Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jaime Cardoso-Ortiz | -- | 2104 | 2022-06-14 01:43:17 | | | |
| 2 | Jason Zhu | -4 word(s) | 2100 | 2022-06-14 03:38:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Noriega, S.; Cardoso-Ortiz, J.; , .; Cuevas, M.D.R.; Flores De La Torre, J.A. Antimicrobial Activity of Nitroaromatic Derivatives. Encyclopedia. Available online: https://encyclopedia.pub/entry/23988 (accessed on 08 February 2026).
Noriega S, Cardoso-Ortiz J, , Cuevas MDR, Flores De La Torre JA. Antimicrobial Activity of Nitroaromatic Derivatives. Encyclopedia. Available at: https://encyclopedia.pub/entry/23988. Accessed February 08, 2026.
Noriega, Saúl, Jaime Cardoso-Ortiz, , Ma. Del Refugio Cuevas, Juan Armando Flores De La Torre. "Antimicrobial Activity of Nitroaromatic Derivatives" Encyclopedia, https://encyclopedia.pub/entry/23988 (accessed February 08, 2026).
Noriega, S., Cardoso-Ortiz, J., , ., Cuevas, M.D.R., & Flores De La Torre, J.A. (2022, June 14). Antimicrobial Activity of Nitroaromatic Derivatives. In Encyclopedia. https://encyclopedia.pub/entry/23988
Noriega, Saúl, et al. "Antimicrobial Activity of Nitroaromatic Derivatives." Encyclopedia. Web. 14 June, 2022.
Copy Citation
Searching for new and efficient pharmaceuticals is a constant struggle for medicinal chemists. New substances are needed in order to treat different pathologies affecting the health of humans and animals, and these new compounds should be safe, effective and have the fewest side effects possible. Some functional groups are known for having biological activity; in this matter, the nitro group (NO2) is an efficient scaffold when synthesizing new bioactive molecules. Nitro compounds display a wide spectrum of activities that include antineoplastic, antibiotic, antihypertensive, antiparasitic, tranquilizers and even herbicides, among others.
antimicrobial
Antibacterial
Antitubercular
Antifungal
Nitroaromatic compounds
1. Introduction
Pharmaceuticals are chemical substances able to cause a local or systemic action in humans and animals. A specific chemical structure is fundamental regarding its activity since the molecule relates to specific targets due to several interactions between the pharmaceutical itself and receptors, enzymes or channels. After absorption, pharmaceuticals may undergo some structural changes with the consecutive change in activity, which can increase, decrease or even change. Since the discovery of chloramphenicol in 1947, nitro containing cycles and heterocycles have been extensively investigated due to their antibacterial activity; however, nitro containing molecules exhibit a wide variety of biological activities that are attractive to medicinal chemists, for example, some are antineoplastic, antibiotic, antihypertensive, antiparasitic agents, tranquilizers and even herbicides, among many others.[1][2][3][4][5][6][7] The nitro group (NO2) is a functional group formed by one nitrogen atom linked to two oxygens, it is a particularly electron-withdrawing moiety since the N has no lone pair, hence it bears a positive charge (Figure 1).[8] The electron-withdrawing effect is easily observed in aromatic rings 1 due to resonance with the nitro group, deactivating certain positions and causing changes in the polarity of molecules, which in some cases favors interaction with nucleophilic sites of protein structures such as enzymes, causing inhibition. The nitro group not only works as a pharmacophore but also as a toxicophore, making it even more interesting for medicinal chemists. In most cases, the nitro group is present in a specific structure but it is not entirely related to the activity; however, it affects pharmacokinetics. The whole activity regarding the nitro group directly (whether beneficial or toxic) depends entirely on the reduction of the nitro itself, accepting up to six electrons in order to form the amine derivative. This reduction occurs through enzymatic reactions using NADH or NADPH as reducing agents. During the reduction of the NO2, some nitroso and hydroxylamine intermediates are formed and react with biomolecules producing undesired toxic and mutagenic effects. Although reduction of the nitro group itself is toxic to humans, in some cases this might be seen as the desired effect for designing and synthesizing new antiparasitic drugs. In some other cases (most of them) the activity of the corresponding molecule is “affected” just by the presence of the NO2 and its electron-withdrawing properties, polarity, or stereochemistry.[9][10][11][12][13][14][15][16][17][18][19]
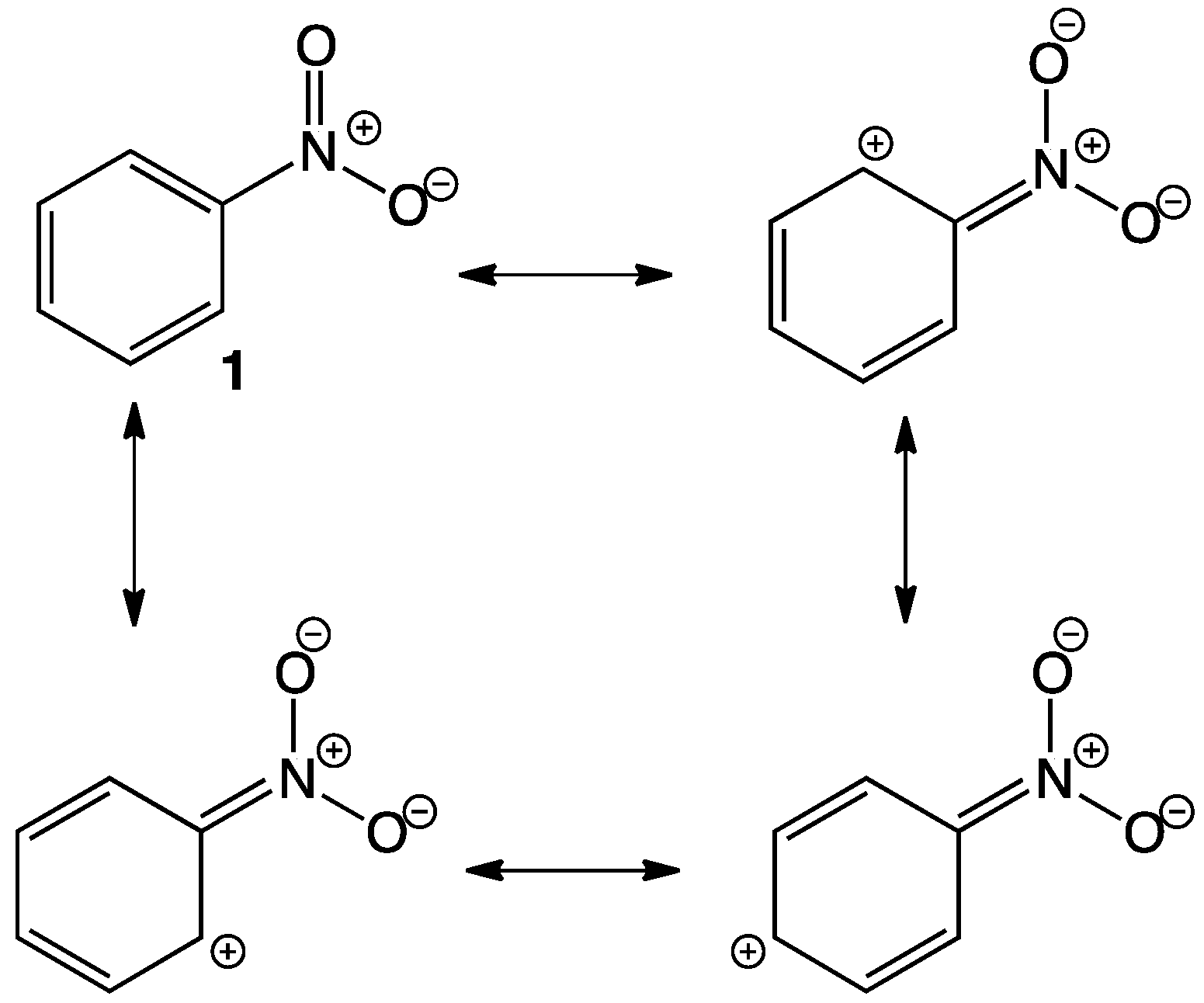
Figure 1. Resonance structures in nitrobenzene.
2. Antimicrobial Activity
Nitro-containing molecules are some of the first lines of treatment for common infections caused by several microorganisms. Metronidazole, chloramphenicol and other nitro derivatives display antimicrobial activity by different mechanisms. One of the most accepted and general models states that when nitro compounds are reduced, they produce toxic intermediates (such as nitroso and superoxide species), then, the reduced nitro species bind covalently to DNA resulting in nuclear damage and cell death.[20] Some examples of molecules that required activation in order to be antimicrobial agents are 5-nitroimidazole derivatives. These compounds undergo intercellular reduction giving rise to a nitro anion radical (NO2−) of short life but a crucial step in the mechanism of action of nitroimidazoles. 5-nitroimidazole is a significant moiety in medicinal chemistry since it is present in commercial drugs such as miconazole, ketoconazole and metronidazole among others.[13][21]
2.1. Antibacterial
The antibacterial activity of nitro-containing molecules is one of the widest effects observed, not only in human or veterinary pharmaceuticals but also in the manufacturing of new materials with antimicrobial properties.[22] Metronidazole is one of the primary pharmaceuticals employed in the treatment of H. pylori, and it contains the moiety 5-nitroimidazole. Unfortunately, it has lost efficiency as this microorganism is developing resistance against traditional treatments. For this reason, several 5-nitroimidazole derivatives have been developed throughout the years in order to overcome this problem, the imidazole base structure is known for having activity against H. pylori as it can be found already existing pharmaceuticals such as metronidazole (Figure 2).[23]
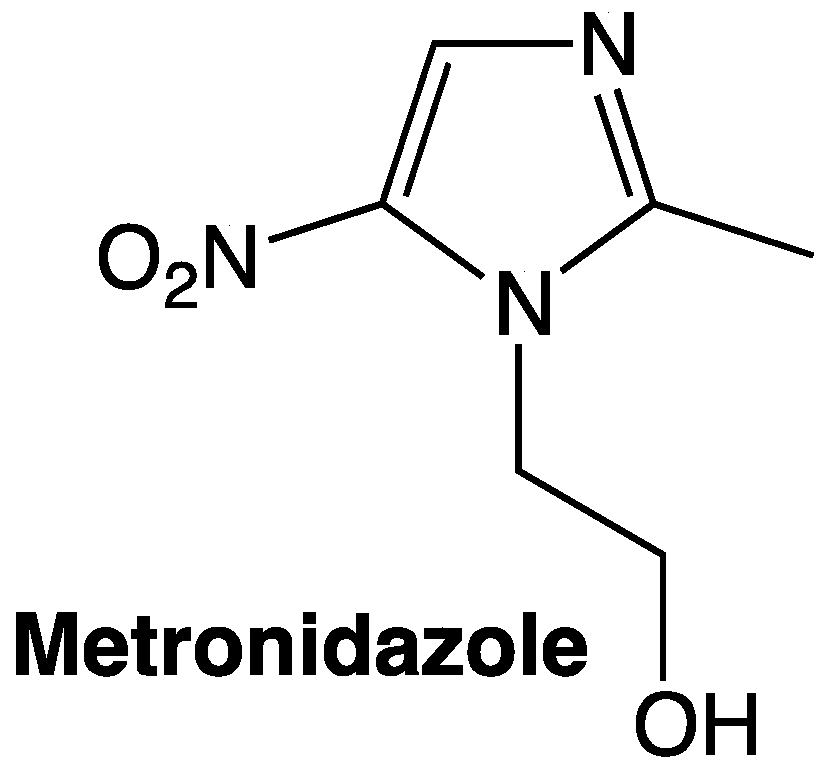
Figure 2. Metronidazole as a 5-nitroimidazole.
Many existing drugs have some degree of nitration in their structures, for example, chloramphenicol or nitrofurantoin. These well-known substances are of common use and will continue so for a while; however, there are many other compounds being developed and tested for their antimicrobial properties. Such is the case of a series of nitrated pyrrolomycins that proved to be efficient against Gram-negative (Pseudomonas aeruginosa) and Gram-positive (Staphylococcus aureus) bacteria.[24] Pyrrolomycins are halogenated organic compounds isolated from Actinosporangium and Streptomyces species that show a variety of biological activities such as neuro and immune-modulatory, antiproliferative, insecticidal and antimicrobial. This latter property is attributed to a protonophoric effect of pyrrolomycins, which is the ability of some molecules to translocate protons from one side to another of biological membranes. Even though pyrrolomycins are potential candidates for developing new antibacterial pharmaceuticals, they are also toxic compounds due to the halogens present in their structure (Cl or Br).[25] By chemically modifying the structure of some pyrrolomycins, researchers are able to obtain new compounds with enhanced antimicrobial activity and low toxicity. One of these modifications includes a nitration step on the pyrrole ring 3a–d, which is easily achieved through an electrophilic aromatic substitution reaction (Figure 3). The C2 position in the pyrrole ring is favored for electrophilic substitution, however, when C2 has either Br or Cl, C1 becomes particularly activated for an electrophilic attack due to the halogen lone pair. The bromine-substituted compound 4c showed the best yield compared to Cl derivatives 4b and 4d, indicating a stronger activation by Br. Experimental results also indicated that the presence of nitro groups (especially in the C2 and C4 positions) in the pyrrole ring enhanced the antibacterial activity 4b (20 μM against S. aureus) and 4d (30 μM against P. aeruginosa). Researchers propose not only a protonophoric effect, but also improved lipophilicity and hence a better interaction with membranes.[24]
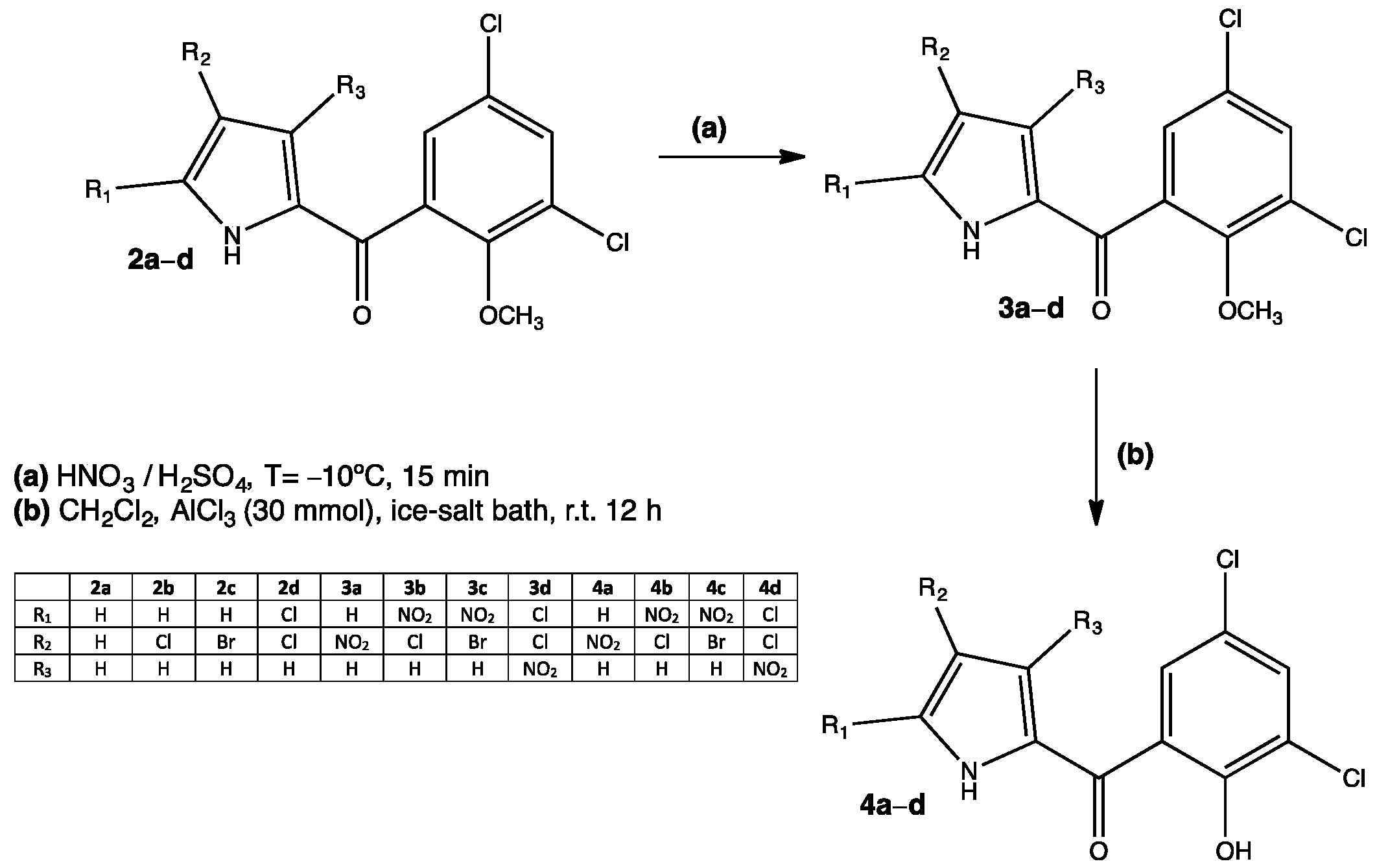
Figure 3. Nitrated pyrrolomycins with potential antibacterial activity.[24]
For many years, the coordination chemistry of transition metals was not considered an important part of medicinal chemistry. However, in recent years, metal complexes with Schiff bases as ligands have gained attention due to their wide potential biological activity. A series of 4-nitro-1,2-phenylendiamine metal complexes were synthesized and tested against Gram-positive microorganisms (Streptococcus mutans and Staphylococcus aureus) Gram-negative (Escherichia coli, Klebsiella pneumonia and Pseudomonas aeruginosa) and the fungus Candida albicans. A total of nine new compounds were obtained but only the Zn(II) complex 5 showed significant activity against Streptococcus mutans (Figure 4). Antibacterial activity was reported as the inhibition zone diameter, with 40.7 mm being the inhibition obtained with the Zn(II) complex. The rest of the compounds with other metals such as Co(II), Cr(III), Fe(III) and Ni(II) showed little or no activity at all.[26] Even when these results might seem discouraging, these kinds of compounds represent new synthetic methods to be addressed in future research or metal complexes with biological activity.
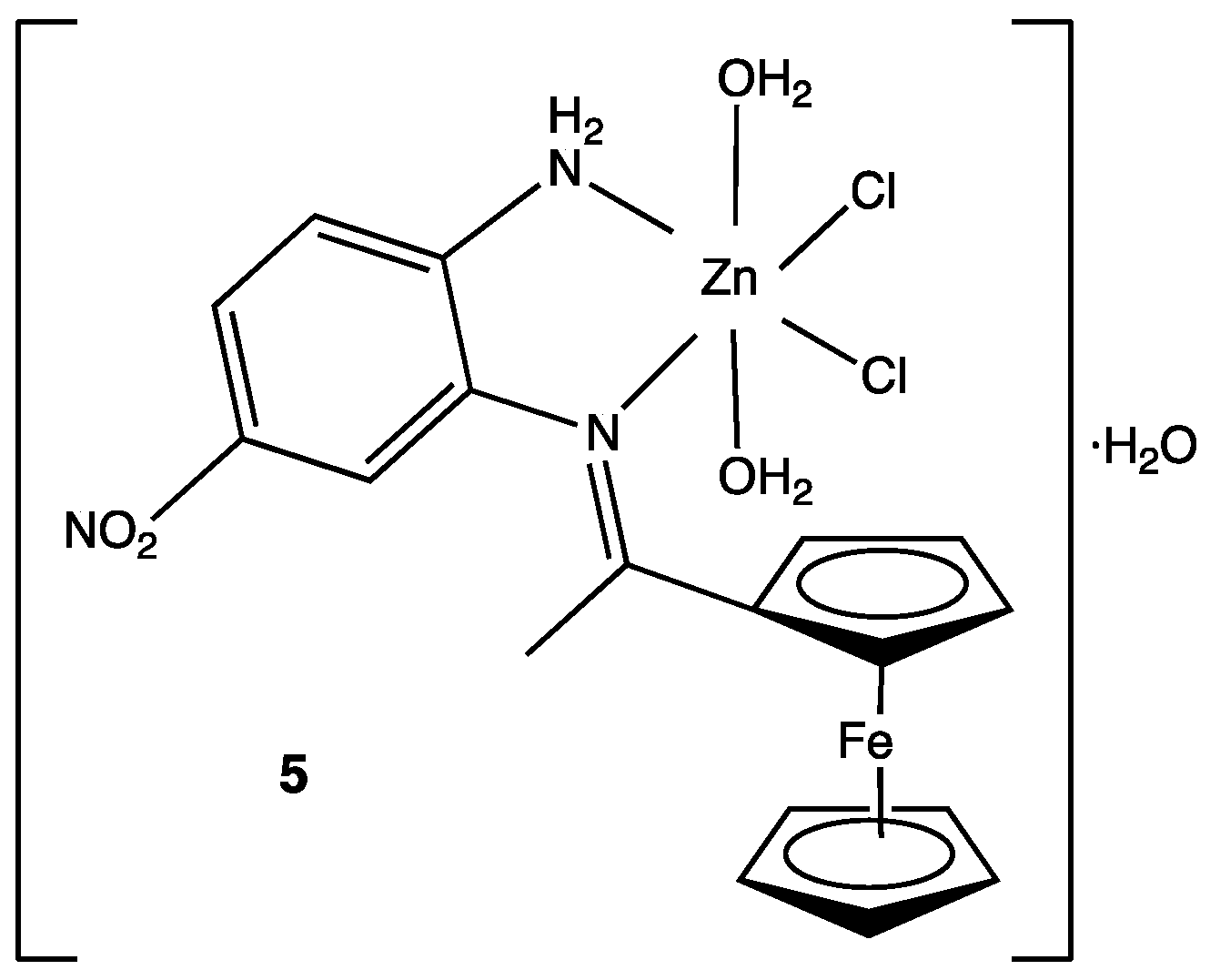
Figure 4. Structure of the Zn(II) complex against Streptococcus mutans.[26]
There is an increasing interest in developing new antimicrobial agents not only because of the growing resistance to antibiotics but also because of the risk of nosocomial infections (infectious diseases acquired within a hospital). In some regions, these types of infections could reach up to 40% of hospitalized patients. Gram-positive bacteria are responsible for more than 90% of nosocomial infections, and Staphylococcus aureus is one of the pathogens listed as a priority for research and development according to the World Health Organization (WHO). In this context, researchers synthesized several nitro derivatives through an oxa-Michael-aldol condensation reaction and tested their potential antimicrobial activity against Staphylococcus aureus and Candida sp., two main microorganisms involved in frequent nosocomial infections (Figure 5). Experimental results indicated that nitro derivatives with some degree of halogenation 9b–9d had the best results with minimum inhibitory concentration (MIC) in the range of 15.6–62.5 μg/mL for S. aureus and minimum fungicidal concentration (MFC) 15–500 62.5 μg/mL in the case of Candida.[27]
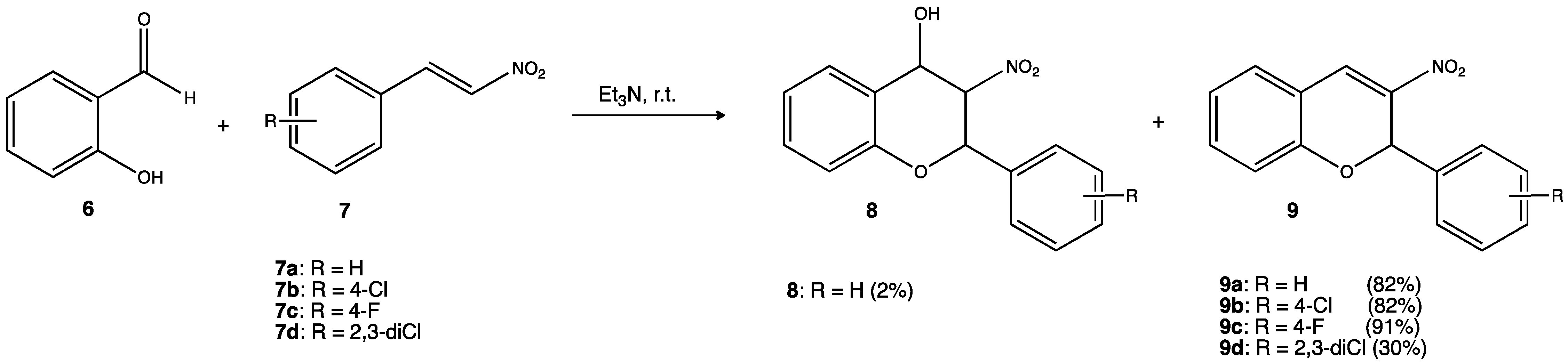
Figure 5. Nitroderivatines containing one F and/or two Cl atoms showed the best activity against S. aureus (15.6–62.5 μg/mL) and Candida sp. (15–500 62.5 μg/mL), respectively.[27]
Pseudomonas aeruginosa is another type of bacteria responsible for nosocomial infections, so it has become important to develop new antibiotics able to inhibit the growth or kill Gram-negative organisms as well. In this matter, heterocycles such as benzothiazoles show antibacterial activity, especially the ones containing the nitro functional group. In a study, several nitrated benzothiazoles were obtained through nitroanilines and KSCN under relatively mild conditions. A total of nine compounds were tested against P. aeruginosa but only three showed significant activity 10–12 (Figure 6), becoming pharmaceutical targets for future research as lead compounds. Inhibition was compared with procaine penicillin obtaining similar inhibition.[28]
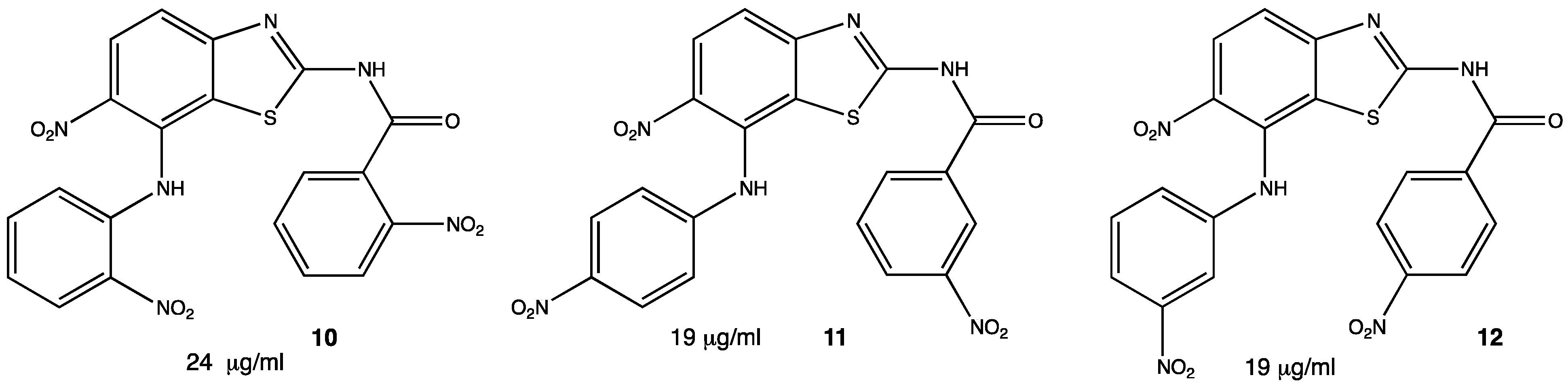
Figure 6. Benzothiazole derivatives against P. aeruginosa.[28]
2.2. Antifungal
Nowadays, fungi resistance is becoming a topic of discussion among medicinal chemists. There are some species resistant to classical treatments such as fluconazole (including C. krusei, and C. albicans), so new molecules are needed in order to limit this growing clinical problem. One of the best methods to obtain new molecules with a desired and specific activity is from already existing molecules that show this particular activity. In this way, researchers are able to obtain new substances by synthesizing derivatives from a parent compound. By these means, a series of fluconazole derivatives 17a–e were prepared to have a piperazine or nitrotriazole moiety in their structure (Figure 7).[29]
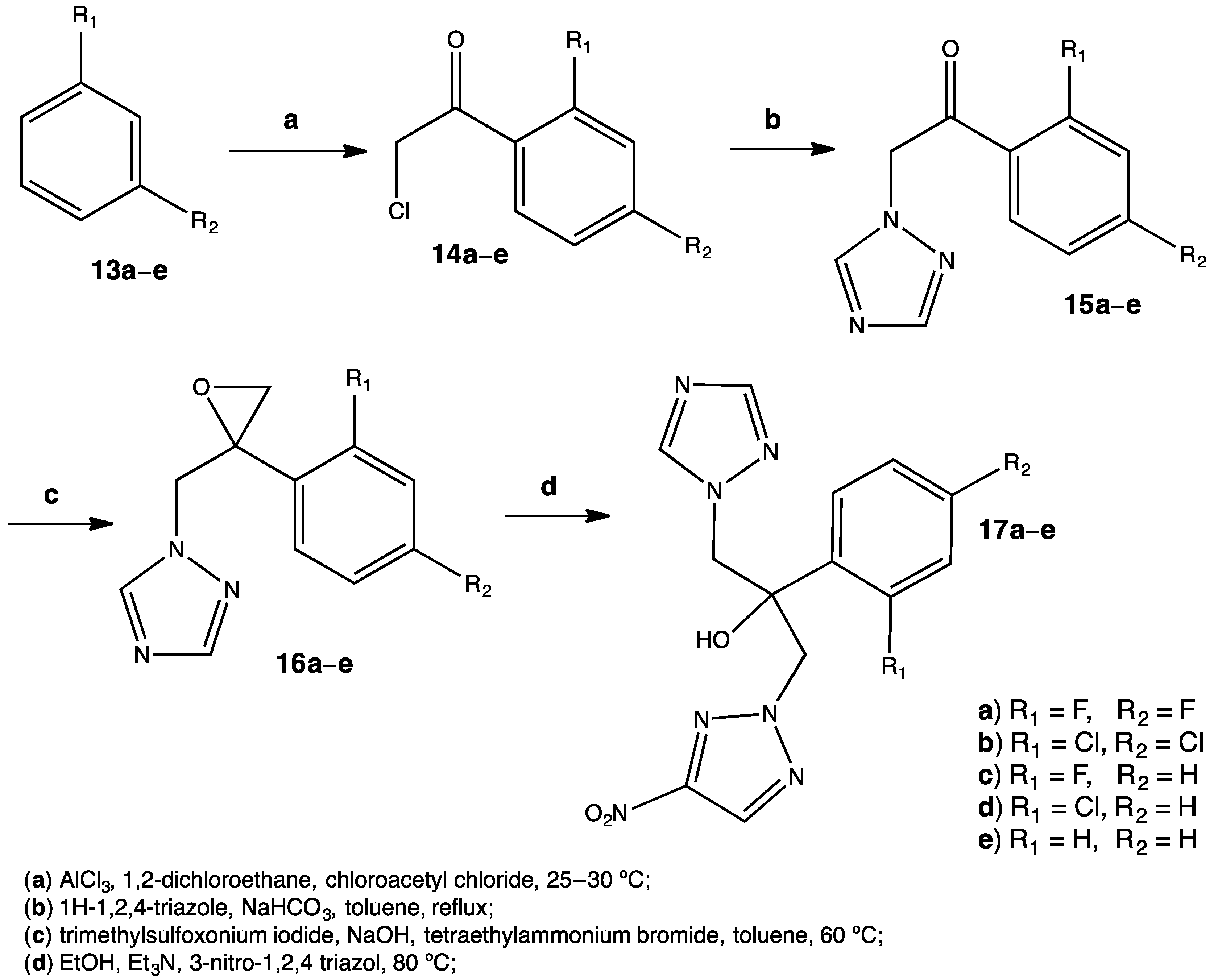
Figure 7. The presence of a nitrotriazole moiety in the fluconazole derivatives enhances antifungal properties.[29]
The nitrotriazole derivatives were obtained through a four-step synthesis under relatively mild conditions. Although reflux was employed, there was no need for temperatures higher than 80 °C. Yields varied from a range of 30% to 56%, but considering the products the results were satisfactory. The best antifungal properties were observed among the molecules baring halogens in the benzene ring, especially Cl. Halogens tend to make compounds more lipophilic and at the same time more polar, improving their pharmacokinetics. It is noteworthy that the nitrotriazole derivative showed better activity against C. krusei (MIC = 1 μM for 17b) compared to the fluconazole standard. The mechanism of action of these fluconazole derivatives involves the inhibition of the 14α-demethylase, an enzyme necessary for the synthesis of ergosterol in fungi.[30] Such antifungal activity observed in the nitro compounds is attributed to an efficient electrostatic interaction between the NO2 and the Fe(II) in the heme group of 14α-demethylase, triggering a strong inhibition of the enzyme. Docking studies were performed in order to complement experimental results.[29]
2.3. Antitubercular
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis, and it is an issue of growing concern for global health because of concomitant infections with HIV. M. tuberculosis has developed resistance to traditional treatments, so it has become imperative to find new substances able to kill or inhibit the growth of this pathogen. Regiments for treatment against TB are long (up to 18 months in the case of multi-resistant bacteria) due to its slow bacterial growth, so it is imperative to develop new drugs targeting different metabolic stages. Some lead compounds have been found such as 5-nitrophenantroline 18 with an MIC value of 0.78 μM against M. tuberculosis;[31] , researchers found that the presence of the NO2 group in the C5 was essential for antitubercular activity (Figure 8). They replaced it with other withdrawing groups, such as cyano, bromo or chloro, and observed ten times less activity with these substituents; furthermore, they also concluded that the NO2 moiety modulates intracellular mechanisms involved in killing bacteria.[31]
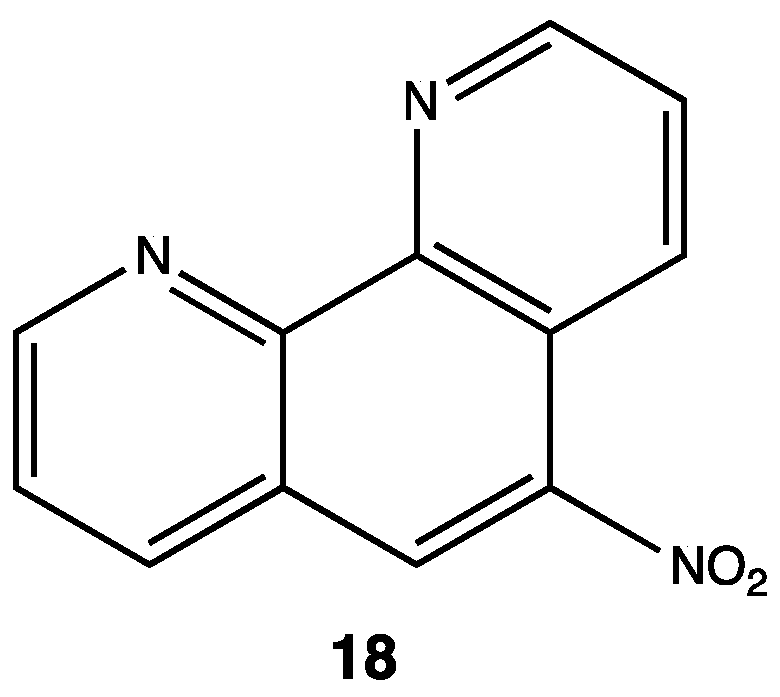
Figure 8. 5-nitro-1,10-phenantroline compounds tested against Mycobacterium tuberculosis.[31]
Some nitro-containing molecules such as 5-nitroimidazooxazine and 5-nitro-imidazooxazole are currently studied in clinical trials in the US.[32] However, there have to be more options available for the treatment of tuberculosis. A promising group of molecules with antitubercular activity is nitrotriazoles; in particular, 3-nitro-1,2,4-triazole-based derivatives 19–23 (Figure 9). It was reported that these classes of compounds inhibit M. tuberculosis in the range of 3–50 μM. Compared to other effective and still used antitubercular agents such as isoniazid, the MICs (minimum inhibitory concentration) observed in nitrotriazoles are not so far from this reference; they are 0.3 μM for isoniazid and 3.0 μM for the most active nitrotriazole. These molecules are effective, but they still need to be further investigated in order to obtain new nitrotriazole derivatives that can compete with lead compounds such as isoniazid or rifampicin.[33][34]
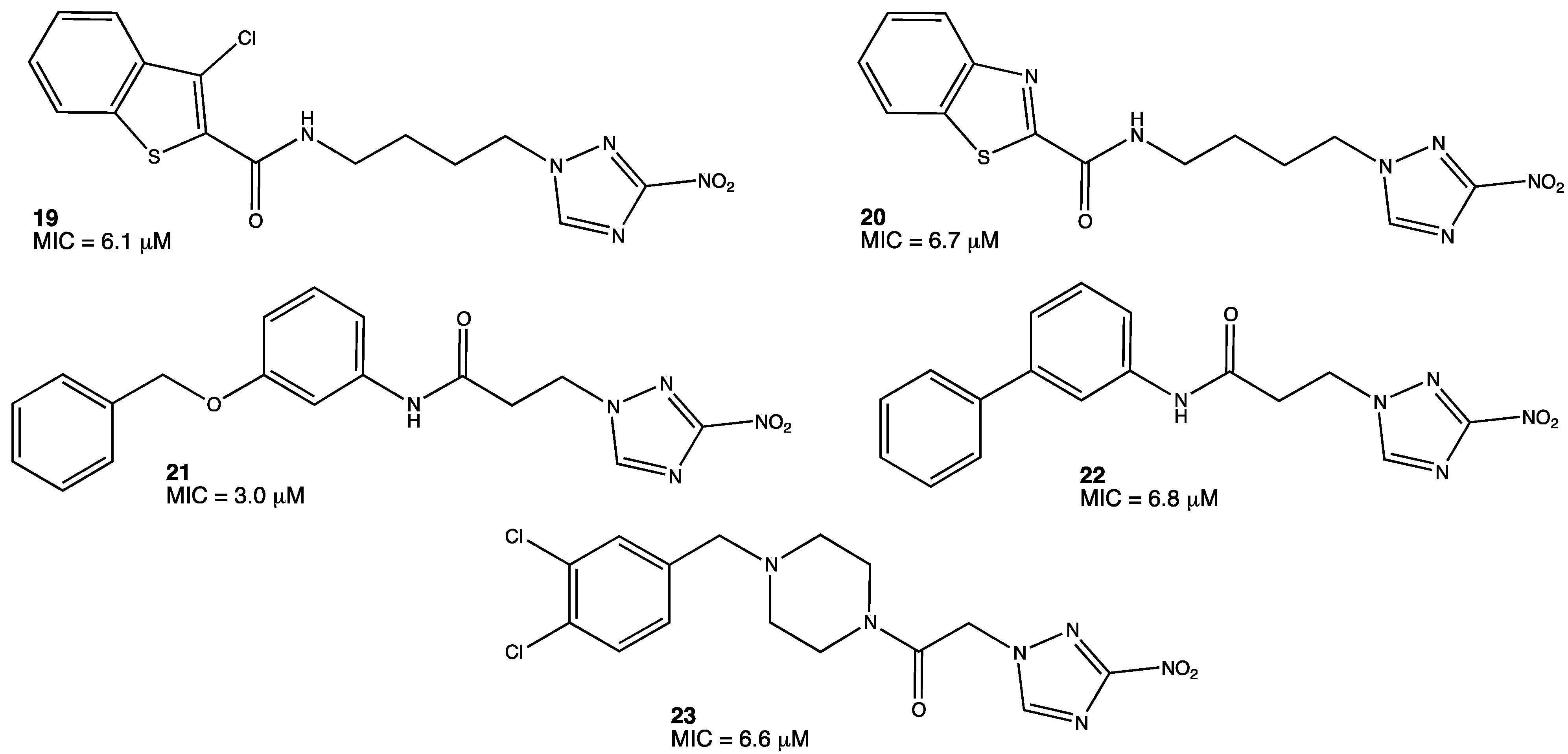
Figure 9. Nitrotriazole derivatives with antitubercular activity.[34]
References
- Kamila Morawska; Katarzyna Jedlińska; Sylwia Smarzewska; Radovan Metelka; Witold Ciesielski; Dariusz Guziejewski; Analysis and DNA interaction of the profluralin herbicide. Environmental Chemistry Letters 2019, 17, 1359-1365, 10.1007/s10311-019-00865-1.
- Sunil Ghatge; Youri Yang; Seonyun Moon; Woo- Young Song; Tae-Young Kim; Kwang- Hyeon Liu; Hor-Gil Hur; A novel pathway for initial biotransformation of dinitroaniline herbicide butralin from a newly isolated bacterium Sphingopyxis sp. strain HMH. Journal of Hazardous Materials 2020, 402, 123510, 10.1016/j.jhazmat.2020.123510.
- Rosario Tavera-Hernández; Manuel Jiménez-Estrada; Jesús J. Alvarado-Sansininea; Antonio Nieto-Camacho; Hugo López-Muñoz; Luis Sánchez-Sánchez; María L. Escobar; Synthesis of Chrysin, Quercetin and Naringin Nitroderivatives: Antiproliferative, Anti-inflammatory and Antioxidant Activity. Letters in Drug Design & Discovery 2021, 18, 795-805, 10.2174/1570180818666210122162313.
- Talita A. Ribeiro; Erik Machado-Ferreira; Lohaine F. Guimarães; Jéssica Cavaleiro; Alan Messala A. Britto; Nátaly Redua; Lucas Miguel Pereira de Souza; André S. Pimentel; Paulo H.S. Picciani; Osvaldo N. Oliveira; et al.Cléber Bonfim BarretoCarlos Augusto G. Soares Novel cytotoxic amphiphilic nitro-compounds derived from a synthetic route for paraconic acids. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 626, 126984, 10.1016/j.colsurfa.2021.126984.
- Aduragbenro D.A. Adedapo; Abayomi M. Ajayi; Nancy Losie Ekwunife; Olufunke O. Falayi; Ademola Oyagbemi; Temidayo Olutayo Omobowale; Adeolu A. Adedapo; Antihypertensive effect of Phragmanthera incana (Schum) Balle on NG-nitro-L-Arginine methyl ester (L-NAME) induced hypertensive rats. Journal of Ethnopharmacology 2020, 257, 112888, 10.1016/j.jep.2020.112888.
- Allison Rice; Yueming Long; S. King; Nitroaromatic Antibiotics as Nitrogen Oxide Sources. Biomolecules 2021, 11, 267, 10.3390/biom11020267.
- Patricia M. Toro; Alejandra Acuña; Mario Mallea; Michel Lapier; Mauricio Moncada; Jonathan Cisterna; Iván Brito; Hugo Klahn; Condensation and substitution products obtained in reactions of isomeric bromo-nitrofuraldehydes with ferrocenylamine: Electrochemistry and anti-parasitic evaluation. Journal of Organometallic Chemistry 2019, 901, 120946, 10.1016/j.jorganchem.2019.120946.
- Anna Jezuita; Krzysztof Ejsmont; Halina Szatylowicz; Substituent effects of nitro group in cyclic compounds. Structural Chemistry 2020, 32, 179-203, 10.1007/s11224-020-01612-x.
- Kunal Nepali; Hsueh-Yun Lee; Jing-Ping Liou; Nitro-Group-Containing Drugs. Journal of Medicinal Chemistry 2018, 62, 2851-2893, 10.1021/acs.jmedchem.8b00147.
- J. Squella; S. Bollo; L. Nunez-Vergara; Recent Developments in the Electrochemistry of Some Nitro Compounds of Biological Significance. Current Organic Chemistry 2005, 9, 565-581, 10.2174/1385272053544380.
- Dorota Olender; Justyna Żwawiak; Lucjusz Zaprutko; Multidirectional Efficacy of Biologically Active Nitro Compounds Included in Medicines. Pharmaceuticals 2018, 11, 54, 10.3390/ph11020054.
- Caroline G. Sanz; Kevin A. Dias; Raphael P. Bacil; Ricardo A.M. Serafim; Leandro H. Andrade; Elizabeth I. Ferreira; Silvia H.P. Serrano; Electrochemical characterization of para- and meta-nitro substituents in aqueous media of new antichagasic pharmaceutical leaders. Electrochimica Acta 2020, 368, 137582, 10.1016/j.electacta.2020.137582.
- Alexandre A. Oliveira; Ana P. A. Oliveira; Lucas L. Franco; Micael O. Ferencs; João F. G. Ferreira; Sofia M. P. S. Bachi; Nivaldo L. Speziali; Luiz M. Farias; Paula P. Magalhães; Heloisa Beraldo; et al. 5-Nitroimidazole-derived Schiff bases and their copper(II) complexes exhibit potent antimicrobial activity against pathogenic anaerobic bacteria. BioMetals 2018, 31, 571-584, 10.1007/s10534-018-0106-6.
- Jadriane A. Xavier; Thaissa L. Silva; Eduardo Caio Torres-Santos; Camila Calado de Vasconcelos; Anastacio Boane; Ricardo Alexandre dos Santos; Andre Felippe A. Xavier; Marília O.F. Goulart; Unveiling the relevance of the redox character of nitroaromatic and nitroheteroaromatic compounds as potential medicines. Current Opinion in Electrochemistry 2021, 29, 100740, 10.1016/j.coelec.2021.100740.
- Rodolfo Rodrigo Florido França; Cheyene Almeida Celestino Menozzi; Frederico Silva Castelo-Branco; Lucas Villas Bôas Hoelz; Nubia Boechat; The Medicinal Chemistry of 3-nitro-1,2,4-triazoles: Focus on Infectious Diseases. Current Topics in Medicinal Chemistry 2021, 21, 2072-2100, 10.2174/1568026621999210902124524.
- Kuldeep Chauhan; Moni Sharma; Priyanka Trivedi; Vinita Chaturvedi; Prem M.S. Chauhan; New class of methyl tetrazole based hybrid of (Z)-5-benzylidene-2-(piperazin-1-yl)thiazol-4(%H)-one as potent antitubercular agents. Bioorganic & Medicinal Chemistry Letters 2014, 24, 4166-4170, 10.1016/j.bmcl.2014.07.061.
- Rajan Sharma; Jacques Joubert; Recent Developments in Drug Design of NO-donor Hybrid Compounds. Mini-Reviews in Medicinal Chemistry 2018, 18, 1175-1198, 10.2174/1389557518666180416150005.
- Hammerich, O. Reduction of Nitro Compounds and Related Substrates. In Organic Electrochemistry: Revised and Expanded, 5th ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1149–1200.
- Nagaraju Kerru; Lalitha Gummidi; Suresh Maddila; Kranthi Kumar Gangu; Sreekantha B. Jonnalagadda; A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909, 10.3390/molecules25081909.
- Joachim Müller; Andrew Hemphill; Norbert Müller; Physiological aspects of nitro drug resistance in Giardia lamblia. International Journal for Parasitology: Drugs and Drug Resistance 2018, 8, 271-277, 10.1016/j.ijpddr.2018.04.008.
- Uzma Salar; Khalid Mohammed Khan; Muhammad Taha; Nor Hadiani Ismail; Basharat Ali; Qurat- Ul Ain; Shahnaz Perveen; Mehreen Ghufran; Abdul Wadood; Biology-oriented drug synthesis (BIODS): In vitro β-glucuronidase inhibitory and in silico studies on 2-(2-methyl-5-nitro-1H-imidazol-1-yl)ethyl aryl carboxylate derivatives. European Journal of Medicinal Chemistry 2016, 125, 1289-1299, 10.1016/j.ejmech.2016.11.031.
- C. Dhivya; S. Anbu Anjugam Vandarkuzhali; N. Radha; Antimicrobial activities of nanostructured polyanilines doped with aromatic nitro compounds. Arabian Journal of Chemistry 2019, 12, 3785-3798, 10.1016/j.arabjc.2015.12.005.
- Elham Ghobadi; Zahra Ghanbarimasir; Saeed Emami; A review on the structures and biological activities of anti-Helicobacter pylori agents. European Journal of Medicinal Chemistry 2021, 223, 113669, 10.1016/j.ejmech.2021.113669.
- Maria Valeria Raimondi; Alessandro Presentato; Giovanna Li Petri; Miriam Buttacavoli; Agnese Ribaudo; Viviana De Caro; Rosa Alduina; Patrizia Cancemi; New Synthetic Nitro-Pyrrolomycins as Promising Antibacterial and Anticancer Agents. Antibiotics 2020, 9, 292, 10.3390/antibiotics9060292.
- Katherine Valderrama; Elizabeth Pradel; Alexander M. Firsov; Hervé Drobecq; Hélène Bauderlique-Le Roy; Baptiste Villemagne; Yuri N. Antonenko; Ruben Christiaan Hartkoorn; Pyrrolomycins Are Potent Natural Protonophores. Antimicrobial Agents and Chemotherapy 2019, 63, e01450-19, 10.1128/aac.01450-19.
- Walaa H. Mahmoud; Reem G. Deghadi; Gehad G. Mohamed; Metal complexes of novel Schiff base derived from iron sandwiched organometallic and 4-nitro-1,2-phenylenediamine: Synthesis, characterization, DFT studies, antimicrobial activities and molecular docking. Applied Organometallic Chemistry 2018, 32, e4289, 10.1002/aoc.4289.
- Jéssica Tauany Andrade; Silmara Lucia Grego Alves; William Gustavo Lima; Carla Daiane Ferreira Sousa; Lucas Fernandes Carmo; Nívea Pereira De Sá; Fernanda Barbara Morais; Susana Johann; José Augusto Ferreira Perez Villar; Jaqueline Maria Siqueira Ferreira; et al. Pharmacologic potential of new nitro-compounds as antimicrobial agents against nosocomial pathogens: design, synthesis, and in vitro effectiveness. Folia Microbiologica 2019, 65, 393-405, 10.1007/s12223-019-00747-7.
- Akhilesh Gupta; Synthesis of Novel Nitro Substituted Benzothiazole Derivatives and Antibacterial activity against Pseudomonas aeruginosa. Research Journal of Pharmacy and Technology 2018, 12, 4663, 10.5958/0974-360x.2019.00803.5.
- Hossein Sadeghpour; Soghra Khabnadideh; Kamiar Zomorodian; Keyvan Pakshir; Khadijeh Hoseinpour; Nabiollah Javid; Ehsan Faghih-Mirzaei; Zahra Rezaei; Design, Synthesis, and Biological Activity of New Triazole and Nitro-Triazole Derivatives as Antifungal Agents. Molecules 2017, 22, 1150, 10.3390/molecules22071150.
- Jakub Suchodolski; Jakub Muraszko; Przemysław Bernat; Anna Krasowska; Lactate Like Fluconazole Reduces Ergosterol Content in the Plasma Membrane and Synergistically Kills Candida albicans. International Journal of Molecular Sciences 2021, 22, 5219, 10.3390/ijms22105219.
- Saqib Kidwai; Chan-Yong Park; Shradha Mawatwal; Prabhakar Tiwari; Myung Geun Jung; Tannu Priya Gosain; Pradeep Kumar; David Alland; Sandeep Kumar; Avinash Bajaj; et al.Yun-Kyung HwangChang Sik SongRohan DhimanIll Young LeeRamanDeep Singh Dual Mechanism of Action of 5-Nitro-1,10-Phenanthroline against Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy 2017, 61, e00969-17, 10.1128/aac.00969-17.
- Lia D'ambrosio; Rosella Centis; Giovanni Sotgiu; Emanuele Pontali; Antonio Spanevello; Giovanni Battista Migliori; New anti-tuberculosis drugs and regimens: 2015 update. ERJ Open Research 2015, 1, 00010-2015, 10.1183/23120541.00010-2015.
- Maria V. Papadopoulou; William D. Bloomer; Howard S. Rosenzweig; Alexander Arena; Francisco Arrieta; Joseph C. J. Rebolledo; Diane K. Smith; Nitrotriazole- and Imidazole-Based Amides and Sulfonamides as Antitubercular Agents. Antimicrobial Agents and Chemotherapy 2014, 58, 6828-6836, 10.1128/aac.03644-14.
- Maria V. Papadopoulou; William D. Bloomer; Howard S. Rosenzweig; The antitubercular activity of various nitro(triazole/imidazole)-based compounds. Bioorganic & Medicinal Chemistry 2017, 25, 6039-6048, 10.1016/j.bmc.2017.09.037.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
14 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




