (OS) time was 13.8 months [25]. In phase III, double-blind, randomized ClarIDHy clinical trial, ivosidenib was administered to patients with IDH1 mutations, while a placebo was administered to those in the control group. The results showed that the PFS of the ivosidenib group was 2.7 months while that of the placebo group was 1.4 months (hazard ratio 0.37, p < 0.001). The ivosidenib group had an ORR of 2.4% and a DCR of 53.2%, whereas the placebo group had an ORR of 0% and a DCR of 27.9% [26], demonstrating the clinical efficacy of ivosidenib. Currently, it is approved for use in patients with IDH1 mutations in acute myeloid leukemia (AML) [27] and CCA [28]. Ivosidenib in combination with nivolumab is under investigation for IDH1 mutant solid cancers (NCT04056910).
In terms of IDH2 mutation, there are ongoing clinical trials on the application of the IDH2 inhibitor enasidenib (AG-221) (NCT02273739) and the pan-IDH inhibitor vorasidenib (AG-881), which inhibit IDH1 and IDH2 simultaneously in the treatment of CCA [24].
With the success of ivosidenib, targeting IDH mutations became possible; however, the activity of ivosidenib is limited, with a low ORR of 2.4%. The PFS benefit from ivosidenib largely results from a DCR of 53.2%; therefore, enhancing the activity of such inhibitors by modifying the structure or combination therapy should be a goal in patients with IDH-mutant CC.
3.3. Neurotrophic Tyrosine Receptor Kinase
Genomic translocation of the neurotrophic tyrosine receptor kinase gene (NTRK), which leads to the constitutive activation of receptor tyrosine kinases, is rare in pan-cancer, including CCA [29]; however, both larotrectinib and entrectinib have been approved for the treatment of cancers harboring NTRK gene rearrangements. Two of the fifty-five patients with various TRK fusion-positive malignancies were enrolled in three trials of larotrectinib treatment [30]. The overall ORR was 75%, and one out of two CCA cases had objective tumor shrinkage. Larotrectinib was approved by the FDA for use in adults and children with solid tumors with NTRK gene fusion in November 2018. Another drug, entrectinib, was approved by the FDA for cancers with an NTRK gene fusion in August 2019.
3.4. Overexpression of EGFR and HER2
Although epidermal growth factor receptor (EGFR) overexpression is rare, human epidermal growth factor receptor 2 (HER2) overexpression is common in CCA (particularly eCCA) and has a prevalence of 11–20% [4][18]. In terms of targeting EGFR, phase III studies on the use of the EGFR inhibitor erlotinib plus gemcitabine and cisplatin revealed that the addition of erlotinib to the gemcitabine/oxaliplatin regimen did not result in better PFS and OS outcomes than gemcitabine/oxaliplatin alone [31]. It is known that EGFR tyrosine kinase inhibitors (EGFR-TKIs) selectively target mutant EGFR better than wild-type EGFR; therefore, using EGFR-TKIs for CCA may not be a good option. In contrast, cetuximab is a monoclonal antibody that targets the wild-type EGFR; however, cetuximab also failed to demonstrate superior efficacy in a randomized phase II study [32][33], although a single-arm phase II study demonstrated promising results [34]. Interestingly, RAS mutation played no role in additional cetuximab treatment, which is a predictive factor for colorectal cancer. The addition of panitumumab, another monoclonal antibody targeting EGFR, to chemotherapy failed to show prolonged OS in a randomized phase II study of 89 patients with KRAS wild-type advanced BTC [35]. Further studies should focus on investigation-predictive biomarkers; otherwise, targeting EGFR may not be a possible strategy for CCA.
The use of trastuzumab, an antibody targeting HER2, in combination with chemotherapy showed a promising response in gallbladder cancer with HER2/neu genetic aberrations or protein overexpression, but no therapeutic effects on CCA in a small retrospective cohort [36]. Currently, there is an ongoing phase I study on the combined use of trastuzumab and tipifarnib (a farnesyltransferase inhibitor of RAS kinase) [37]. In addition, trastuzumab deruxtecan (DS-8201) is currently being studied in CCA patients with HER2 alterations [38]. DS-8201 has been approved for breast cancer with amplified/overexpressed HER2 [39] and has been widely studied in lung cancer, gastric cancer, and other solid cancer with HER2 alterations. Neratinib is an oral, irreversible, pan-HER tyrosine kinase inhibitor and is under investigation in a basket SUMMIT study (NCT01953926), which demonstrated that neratinib has encouraging clinical activity in multiple types of HER2-mutant solid tumor malignancies. Unfortunately, the study of BTC cohort did not meet its prespecified criteria for further expansion. The ORR was 16% and DCR was 28% among 25 BTC patients with HER-2 mutation. A subset of patients had tumor response and durable disease control, suggesting its antitumor activity in this rare population [40].
References
- Wu, C.E.; Yeh, C.N. Cutting Edge Research for Exploration of Biomolecules for Gemcitabine-Based Chemo-Resistant Advanced Bile Duct Cancer: From Basic Study to Clinical Trial. Biomolecules 2021, 11, 1626.
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184.
- Khan, S.A.; Toledano, M.; Taylor-Robinson, S. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB 2008, 10, 77–82.
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42.
- Hung, T.H.; Hung, J.; Wu, C.; Huang, Y.; Lee, C.; Yeh, C.; Chung, Y.; Lo, F.; Lai, L.; Tung, J.K.; et al. Globo H Is a Promising Theranostic Marker for Intrahepatic Cholangiocarcinoma. Hepatol. Commun. 2022, 6, 194–208.
- Jan, Y.-Y.; Yeh, C.-N.; Yeh, T.-S.; Tsann-Long, H.; Chen, M.F. Clinicopathological factors predicting long-term overall survival after hepatectomy for peripheral cholangiocarcinoma. World J. Surg. 2005, 29, 894–898.
- Mahipal, A.; Tella, S.H.; Kommalapati, A.; Anaya, D.; Kim, R. FGFR2 genomic aberrations: Achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat. Rev. 2019, 78, 1–7.
- Valle, J. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 363, 198.
- Kim, Y.J.; Im, S.-A.; Kim, H.G.; Oh, S.Y.; Lee, K.W.; Choi, I.S.; Oh, D.Y.; Lee, S.H.; Kim, J.H.; Kim, D.W.; et al. A phase II trial of TS-1 and cisplatin in patients with metastatic or relapsed biliary tract cancer(BTC). Ann. Oncol. 2006, 17, 319.
- Chiang, N.J.; Chen, M.; Yang, S.; Hsu, C.; Yen, C.; Tsou, H.; Su, Y.; Chen, J.; Shan, Y.; Chen, L. Multicentre, phase II study of gemcitabine and S-1 in patients with advanced biliary tract cancer: TG1308 study. Liver Int. 2020, 40, 2535–2543.
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 2019, 30, 1950–1958.
- Ciardiello, D.; Maiorano, B.A.; Parente, P.; Rodriquenz, M.G.; Latiano, T.P.; Chiarazzo, C.; Pazienza, V.; Guerrera, L.P.; Amoruso, B.; Normanno, N.; et al. Immunotherapy for Biliary Tract Cancer in the Era of Precision Medicine: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 820.
- Manne, A.; Woods, E.; Tsung, A.; Mittra, A. Biliary Tract Cancers: Treatment Updates and Future Directions in the Era of Precision Medicine and Immuno-Oncology. Front. Oncol. 2021, 11, 768009.
- DiPeri, T.P.; Evans, K.W.; Tzeng, C.-W.D.; Kwong, L.; Kahle, M.P.; Zheng, X.; Li, D.; Cao, H.S.T.; Vu, T.; Kim, S.; et al. The MD Anderson patient-derived xenograft series for modeling precision oncology in biliary tract cancers. Cancer Res. 2021, 81, 2710.
- Cao, J.; Hu, J.; Liu, S.; Meric-Bernstam, F.; Abdel-Wahab, R.; Xu, J.; Li, Q.; Yan, M.; Feng, Y.; Lin, J.; et al. Intrahepatic Cholangiocarcinoma: Genomic Heterogeneity Between Eastern and Western Patients. JCO Precis. Oncol. 2020, 4, 557–569.
- Casadio, M.; Biancaniello, F.; Overi, D.; Venere, R.; Carpino, G.; Gaudio, E.; Alvaro, D.; Cardinale, V. Molecular Landscape and Therapeutic Strategies in Cholangiocarcinoma: An Integrated Translational Approach towards Precision Medicine. Int. J. Mol. Sci. 2021, 22, 5613.
- Aitcheson, G.; Mahipal, A.; John, B.V. Targeting FGFR in intrahepatic cholangiocarcinoma : Leading the way for precision medicine in biliary tract cancer ? Expert Opin. Investig. Drugs 2021, 30, 463–477.
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision medicine in cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40.
- Yue, S.; Li, Y.; Chen, X.; Wang, J.; Li, M.; Chen, Y.; Wu, D. FGFR-TKI resistance in cancer: Current status and perspectives. J. Hematol. Oncol. 2021, 14, 23.
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684.
- Silverman, I.M.; Hollebecque, A.; Friboulet, L.; Owens, S.; Newton, R.C.; Zhen, H.; Féliz, L.; Zecchetto, C.; Melisi, D.; Burn, T.C. Clinicogenomic Analysis of FGFR2-Rearranged Cholangiocarcinoma Identifies Correlates of Response and Mechanisms of Resistance to Pemigatinib. Cancer Discov. 2021, 11, 326–339.
- Rizzo, A.; Ricci, A.D.; Brandi, G. Pemigatinib: Hot topics behind the first approval of a targeted therapy in cholangiocarcinoma. Cancer Treat. Res. Commun. 2021, 27, 100337.
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Biological Role and Therapeutic Potential of IDH Mutations in Cancer. Cancer Cell 2018, 34, 186–195.
- Rizzo, A.; Ricci, A.D.; Brandi, G. IDH inhibitors in advanced cholangiocarcinoma: Another arrow in the quiver? Cancer Treat. Res. Commun. 2021, 27, 100356.
- Lowery, M.; A Burris, H.; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; A Maher, E.; Gore, L.; Hollebecque, A.; et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720.
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807.
- Stemer, G.; Rowe, J.M.; Ofran, Y. Efficacy and Safety Profile of Ivosidenib in the Management of Patients with Acute Myeloid Leukemia (AML): An Update on the Emerging Evidence. Blood Lymphat. Cancer 2021, 11, 41–54.
- Casak, S.J.; Pradhan, S.; Fashoyin-Aje, L.A.; Ren, Y.; Shen, Y.L.; Xu, Y.; Chow, E.C.; Xiong, Y.; Zirklelbach, J.F.; Liu, J.; et al. FDA Approval Summary: Ivosidenib for the treatment of patients with advanced unresectable or metastatic, chemotherapy refractory cholangiocarcinoma with an IDH1 mutation. Clin. Cancer Res. 2022, 4462, OF1–OF5.
- Ross, J.S.; Wang, K.; Gay, L.; Al-Rohil, R.; Rand, J.V.; Jones, D.M.; Lee, H.J.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014, 19, 235–242.
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739.
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Jang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188.
- Chen, J.S.; Hsu, C.-H.; Chiang, N.J.; Tsai, C.S.; Tsou, H.H.; Huang, S.F.; Bai, L.Y.; Chang, I.C.; Shiah, H.S.; Ho, C.L.; et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann. Oncol. 2015, 26, 943–949.
- Malka, D.; Cervera, P.; Foulon, S.; Trarbach, T.; de la Fouchardière, C.; Boucher, E.; Fartoux, L.; Faivre, S.; Blanc, J.-F.; Viret, F.; et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014, 15, 819–828.
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148.
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (V ecti-BIL study). Cancer 2016, 122, 574–581.
- Javle, M.; Churi, C.; Kang, H.C.; Shroff, R.; Janku, F.; Surapaneni, R.; Zuo, M.; Barrera, C.; Alshamsi, H.; Krishnan, S.; et al. HER2/neu-directed therapy for biliary tract cancer. J. Hematol. Oncol. 2015, 8, 58.
- Yang, W.; Sun, Y. Promising Molecular Targets for the Targeted Therapy of Biliary Tract Cancers: An Overview. Onco. Targets Ther. 2021, 14, 1341–1366.
- Koeberle, D.; Fritsch, R. Targeting HER2 in Biliary Tract Carcinomas: Challenges and Opportunities. Oncol. Res. Treat. 2021, 44, 1–3.
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621.
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
802
Revisions:
3 times
(View History)
Update Date:
14 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




