Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kasum Azim | -- | 1544 | 2022-06-14 02:59:49 | | | |
| 2 | Camila Xu | Meta information modification | 1544 | 2022-06-14 03:06:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Azim, K.; Butt, A.; , .; Fulton, D. Subventricular Zone in the Aging Brain. Encyclopedia. Available online: https://encyclopedia.pub/entry/23989 (accessed on 08 February 2026).
Azim K, Butt A, , Fulton D. Subventricular Zone in the Aging Brain. Encyclopedia. Available at: https://encyclopedia.pub/entry/23989. Accessed February 08, 2026.
Azim, Kasum, Arthur Butt, , Daniel Fulton. "Subventricular Zone in the Aging Brain" Encyclopedia, https://encyclopedia.pub/entry/23989 (accessed February 08, 2026).
Azim, K., Butt, A., , ., & Fulton, D. (2022, June 14). Subventricular Zone in the Aging Brain. In Encyclopedia. https://encyclopedia.pub/entry/23989
Azim, Kasum, et al. "Subventricular Zone in the Aging Brain." Encyclopedia. Web. 14 June, 2022.
Copy Citation
The subventricular zone (SVZ) is the largest and most active germinal zone in the adult forebrain. Neural stem cells (NSCs) of the SVZ generate olfactory interneurons throughout life and retain the intrinsic ability to generate oligodendrocytes (OLs), the myelinating cells of the central nervous system.
oligodendrogenesis
subventricular zone
aging
multiple sclerosis
1. CNS (re)Myelination Efficiency Declines Significantly with Age
In the central nervous system (CNS), oligodendrocytes (OLs) enable rapid axonal conduction of electrical impulses by producing myelin, a lipid-rich membrane that acts as an axonal insulator and contributes to their metabolic support [1]. In both mice and humans, the bulk of oligodendrogenesis and thus myelin deposition takes place by oligodendrocyte progenitor cells (OPCs) during adolescence and young adulthood, but the addition of newly generated OLs and myelin replenishment are life-long processes [2]. Importantly, OPCs persist throughout the parenchyma of the adult brain and spinal cord and are committed to the life-long generation of OLs, which is a dynamic process that can be modulated to meet local requirements, such as myelin remodelling in response to changes in neuronal activity or myelin loss due to pathology (reviewed extensively elsewhere, for example [3]). By a process of self-replication, OPCs generate OLs by differentiation along the lineage whilst maintaining a relatively stable population of slowly proliferating parenchymal OPCs [4][5][6]. Compensatory OPC proliferation and differentiation are rapidly induced in response to loss of oligodendroglial lineage cells, ensuring both OPC population homeostasis and myelin repair. However, despite relatively stable densities of parenchymal OPCs, oligodendrogenesis declines with age, at least in part due to decreasing responsiveness to pro-differentiation signals [3][7][8]. Other physiological functions of OPCs also undergo age-related deterioration, such as the regulation of neurotransmission [9][10] and potentially the maintenance of homeostatic microglial phenotypes [11]. The reduced ability of aged OPCs to respond to and compensate for myelin loss results in the ultimate failure of effective remyelination, as observed in relapse-remitting multiple sclerosis (MS), which is characterised by efficient remyelination (remittance) in younger individuals, and very often secondary progressive MS at later stages of the disease. Notably, there is accumulating evidence that OPCs derived from neural stem cells (NSCs) of the subventricular zone (SVZ) play a major role in replenishing parenchymal OPCs and supporting myelin repair in the forebrain.
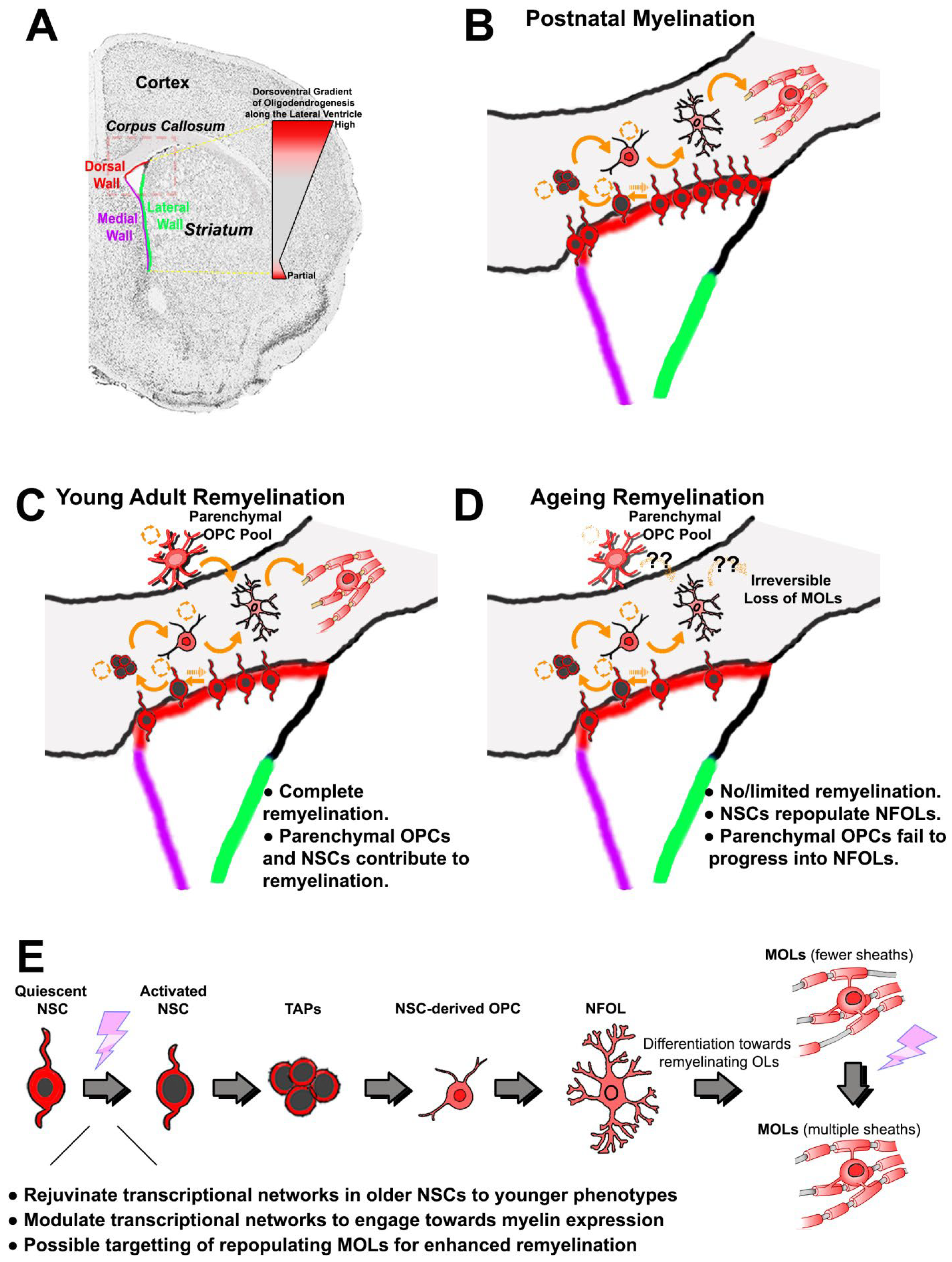 Figure 1. Forebrain oligodendrogenesis and remyelination efficiencies in young versus aged adults. (A) Coronal brain section counterstained for nuclei; the corpus callosum is evident as light grey, and the SVZ zones and other regions of interest are indicated. The dorsoventral gradient of oligodendrogenesis in the SVZ is illustrated; OPCs are generated primarily from NSCs in the dorsal microdomain, and at lower rates in the most ventral regions of the SVZ. This preferential generation of OLs from the dorsal SVZ persists in adulthood and is increased following demyelination. (B) During postnatal development, the majority of OLs in the dorsal forebrain are derived from NSC located in the dorsal SVZ that progress through a number of distinct differentiation stages in response to intrinsic and extrinsic cues (see (E) for explanations of pictograms of the differentiation stages): quiescent NSCs have small nuclei and in response to appropriate stimuli can transform into activated NSCs that have larger nuclei; activated NSCs generate transiently amplifying progenitors (TAPs), which is a pre-OPC stage that gives rise to migratory and proliferative OPCs with a simple processing-bearing morphology; OPCs migrate to their final sites, where they undergo self-replication and generate newly formed (NF)OLs, which have a complex process-bearing morphology and are non-proliferative; NFOLs differentiate into mature myelinating (M)OLs; slowly proliferating parenchymal OPCs with a highly complex ramified morphology persist after the main developmental period of myelination. (C) In young adults, demyelinating insults trigger efficient remyelination by parenchymal OPCs that are located at or near to the lesion site. Additionally, morphologically simpler and highly migratory OPCs are recruited from dorsal NSCs of the SVZ to replenish parenchymal OPCs and contribute to remyelination. (D) The aged brain is characterised by inefficient regeneration of MOLs both from parenchymal and SVZ-derived OPCs, resulting in impaired remyelination; in the aged SVZ, dorsal NSCs are able to regenerate NFOLs, but these fail to progress into remyelinating MOLs, suggesting a deficiency of appropriate extrinsic stimuli (indicated by ‘?’). (E) Identifying the transcriptional networks that regulate each stage of oligodendrogenesis from dorsal NSCs will enable the development of targeted therapies that rejuvenate aged NSCs and stimulate replenishment of OPCs to promote remyelination and repair in the aged brain. Abbreviations: NSC = neural stem cell; TAPs = transiently amplifying progenitors (pre-OPC stage); OPC = oligodendrocyte precursor cell; NFOL = non-myelin forming oligodendrocyte; MOL = mature oligodendrocyte.
Figure 1. Forebrain oligodendrogenesis and remyelination efficiencies in young versus aged adults. (A) Coronal brain section counterstained for nuclei; the corpus callosum is evident as light grey, and the SVZ zones and other regions of interest are indicated. The dorsoventral gradient of oligodendrogenesis in the SVZ is illustrated; OPCs are generated primarily from NSCs in the dorsal microdomain, and at lower rates in the most ventral regions of the SVZ. This preferential generation of OLs from the dorsal SVZ persists in adulthood and is increased following demyelination. (B) During postnatal development, the majority of OLs in the dorsal forebrain are derived from NSC located in the dorsal SVZ that progress through a number of distinct differentiation stages in response to intrinsic and extrinsic cues (see (E) for explanations of pictograms of the differentiation stages): quiescent NSCs have small nuclei and in response to appropriate stimuli can transform into activated NSCs that have larger nuclei; activated NSCs generate transiently amplifying progenitors (TAPs), which is a pre-OPC stage that gives rise to migratory and proliferative OPCs with a simple processing-bearing morphology; OPCs migrate to their final sites, where they undergo self-replication and generate newly formed (NF)OLs, which have a complex process-bearing morphology and are non-proliferative; NFOLs differentiate into mature myelinating (M)OLs; slowly proliferating parenchymal OPCs with a highly complex ramified morphology persist after the main developmental period of myelination. (C) In young adults, demyelinating insults trigger efficient remyelination by parenchymal OPCs that are located at or near to the lesion site. Additionally, morphologically simpler and highly migratory OPCs are recruited from dorsal NSCs of the SVZ to replenish parenchymal OPCs and contribute to remyelination. (D) The aged brain is characterised by inefficient regeneration of MOLs both from parenchymal and SVZ-derived OPCs, resulting in impaired remyelination; in the aged SVZ, dorsal NSCs are able to regenerate NFOLs, but these fail to progress into remyelinating MOLs, suggesting a deficiency of appropriate extrinsic stimuli (indicated by ‘?’). (E) Identifying the transcriptional networks that regulate each stage of oligodendrogenesis from dorsal NSCs will enable the development of targeted therapies that rejuvenate aged NSCs and stimulate replenishment of OPCs to promote remyelination and repair in the aged brain. Abbreviations: NSC = neural stem cell; TAPs = transiently amplifying progenitors (pre-OPC stage); OPC = oligodendrocyte precursor cell; NFOL = non-myelin forming oligodendrocyte; MOL = mature oligodendrocyte.
2. Oligodendrogenesis in the SVZ Is Spatially and Temporally Conserved across Species
The majority of OLs found in the adult mouse forebrain originate postnatally from neural stem cells (NSCs) that reside in the subventricular zone (SVZ), via a defined series of differentiation steps (see reviews [12][13] (Figure 1A,B)), and it is established that the early postnatal period is critical for OL specification and myelination [14][15][16]. Notably, the murine SVZ is spatially heterogeneous and contains specific microdomains of NSCs that are biased to give rise to defined lineages, depending on intrinsic and extrinsic factors, with OPCs being derived primarily from NSCs located in the dorsal SVZ [17][18]. Importantly, studies performed by Zecevic and co-workers have demonstrated an equivalent spatial organisation of human SVZ-NSCs during early development, where oligodendrogenesis emerges largely from more dorsal NSC subpopulations [19][20]. More recent studies have confirmed these findings in primates, guided by the expression of pallial transcriptional cues in NSCs such as Hopx [21], and humans, where radial glia switch developmentally from neurogenesis to oligodendrogenesis [22]. Thus, it is apparent that SVZ microdomains observed in the mouse are representative of species with more complex brain architectures, including humans [19][20][23]. This organisation persists after postnatal development and is important for adult OL regeneration [24][25][26][27][28]. Single-cell RNA sequencing (ScRNA-seq), coupled with long-term genetic fate mapping approaches, have characterised NSCs residing within the most lateral and ventral aspects of the rodent SVZ and show that they become increasingly quiescent during aging [29][30][31][32]. Analyses of aged dorsal NSCs at the single-cell RNA level are still lacking, but meta-analyses of published datasets support their life-long persistence and indicate they are largely quiescent under physiological conditions but can be stimulated in pathological contexts [32][33]. In humans, NSCs identified by their expression of β4 tubulin persist after postnatal development in all domains of the SVZ and, although they do not express most markers that are used to identify rodent NSCs, human NSCs do however appear to express GFAP-delta and cell surface receptors responsive to FGF2 and EGF, as in rodent NSCs [34][35][36]. Moreover, a recent elegant ScRNA-seq study using the broad progenitor marker CD271 demonstrated NSCs persist in the human dorsal SVZ of healthy individuals aged between 72 and 96 years [37]. Meta-analysis and integration with single cell datasets of human oligodendroglia [38], revealed that these dorsal SVZ-derived NSCs have a pro-oligodendroglial phenotype.
 Figure 1. Forebrain oligodendrogenesis and remyelination efficiencies in young versus aged adults. (A) Coronal brain section counterstained for nuclei; the corpus callosum is evident as light grey, and the SVZ zones and other regions of interest are indicated. The dorsoventral gradient of oligodendrogenesis in the SVZ is illustrated; OPCs are generated primarily from NSCs in the dorsal microdomain, and at lower rates in the most ventral regions of the SVZ. This preferential generation of OLs from the dorsal SVZ persists in adulthood and is increased following demyelination. (B) During postnatal development, the majority of OLs in the dorsal forebrain are derived from NSC located in the dorsal SVZ that progress through a number of distinct differentiation stages in response to intrinsic and extrinsic cues (see (E) for explanations of pictograms of the differentiation stages): quiescent NSCs have small nuclei and in response to appropriate stimuli can transform into activated NSCs that have larger nuclei; activated NSCs generate transiently amplifying progenitors (TAPs), which is a pre-OPC stage that gives rise to migratory and proliferative OPCs with a simple processing-bearing morphology; OPCs migrate to their final sites, where they undergo self-replication and generate newly formed (NF)OLs, which have a complex process-bearing morphology and are non-proliferative; NFOLs differentiate into mature myelinating (M)OLs; slowly proliferating parenchymal OPCs with a highly complex ramified morphology persist after the main developmental period of myelination. (C) In young adults, demyelinating insults trigger efficient remyelination by parenchymal OPCs that are located at or near to the lesion site. Additionally, morphologically simpler and highly migratory OPCs are recruited from dorsal NSCs of the SVZ to replenish parenchymal OPCs and contribute to remyelination. (D) The aged brain is characterised by inefficient regeneration of MOLs both from parenchymal and SVZ-derived OPCs, resulting in impaired remyelination; in the aged SVZ, dorsal NSCs are able to regenerate NFOLs, but these fail to progress into remyelinating MOLs, suggesting a deficiency of appropriate extrinsic stimuli (indicated by ‘?’). (E) Identifying the transcriptional networks that regulate each stage of oligodendrogenesis from dorsal NSCs will enable the development of targeted therapies that rejuvenate aged NSCs and stimulate replenishment of OPCs to promote remyelination and repair in the aged brain. Abbreviations: NSC = neural stem cell; TAPs = transiently amplifying progenitors (pre-OPC stage); OPC = oligodendrocyte precursor cell; NFOL = non-myelin forming oligodendrocyte; MOL = mature oligodendrocyte.
Figure 1. Forebrain oligodendrogenesis and remyelination efficiencies in young versus aged adults. (A) Coronal brain section counterstained for nuclei; the corpus callosum is evident as light grey, and the SVZ zones and other regions of interest are indicated. The dorsoventral gradient of oligodendrogenesis in the SVZ is illustrated; OPCs are generated primarily from NSCs in the dorsal microdomain, and at lower rates in the most ventral regions of the SVZ. This preferential generation of OLs from the dorsal SVZ persists in adulthood and is increased following demyelination. (B) During postnatal development, the majority of OLs in the dorsal forebrain are derived from NSC located in the dorsal SVZ that progress through a number of distinct differentiation stages in response to intrinsic and extrinsic cues (see (E) for explanations of pictograms of the differentiation stages): quiescent NSCs have small nuclei and in response to appropriate stimuli can transform into activated NSCs that have larger nuclei; activated NSCs generate transiently amplifying progenitors (TAPs), which is a pre-OPC stage that gives rise to migratory and proliferative OPCs with a simple processing-bearing morphology; OPCs migrate to their final sites, where they undergo self-replication and generate newly formed (NF)OLs, which have a complex process-bearing morphology and are non-proliferative; NFOLs differentiate into mature myelinating (M)OLs; slowly proliferating parenchymal OPCs with a highly complex ramified morphology persist after the main developmental period of myelination. (C) In young adults, demyelinating insults trigger efficient remyelination by parenchymal OPCs that are located at or near to the lesion site. Additionally, morphologically simpler and highly migratory OPCs are recruited from dorsal NSCs of the SVZ to replenish parenchymal OPCs and contribute to remyelination. (D) The aged brain is characterised by inefficient regeneration of MOLs both from parenchymal and SVZ-derived OPCs, resulting in impaired remyelination; in the aged SVZ, dorsal NSCs are able to regenerate NFOLs, but these fail to progress into remyelinating MOLs, suggesting a deficiency of appropriate extrinsic stimuli (indicated by ‘?’). (E) Identifying the transcriptional networks that regulate each stage of oligodendrogenesis from dorsal NSCs will enable the development of targeted therapies that rejuvenate aged NSCs and stimulate replenishment of OPCs to promote remyelination and repair in the aged brain. Abbreviations: NSC = neural stem cell; TAPs = transiently amplifying progenitors (pre-OPC stage); OPC = oligodendrocyte precursor cell; NFOL = non-myelin forming oligodendrocyte; MOL = mature oligodendrocyte.The causes of age-related quiescence of SVZ-NSC and their progeny are unresolved, but there is evidence of an important role for canonical Wnt signalling, which maintains the dorsal identity of the SVZ throughout life in the mammalian brain (reviewed in [12]). The aged SVZ expresses the inhibitory ligands secreted frizzled-related proteins (SFRPs), which are potent inhibitors of the canonical Wnt pathway and limit both neurogenesis and oligodendrogenesis [32][38]. Attenuation of SFRPs in human IPSCs lines [38] and mouse models of demyelination [39] corroborate that repression of canonical Wnt signalling is an important factor in driving SVZ-NSCs and their progeny towards oligodendrogenesis. These studies amongst others confirm that life-long oligodendrogenesis occurs in the human SVZ, but progress in the understanding of these processes has been limited by several challenges, not least the difficulty of systematic sampling of human tissue due to the large area to cover and the challenge of assessing current gold-standard markers with high confidence on preserved human tissue. Furthermore, as noted above, the antigenicity of human and rodent SVZ-derived NSC differs considerably, and defined markers that distinguish human NSC from astrocytic lineages are required to enable accurate studies of human populations in the context of brain aging, disease, and trauma.
3. Recruitment of SVZ NSCs for Oligodendroglial Replacement
Following myelin loss in the rodent CNS, it has been demonstrated that OPCs located within and adjacent to demyelinated lesions proliferate, migrate, and differentiate into remyelinating OLs [13][40]. This process is pronounced and efficient in early disease stages and in young adults (Figure 1C). OLs that persist within demyelinated lesions are also capable of remyelination [41][42][43][44] (reviewed in detail elsewhere, see, for example, [40]). Studies using 14C levels in humans concluded that surviving OLs rather than OPCs play the major role in remyelination in MS [41][42], except in very aggressive forms of the disease where OPCs are more important [45]. The understanding of these processes comes from studies in rodent models, and fate-mapping studies demonstrate unequivocally that remyelination in the adult brain is from both parenchymal and SVZ-derived OPCs. However, in older rodents, recruitment of OPCs and their differentiation into MOLs is incomplete, involving multiple processes [7][9][46][47]. The ultimate failure of remyelination coupled to continuous loss of myelin with time is the basis of progressive disability in MS patients [41][42][43]. Consistent with this, studies of human post-mortem tissue have shown that OPCs are present in active and remyelinating lesions, and that they are stunted in chronic lesions. Thus, there is a clear and unmet need to develop new therapies to rejuvenate OPCs and stimulate remyelination in the aged brain at later stages of MS.
References
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431.
- Hill, R.A.; Li, A.M.; Grutzendler, J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 2018, 21, 683–695.
- Rivera, A.D.; Azim, K.; Macchi, V.; Porzionato, A.; Butt, A.M.; De Caro, R. Epidermal Growth Factor Pathway in the Age-Related Decline of Oligodendrocyte Regeneration. Front. Cell. Neurosci. 2022, 16, 838007.
- Butt, A.M.; Papanikolaou, M.; Rivera, A. Physiology of Oligodendroglia. Adv. Exp. Med. Biol. 2019, 1175, 117–128.
- Akay, L.A.; Effenberger, A.H.; Tsai, L.H. Cell of all trades: Oligodendrocyte precursor cells in synaptic, vascular, and immune function. Genes Dev. 2021, 35, 180–198.
- Kula, B.; Chen, T.J.; Kukley, M. Glutamatergic signaling between neurons and oligodendrocyte lineage cells: Is it synaptic or non-synaptic? GLIA 2019, 67, 2071–2091.
- Neumann, B.; Baror, R.; Zhao, C.; Segel, M.; Dietmann, S.; Rawji, K.S.; Foerster, S.; McClain, C.R.; Chalut, K.; van Wijngaarden, P.; et al. Metformin Restores CNS Remyelination Capacity by Rejuvenating Aged Stem Cells. Cell Stem. Cell 2019, 25, 473–485.e478.
- Rivera, A.D.; Chacon-De-La-Rocha, I.; Pieropan, F.; Papanikolau, M.; Azim, K.; Butt, A.M. Keeping the ageing brain wired: A role for purine signalling in regulating cellular metabolism in oligodendrocyte progenitors. Pflug. Arch. Eur. J. Physiol. 2021, 473, 775–783.
- Chacon-De-La-Rocha, I.; Fryatt, G.; Rivera, A.D.; Verkhratsky, A.; Raineteau, O.; Gomez-Nicola, D.; Butt, A.M. Accelerated Dystrophy and Decay of Oligodendrocyte Precursor Cells in the APP/PS1 Model of Alzheimer’s-Like Pathology. Front. Cell. Neurosci. 2020, 14, 575082.
- Vanzulli, I.; Papanikolaou, M.; De-La-Rocha, I.C.; Pieropan, F.; Rivera, A.D.; Gomez-Nicola, D.; Verkhratsky, A.; Rodriguez, J.J.; Butt, A.M. Disruption of oligodendrocyte progenitor cells is an early sign of pathology in the triple transgenic mouse model of Alzheimer’s disease. Neurobiol. Aging 2020, 94, 130–139.
- Liu, Y.; Aguzzi, A. NG2 glia are required for maintaining microglia homeostatic state. GLIA 2020, 68, 345–355.
- Azim, K.; Berninger, B.; Raineteau, O. Mosaic Subventricular Origins of Forebrain Oligodendrogenesis. Front. Neurosci. 2016, 10, 107.
- El Waly, B.; Macchi, M.; Cayre, M.; Durbec, P. Oligodendrogenesis in the normal and pathological central nervous system. Front. Neurosci. 2014, 8, 145.
- Liu, R.; Jia, Y.; Guo, P.; Jiang, W.; Bai, R.; Liu, C. In Vivo Clonal Analysis Reveals Development Heterogeneity of Oligodendrocyte Precursor Cells Derived from Distinct Germinal Zones. Adv. Sci. 2021, 8, e2102274.
- Tong, C.K.; Fuentealba, L.C.; Shah, J.K.; Lindquist, R.A.; Ihrie, R.A.; Guinto, C.D.; Rodas-Rodriguez, J.L.; Alvarez-Buylla, A. A Dorsal SHH-Dependent Domain in the V-SVZ Produces Large Numbers of Oligodendroglial Lineage Cells in the Postnatal Brain. Stem. Cell Rep. 2015, 5, 461–470.
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179.
- Azim, K.; Hurtado-Chong, A.; Fischer, B.; Kumar, N.; Zweifel, S.; Taylor, V.; Raineteau, O. Transcriptional Hallmarks of Heterogeneous Neural Stem Cell Niches of the Subventricular Zone. Stem. Cells 2015, 33, 2232–2242.
- Azim, K.; Rivera, A.; Raineteau, O.; Butt, A.M. GSK3beta regulates oligodendrogenesis in the dorsal microdomain of the subventricular zone via Wnt-beta-catenin signaling. GLIA 2014, 62, 778–779.
- Rakic, S.; Zecevic, N. Early oligodendrocyte progenitor cells in the human fetal telencephalon. GLIA 2003, 41, 117–127.
- Jakovcevski, I.; Filipovic, R.; Mo, Z.; Rakic, S.; Zecevic, N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front. Neuroanat. 2009, 3, 5.
- Rash, B.G.; Duque, A.; Morozov, Y.M.; Arellano, J.I.; Micali, N.; Rakic, P. Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc. Natl. Acad. Sci. USA 2019, 116, 7089–7094.
- Fu, Y.; Yang, M.; Yu, H.; Wang, Y.; Wu, X.; Yong, J.; Mao, Y.; Cui, Y.; Fan, X.; Wen, L.; et al. Heterogeneity of glial progenitor cells during the neurogenesis-to-gliogenesis switch in the developing human cerebral cortex. Cell Rep. 2021, 34, 108788.
- Azim, K.; Zweifel, S.; Klaus, F.; Yoshikawa, K.; Amrein, I.; Raineteau, O. Early decline in progenitor diversity in the marmoset lateral ventricle. Cereb Cortex 2013, 23, 922–931.
- Azim, K.; Angonin, D.; Marcy, G.; Pieropan, F.; Rivera, A.; Donega, V.; Cantu, C.; Williams, G.; Berninger, B.; Butt, A.M.; et al. Pharmacogenomic identification of small molecules for lineage specific manipulation of subventricular zone germinal activity. PLoS Biol. 2017, 15, e2000698.
- Azim, K.; Raineteau, O.; Butt, A.M. Intraventricular injection of FGF-2 promotes generation of oligodendrocyte-lineage cells in the postnatal and adult forebrain. GLIA 2012, 60, 1977–1990.
- Kang, W.; Nguyen, K.C.Q.; Hebert, J.M. Transient Redirection of SVZ Stem Cells to Oligodendrogenesis by FGFR3 Activation Promotes Remyelination. Stem. Cell Rep. 2019, 12, 1223–1231.
- Embalabala, R.J.; Brockman, A.A.; Jurewicz, A.R.; Kong, J.A.; Ryan, K.; Guinto, C.D.; Alvarez-Buylla, A.; Chiang, C.; Ihrie, R.A. GLI3 is Required for OLIG2+ Progeny Production in Adult Dorsal Neural Stem Cells. Cells 2022, 11, 218.
- Vancamp, P.; Gothie, J.D.; Luongo, C.; Sebillot, A.; Le Blay, K.; Butruille, L.; Pagnin, M.; Richardson, S.J.; Demeneix, B.A.; Remaud, S. Gender-specific effects of transthyretin on neural stem cell fate in the subventricular zone of the adult mouse. Sci. Rep. 2019, 9, 19689.
- Bast, L.; Calzolari, F.; Strasser, M.K.; Hasenauer, J.; Theis, F.J.; Ninkovic, J.; Marr, C. Increasing Neural Stem Cell Division Asymmetry and Quiescence Are Predicted to Contribute to the Age-Related Decline in Neurogenesis. Cell Rep. 2018, 25, 3231–3240.e3238.
- Calzolari, F.; Michel, J.; Baumgart, E.V.; Theis, F.; Gotz, M.; Ninkovic, J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 2015, 18, 490–492.
- Dulken, B.W.; Buckley, M.T.; Negredo, P.N.; Saligrama, N.; Cayrol, R.; Leeman, D.S.; George, B.M.; Boutet, S.C.; Hebestreit, K.; Pluvinage, J.V.; et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 2019, 571, 205–210.
- Kalamakis, G.; Brune, D.; Ravichandran, S.; Bolz, J.; Fan, W.; Ziebell, F.; Stiehl, T.; Catala-Martinez, F.; Kupke, J.; Zhao, S.; et al. Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407–1419.e1414.
- Borrett, M.J.; Innes, B.T.; Jeong, D.; Tahmasian, N.; Storer, M.A.; Bader, G.D.; Kaplan, D.R.; Miller, F.D. Single-Cell Profiling Shows Murine Forebrain Neural Stem Cells Reacquire a Developmental State when Activated for Adult Neurogenesis. Cell Rep. 2020, 32, 108022.
- Wu, C.; Chang, A.; Smith, M.C.; Won, R.; Yin, X.; Staugaitis, S.M.; Agamanolis, D.; Kidd, G.J.; Miller, R.H.; Trapp, B.D. Beta4 tubulin identifies a primitive cell source for oligodendrocytes in the mammalian brain. J. Neurosci. 2009, 29, 7649–7657.
- Roelofs, R.F.; Fischer, D.F.; Houtman, S.H.; Sluijs, J.A.; Van Haren, W.; Van Leeuwen, F.W.; Hol, E.M. Adult human subventricular, subgranular, and subpial zones contain astrocytes with a specialized intermediate filament cytoskeleton. GLIA 2005, 52, 289–300.
- Van den Berge, S.A.; Middeldorp, J.; Zhang, C.E.; Curtis, M.A.; Leonard, B.W.; Mastroeni, D.; Voorn, P.; van de Berg, W.D.; Huitinga, I.; Hol, E.M. Longterm quiescent cells in the aged human subventricular neurogenic system specifically express GFAP-delta. Aging Cell 2010, 9, 313–326.
- Donega, V.; Burm, S.M.; van Strien, M.E.; van Bodegraven, E.J.; Paliukhovich, I.; Geut, H.; van de Berg, W.D.J.; Li, K.W.; Smit, A.B.; Basak, O.; et al. Transcriptome and proteome profiling of neural stem cells from the human subventricular zone in Parkinson’s disease. Acta Neuropathol. Commun. 2019, 7, 84.
- Donega, V.; van der Geest, A.T.; Sluijs, J.A.; van Dijk, R.E.; Wang, C.C.; Basak, O.; Pasterkamp, R.J.; Hol, E.M. Single-cell profiling of human subventricular zone progenitors identifies SFRP1 as a target to re-activate progenitors. Nat. Commun. 2022, 13, 1036.
- Huang, S.; Choi, M.H.; Huang, H.; Wang, X.; Chang, Y.C.; Kim, J.Y. Demyelination Regulates the Circadian Transcription Factor BMAL1 to Signal Adult Neural Stem Cells to Initiate Oligodendrogenesis. Cell Rep. 2020, 33, 108394.
- Cayre, M.; Falque, M.; Mercier, O.; Magalon, K.; Durbec, P. Myelin Repair: From Animal Models to Humans. Front. Cell. Neurosci. 2021, 15, 604865.
- Jäkel, S.; Agirre, E.; Falcao, A.M.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547.
- Falcao, A.M.; van Bruggen, D.; Marques, S.; Meijer, M.; Jäkel, S.; Agirre, E.; Samudyata; Floriddia, E.M.; Vanichkina, D.P.; Ffrench-Constant, C.; et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 2018, 24, 1837–1844.
- Patrikios, P.; Stadelmann, C.; Kutzelnigg, A.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Bruck, W.; Lucchinetti, C.; Lassmann, H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 2006, 129, 3165–3172.
- Patani, R.; Balaratnam, M.; Vora, A.; Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 2007, 33, 277–287.
- Yeung, M.S.Y.; Djelloul, M.; Steiner, E.; Bernard, S.; Salehpour, M.; Possnert, G.; Brundin, L.; Frisen, J. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 2019, 566, 538–542.
- Rivera, A.D.; Pieropan, F.; Chacon-De-La-Rocha, I.; Lecca, D.; Abbracchio, M.P.; Azim, K.; Butt, A.M. Functional genomic analyses highlight a shift in Gpr17-regulated cellular processes in oligodendrocyte progenitor cells and underlying myelin dysregulation in the aged mouse cerebrum. Aging Cell 2021, 20, e13335.
- Segel, M.; Neumann, B.; Hill, M.F.E.; Weber, I.P.; Viscomi, C.; Zhao, C.; Young, A.; Agley, C.C.; Thompson, A.J.; Gonzalez, G.A.; et al. Author Correction: Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 2019, 573, E3.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
14 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




