
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gerardo Gómez Millán | + 4094 word(s) | 4094 | 2020-09-30 06:08:01 | | | |
| 2 | Rita Xu | -1415 word(s) | 2679 | 2020-10-10 06:12:15 | | |
Video Upload Options
Furfuryl alcohol (FuOH, C4H3OCH2OH, 2-furylmethanol, 2-furancarbinol) has applications in the fabrication of foundry resins, the ingredient production of P-series fuels, in liquid alkanes and in food production. It is also a very important intermediate in fine chemical synthesis and the polymer industry, and it is used as a chemical intermediate for the synthesis of lysine, vitamin C and levulinic acid and employed as a lubricant and as a dispersing agent.
1. Introduction
Owing to the continuous global demand and concerns related to chemicals, fuels and materials produced from the oil industry (i.e., coal, natural gas and gasoline), which currently supplies most of these substances consumed on the planet, and the dependency of the global economy on them, alternative renewable resources have gained momentum in industry and academia. In this sense, lignocellulosic biomass is becoming an attractive alternative to substitute fossil derivatives in the production of fuels and chemicals in liquid, solid and gas form [1]. Furthermore, this type of biomass is the most abundant (after atmospheric CO2), non-contaminant, inexpensive renewable carbon source. Valorization of by-products from the pulp and paper industry emerges as a notably promising feedstock, considering that it does not compete with food consumption. Moreover, pulp and paper mills in the Nordics are struggling to keep profiting as a consequence of the digitalization of literature, the climate crisis and especially the competitive growing market in equatorial and sub-equatorial regions with larger tree-growing rates and low-cost labor [2]. This current tendency can bring new markets to current forest firms to further expand their product portfolios with biobased chemicals and biofuels, as an extension to their cellulose-based products, such as paper and packaging materials. This situation forces the shift of their bulk production of paper-grade pulp on the way to other products with lower production volumes but higher profit, such as bio-oil from lignin [3], and value-added chemicals like xylitol [4], furfural (FUR), 5-hydroxymethylfurfural (HMF) and acetic acid from hydrolysate liquor from the dissolving pulp production [5][6].
The peculiar layout of biomass (highly oxygenated compounds) causes its conversion into chemicals and fuels to be energy-intensive and comprises profound chemical transformations [7]. One option to handle the complex matrix of biomass feedstock considers its conversion into simpler fractions, which could be further transformed downstream. Promising biomass-derived molecules have recently been highlighted, the so-called building blocks or platform molecules [8], which consist of numerous functionalities in their structures, and therefore can be further converted to a broad spectrum of useful chemical compounds. Among the various appealing biobased platform molecules, furanic compounds like FUR, HMF, furan-2,5-dicarboxylic acid, ethanol, glycerol, isoprene, sorbitol, xylitol, lactic acid, succinic acid and levulinic acid can be produced from C5 and C6 sugars, which are incorporated in the hemicellulose fraction of the lignocellulosic biomass [9]. In the case of FUR, more than 70% of its market is devoted to the synthesis of furfuryl alcohol (FuOH), whose market is growing continuously [10]. Other important and widely utilized FUR derivatives in the chemical industry are tetrahydrofurfuryl alcohol (THFA) and 2-methylfuran (MF).
Furfuryl alcohol (FuOH, C4H3OCH2OH, 2-furylmethanol, 2-furancarbinol) has applications in the fabrication of foundry resins, the ingredient production of P-series fuels, in liquid alkanes and in food production [11][12][13]. It is also a very important intermediate in fine chemical synthesis and the polymer industry, and it is used as a chemical intermediate for the synthesis of lysine, vitamin C and levulinic acid and employed as a lubricant and as a dispersing agent [14].
In order to synthesize FuOH from FUR, FUR typically is reduced at 120 °C under atmospheric pressure [15]. Currently, industrial production of derivates from FUR has been achieved on the Cu-Cr catalyst [16], which involves various drawbacks, such as harsh conditions regarding the high H2 pressure (3 MPa), high reaction temperature (403–473 K) and high toxicity of chromium compounds [17][18], and Cu-chromite goes through rapid deactivation associated with coke formation or via a change in the Cu oxidation state during reaction [19][20][21]. The first reported laboratory synthesis of FuOH was in 1864 using amalgam to reduce FUR [22]. Further studies were performed due to its presence in coffee beans [23]. Its commercial feasibility was investigated in 1934 by the Quaker Oats Company in the United States, which achieved a 99% conversion of FUR to FuOH [24]. Wojcik [25] reported in 1948 that the copper-chromium oxide catalyst yields around 96–99% FuOH in theory at 175 °C and the catalyst has little or no effect on the furan ring at 175 °C.
In addition, under liquid-phase and gas-phase systems, the chromium-based catalyst suffers from deactivation. Hence, various types of chromium-free catalysts, including noble metals (Pt, Pd, Ru, Rh and Ir) [26][27][28][29][30], non-noble metals (Fe, Co, Ni and Cu) [31][32][33][34] and alloyed bimetallic metals (Pt-, Ni-, Fe- and Cu-M) [35][36][37][38] have been developed to study the hydrogenation of FUR in liquid phase [39]. Moreover, liquid-phase systems are preferred, since the hydrogenation of FUR in gas phase typically results in a higher amount of by-products and higher energy requirements, due to the vaporization of FUR. Besides, it is desirable to produce FuOH in aqueous solution at mild conditions and utilize less toxic components. Hydrogenation is the most fundamental reaction in reductive conversions. The products of the hydrogenation of FUR are FuOH, MF, tetrahydrofurfural and tetrahydrofurfuryl alcohol [40] (Figure 1). Moreover, xylose and its isomerization product, xylulose, can be directly hydrogenated to xylitol on metal sites, or converted into glyceraldehyde via tetro-aldol condensation, and ultimately reduced to glycerol and ethylene glycol [41]. Additionally, xylose can be converted to xylonic acid with in situ cofactor regeneration catalyzed by co-immobilized xylose dehydrogenase and alcohol dehydrogenase [42]; it can also be converted to levulinic acid employing HY zeolite [43] FUR, an intermediate product, can be converted into some useful chemicals through selective hydrogenation that starts by the reduction reaction of the carbonyl group and/or the furan ring, including FuOH, tetrahydrofuran (THF), tetrahydrofurfural, THFA and furan. Meanwhile, FuOH, may also continue to undergo side reactions, yielding levulinic acid and hydrogenolysis of the C‐O bond (hydrodeoxygenation) forming MF notably at temperatures above 200 °C [44]. Additionally, many other compounds derived from secondary reactions like hydrogenolysis of the C‐O bond, decarbonylation, hydrogenation and furan ring opening can occur, such as 2-methyltetrahydrofuran (MTHF), 2-pentanone (PN) and 2-pentanol (POL). Furthermore, when alcohols are present (methanol, ethanol, isopropanol), etherification and acetalization products may be formed such as 2-furaldehyde dimethyl acetal [40], difurfuryl ether [45] and 2-furaldehyde diethyl acetal [46][47][48][49]. Along these lines, in order to produce FuOH efficiently and selectively, it is decisive to avert over-hydrogenation by selectively hydrogenating the C=O bonds rather than the C=C bonds, and unselectively cracking the C‐C bonds. The coking process via the condensation of xylose and/or FUR derivatives also reduces the carbon efficiency [27]. The selective hydrogenation of FUR towards FuOH depends on various elements governing the intramolecular selectivity of the hydrogenation of α,β-unsaturated aldehydes [50], such as metal–support interaction, electronic and steric influence of the support, morphology of the metal particles, selective poisoning, influence and nature of the second metal, pressure and the steric effects of substituents at the conjugated double bond.
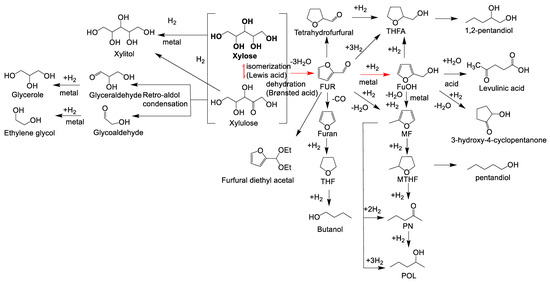
Figure 1. Possible reaction routes of the one-pot transformation of xylose to furfuryl alcohol. Adapted from [37][51][52][53].
2. Patents on Furfuryl Alcohol Formation
The top five assignees registering patents on FuOH have been Quaker Oats Company (Chicago, IL, USA), Texaco Inc. (San Ramon, CA, USA), Halliburton Energy Services, Inc. (Houston, TX, USA), Jinan Shengquan Group Co. Ltd. (Shandong, Jinan, China) and Kao Corporation (Chuo City, Tokyo, Japan). Additionally, other companies in the USA and China have registered patents in FuOH. Most of the patents related to FuOH are associated with the manufacture of resins, binders, molds, foams, coatings, polymers and waxes [54][55][56][57][58][59][60].
Quaker Oats Co. began patenting their knowledge on FuOH by a continuous process to produce FuOH from FUR in the vapor phase employing CuO and Na2O-SiO2, yielding around 99% FuOH [61][62]. Furthermore, a patent submitted by Lillwitz in 1978 [63] describes the production of FuOH using HMF as the feedstock in liquid phase with Pd and Rh at ≥135 °C. FuOH is continuously extracted and the pH value is in the range of 6.5 to 9.0. The highest yield obtained was 79% with an HMF conversion of 87% in a continuous flow without the presence of solvents at 200 °C and 0.02 MPa. A method for the catalytic conversion of FUR to FuOH involving a Ru-supported and N-doped graphene material has been recently patented [64]. Furthermore, a patent reports the formation of FuOH from FUR in liquid phase employing a copper-aluminium alloy and 5.5% of Ni-Fe at 130–140 °C with a H2 pressure of 3 MPa, that results in a FUR conversion of 99.5% and a selectivity to FuOH of 97.6% [65]. In contrast, 2-zirconium hydroxyphosphinyl acetate has been employed to convert FUR (98.1%) to FuOH (96.5% yield) at 150 °C in 1.5 h [66]. In this application, the inventors used isopropanol as a hydrogen source and as a solvent. Furthermore, the catalyst was reused three times, leading to a reduction in the catalytic activity to 92.5% FuOH yield. In a similar invention, ZrO2@SBA-15 was used as a catalyst to form FuOH from FUR by transfer hydrogenation in a reaction temperature range of 130–160 °C and a hydrogenation reaction time of 1–4 h [67].
Additionally, a process of especial interest to obtain FuOH through multifunctional catalysts from carbohydrates (xylose) derived from lingocellulosic material was developed by Fraga and Perez in 2013 [68]. Even though various catalysts were reported, such as Pt/SiO2-SO3H, Pt/ZrO2-SO42- and Pt/ZrO2, the patent claims that the highest selectivity to FuOH of 93% with a conversion of 19% is reached using a Pt/SBA-15-SO3H catalyst at 130 °C, 3 MPa and a reaction time of 90 min.
3. Formation of Furfuryl Alcohol from Xylose in One-Pot Reactions
Recent advances in one-pot cascade conversion of xylose to FuOH over solid acid catalysts have attracted much attention from the industry and academia. The synthesis of FuOH from xylose employing bifunctional catalysts that incorporates acid and metal sites in one reactor brings challenges in avoiding side reactions to optimize the yield of FuOH. Furthermore, most of the studies of one-pot conversion of xylose to FuOH over bifunctional catalysts involve precious metals like Pt and Pd, and metal oxides and mesoporous silica with acid sites, such as sulphate or sulfonic groups. Nevertheless, the adequate conversion of xylose to FuOH adopting a one-step process is very attractive as it is more cost-effective and the energy-intensive separation of FUR might be avoided.
In one of the pioneering works of the one-step production of FuOH from xylose, Perez and Fraga [69] investigated a dual catalyst system consisting of Pt/SiO2 and sulfated ZrO2 as metal and acid catalysts, respectively. The highest selectivity to FuOH (51%) is achieved at 130 °C in 6 h employing a 1:3 aqueous to 2-propanol phase ratio at a xylose conversion of 65% (Table 2). Under these experimental conditions, reusability tests were performed. However, after the first reusability cycle, the selectivity to FuOH declined progressively after each run, reaching 29% in the third cycle. The authors suggested that the solid acid catalyst underwent deactivation, due to the unaffected formation of other products, which are dependent on metal sites (either SiO2 or Pt). Additionally, a multifunctional catalyst based on sulphated zirconia was investigated in the one-pot formation of FuOH from xylose [70]. The highest selectivity to FuOH (27%) was obtained at a xylose conversion of 32% with an acid/metal ratio of 142 at 130 °C and 3 MPa. An interesting effect that was studied in the article shows the role of isolated metal centers, which afford the production of xylitol, whereas the presence of sole acid sites leads to the formation of FUR. A following paper from the same research group reported a high selectivity to FuOH (75%) over a metal-free catalyst (zeolite beta) via MPV [71]. This high selectivity to FuOH was linked to the configuration of tetrahedral-framework Al centers tailored by the Al-O-Si bond distance and the characteristic typology of the catalyst. In another contribution from Fraga’s group, multiwalled carbon nanotubes-supported noble metal catalysts (Ru, Pt, Au, Pd and Rh) were assessed for the one-pot conversion of xylose in aqueous phase [72]. Under the experimental conditions (6 h at 130 °C, 3 MPa H2, using water/2-propanol (1:1)), Ru displayed the highest catalytic activity to hydrogenate xylose to xylitol and FuOH, providing 84% and 9% yield, respectively, at 100% xylose conversion. The highest FuOH yield was obtained with the Pd-functionalized catalyst (12%) at a 66% xylose conversion. The authors also studied SBA-15 catalysts incorporating Al as a heteroatom at different Si/Al ratios ([Al]-SBA-15) in the formation of FuOH from xylose [73]. The alterations to the surface of [Al]-SBA-15 guided the product distribution, FuOH being the main product and only FUR in a minor quantity despite the Si/Al ratio. All the modified mesoporous catalysts reached selectivities to FuOH of around 90%. Reusability tests were completed at 130 °C in 4 h using a water/2-propanol medium (1:1), where it can be seen that after three reusability cycles, the catalytic activity loss is insignificant (pentose conversion remains around 15%). However, the selectivity to FuOH decreased from 90 to 80%, and the selectivity to FUR increased from 10 to 20%. This effect might be a result of the Lewis acid sites losing activity after each run that results in lower conversions of the adsorbed FUR intermediate favoring the aldehyde to desorb. This developed system was designed because it requires neither molecular hydrogen nor noble metal sites for xylose conversion to FuOH, which raises costs. Brønsted acid sites come across to be active for the pentose dehydration reaction, whereas Lewis acid sites promote the transfer hydrogenation of the adsorbed FUR intermediate to FuOH [73].
Deng et al. [51] synthesized and employed a bifunctional Cu/SBA-15-SO3H catalyst to form FuOH from xylose in a one-pot catalytic system. The highest FuOH yield (63%) was obtained at 4 MPa, 140 °C and 6 h in a biphasic water/n-butanol solvent mixture at a total xylose conversion. Under these experimental conditions, the authors also identified three main side-products, xylitol, FUR and xylulose. They observed that the relative high hydrogen pressure led to the side hydrogenation reaction of xylose to xylitol and the relative high reaction temperature led to the further hydrogenation to MF. Nevertheless, a study on the hydrothermal stability of the catalyst is missing and it would be of great concern to observe the catalytic activity of the functionalized Cu/SBA-15 through several reusability cycles under the same experimental conditions.
Canhaci et al. [74] converted xylose to FuOH on a single organic–inorganic hybrid mesoporous silica-supported catalyst. They employed Pt/SBA-15-SO3H bearing different acid/metal site ratios and found negligible sole sugar dehydration to FUR and a striking production of FuOH. When they tested the catalyst reusability, the catalytic activity decreased and the product distribution changed after each reaction cycle. Nevertheless, their work demonstrated that sulfonated ordered mesoporous silica-supported catalysts deliver active and highly selective systems for the generation of FuOH from xylose. High selectivities were accomplished (83–87%) in this system.
Cui et al. [75] converted xylose to FuOH and MF. Firstly, they dehydrated xylose to FUR using a Hβ zeolite catalyst in a fixed-bed reactor with a high xylose conversion (>99%) and FUR yield (87.6%) when using γ-butyrolactone (GBL) and water. Secondly, they added the ternary Cu/ZnO/Al2O3 catalyst, which they reported in previous work to form MF from FUR [76][77], to hydrogenate FUR. A high yield of FuOH (87.2%) and MF were obtained at 150 °C and 190 °C, respectively. After a time-on-stream of 162 h in the reactor, a decline in the yield of FuOH was observed, due to the deactivation of the Hβ zeolite catalyst, but after reactivation, the catalytic activity could be recovered.
Furthermore, Xu et al. [78] used formic acid as both an acid catalyst and as a hydrogen donor together with a mesoporous N-doped carbon-confined Co catalyst (Co-N-C) to convert xylose to FuOH. They reported a 69.5% FuOH yield at 160 °C in 3 h from xylose, and after five reusability cycles, it was observed that the Co-N-C catalyst possesses high stability for the xylose conversion. Moreover, Ordomsky et al. [79] developed a biphasic system to dehydrate xylose and hydrogenate FUR employing Amberlyst-15 and a hydrophobic Ru/C catalyst, which is located in the organic phase. However, due to the experimental conditions, the main products of xylose dehydration with hydrogenation of FUR are THFA, γ-valerolactone, levulinic acid and pentanediols. The low amount of FuOH formed under these conditions could be the result of the high pressure (4 MPa) and high temperature (165 °C), which could have hydrogenated FuOH further to THFA.
References
- Serrano-Ruiz, J.; Luque, R.; Campelo, J.M.; Romero, A.A. Continuous-Flow Processes in Heterogeneously Catalyzed Transformations of Biomass Derivatives into Fuels and Chemicals. Challenges 2012, 3, 114–132.
- Lê, H.Q. Wood Biorefinery Concept Based on γ-Valerolactone/Water Fractionation. Ph.D. Thesis, Aalto University, Espoo, Finland, 2018.
- Hashmi, S.F.; Meriö-Talvio, H.; Hakonen, K.J.; Ruuttunen, K.; Sixta, H. Hydrothermolysis of organosolv lignin for the production of bio-oil rich in monoaromatic phenolic compounds. Fuel Process. Technol. 2017, 168, 74–83.
- Venkateswar Rao, L.; Goli, J.K.; Gentela, J.; Koti, S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour. Technol. 2016, 213, 299–310.
- Gómez Millán, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent Advances in the Catalytic Production of Platform Chemicals from Holocellulosic Biomass. ChemCatChem 2019, 11, 2022–2042.
- Ståhlberg, T.; Fu, W.; Woodley, J.M.; Riisager, A. Synthesis of 5-(Hydroxymethyl)furfural in Ionic Liquids: Paving the Way to Renewable Chemicals. ChemSusChem 2011, 4, 451–458.
- Zhu, P.; Abdelaziz, O.Y.; Hulteberg, C.P.; Riisager, A. New synthetic approaches to biofuels from lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2020, 21, 16–21.
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Oak Ridge, TN, US Department of Energy (US): 2004.
- Bozell, J.J.; Petersen, G.R.; Technology development for the production of biobased products from biorefinery carbohydrates- the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 4, 539–554.
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676.
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic upgrading of lactic acid to fuels and chemicals by dehydration/hydrogenation and C-C coupling reactions. Green Chem. 2009, 11, 1101–1104.
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183.
- Weingarten, R.; Cho, J.; Conner, W.C.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423–1429.
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224.
- Heng, Z.; Grinstaff, M.W. Recent Advances in Glycerol Polymers: Chemistry and Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 1906–1924.
- Adkins, H.; Connor, R. The catalytic hydrogenation of organic compounds over copper chromite. J. Am. Chem. Soc. 1931, 53, 1091–1095.
- Nagaraja, B.M.; Padmasri, A.H.; David Raju, B.; Rama Rao, K.S. Vapor phase selective hydrogenation of furfural to furfuryl alcohol over Cu–MgO coprecipitated catalysts. J. Mol. Catal. A Chem. 2007, 265, 90–97.
- Seo, G.; Chon, H. Hydrogenation of furfural over copper-containing catalysts. J. Catal. 1981, 67, 424–429.
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419.
- Zhang, H.; Lei, Y.; Kropf, A.J.; Zhang, G.; Elam, J.W.; Miller, J.T.; Sollberger, F.G.; Ribeiro, F.H.; Akatay, M.C.; Stach, E.A.; Dumesic, J.A.; Marshall, C.L. Enhancing the stability of copper chromite catalysts for the selective hydrogenation of furfural using ALD overcoating. J. Catal. 2014, 317, 284–292.
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobolapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345.
- NIIR Board of Consultants and Engineers. Synthetic Resins Technology Handbook; ASIA PACIFIC BUSINESS PRESS Inc.: Kamla Nagar, Delhi, India, 2005; p. 588.
- Erdmann, E. Zur Charakteristik des Furfuralkohols. Ber. Dtsch. Chem. Ges. 1902, 35, 1855–1862.
- Industrial Development of Furfuryl Alcohol. Available online: http://www.furan.com/furfuryl_alcohol_historical_overview.html (accessed on 23 May 2020).
- Wojcik, B.H. Catalytic Hydrogenation of Furan Compounds. Ind. Eng. Chem. 1948, 40, 210–216.
- Tamura, M.; Tokonami, K.; Nakagawa, Y.; Tomishige, K. Rapid synthesis of unsaturated alcohols under mild conditions by highly selective hydrogenation. Chem. Commun. 2013, 49, 7034–7036.
- Kijeński, J.; Winiarek, P.; Paryjczak, T.; Lewicki, A.; Mikołajska, A. Platinum deposited on monolayer supports in selective hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A 2002, 233, 171–182.
- Pang, S.H.; Medlin, J.W. Adsorption and Reaction of Furfural and Furfuryl Alcohol on Pd(111): Unique Reaction Pathways for Multifunctional Reagents. ACS Catal. 2011, 1, 1272–1283.
- Yuan, Q.; Zhang, D.; van Haandel, L.; Ye, F.; Xue, T.; Hensen, E.J.M.; Guan, Y. Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs. J. Mol. Catal. A Chem. 2015, 406, 58–64.
- Yu, W.; Tang, Y.; Mo, L.; Chen, P.; Lou, H.; Zheng, X. Bifunctional Pd/Al-SBA-15 catalyzed one-step hydrogenation–esterification of furfural and acetic acid: A model reaction for catalytic upgrading of bio-oil. Catal. Commun. 2011, 13, 35–39.
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient Synthesis of Furfuryl Alcohol from H2-Hydrogenation/Transfer Hydrogenation of Furfural Using Sulfonate Group Modified Cu Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180.
- Sharma, R.V.; Das, U.; Sammynaiken, R.; Dalai, A.K. Liquid phase chemo-selective catalytic hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A 2013, 454, 127–136.
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13.
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of Furfural over Supported Metal Catalysts: A Comparative Study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791.
- Merlo, A.B.; Vetere, V.; Ruggera, J.F.; Casella, M.L. Bimetallic PtSn catalyst for the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. Catal. Commun. 2009, 10, 1665–1669.
- Li, H.; Luo, H.; Zhuang, L.; Dai, W.; Qiao, M. Liquid phase hydrogenation of furfural to furfuryl alcohol over the Fe-promoted Ni-B amorphous alloy catalysts. J. Mol. Catal. A Chem. 2003, 203, 267–275.
- Chen, X.; Li, H.; Luo, H.; Qiao, M. Liquid phase hydrogenation of furfural to furfuryl alcohol over Mo-doped Co-B amorphous alloy catalysts. Appl. Catal. A 2002, 233, 13–20.
- Fulajtárova, K.; Soták, T.; Hronec, M.; Vávra, I.; Dobročka, E.; Omastová, M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd–Cu catalysts. Appl. Catal. A 2015, 502, 78–85.
- Baijun, L.; Lianhai, L.; Bingchun, W.; Tianxi, C.; Iwatani, K. Liquid phase selective hydrogenation of furfural on Raney nickel modified by impregnation of salts of heteropolyacids. Appl. Catal. A 1998, 171, 117–122.
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668.
- Du, H.; Ma, X.; Jiang, M.; Yan, P.; Zhao, Y.; Zhang, Z.C. Efficient Ni/SiO2 catalyst derived from nickel phyllosilicate for xylose hydrogenation to xylitol. Catal. Today 2020, in press.
- Bachosz, K.; Synoradzki, K.; Staszak, M.; Pinelo, M.; Meyer, A.S.; Zdarta, J., et al. Bioconversion of xylose to xylonic acid via co-immobilized dehydrogenases for conjunct cofactor regeneration. Bioorg. Chem. 2019, 93, 102747.
- (Chamnankid, B.; Ratanatawanate, C., Faungnawakij, K. Conversion of xylose to levulinic acid over modified acid functions of alkaline-treated zeolite Y in hot-compressed water. Chem. Eng. J. 2014, 258, 341–347.
- Rao, R.S.; Baker, R.T.; Vannice, M.A. Furfural hydrogenation over carbon-supported copper. Catal. Lett. 1999, 60, 51–57.
- O’Driscoll, Á.; Leahy, J.J.; Curtin, T. The influence of metal selection on catalyst activity for the liquid phase hydrogenation of furfural to furfuryl alcohol. Catal. Today 2017, 279, 194–201.
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal. B 2016, 180, 580–585.
- Lee, J.; Burt, S.P.; Carrero, C.A.; Albarubio, A.C.; Ro, I.; Oneill, B.J.; Kim, H.J.; Jackson, D.H.K.; Kuech, T.F.; Hermans, I.; Dumesic, J.A.; Huber, G.W. Stabilizing cobalt catalysts for aqueous-phase reactions by strong metal-support interaction. J. Catal. 2015, 330, 19–27.
- Mironenko, R.M.; Belskaya, O.B.; Gulyaeva, T.I.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Lavrenov, A.V.; Likholobov, V.A. Effect of the nature of carbon support on the formation of active sites in Pd/C and Ru/C catalysts for hydrogenation of furfural. Catal. Today 2015, 249, 145–152.
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT). Appl. Catal. A 2018, 550, 1–10.
- Claus, P. Selective hydrogenation of α,β-unsaturated aldehydes and other C=O and C=C bonds containing compounds. Top. Catal. 1998, 5, 51–62.
- Deng, T.; Xu, G.; Fu, Y. One-pot cascade conversion of xylose to furfuryl alcohol over a bifunctional Cu/SBA-15-SO3H catalyst. Chin. J. Catal. 2020, 41, 404–414.
- Jouve, A.; Cattaneo, S.; Delgado, D.; Scotti, N.; Evangelisti, C.; Nieto, J.M.L.; Prati, L. Furfural Hydrogenation on Modified Niobia. Appl. Sci. 2019, 9, 2287.
- Jaatinen, S.K.; Karinen, R.S.; Lehtonen, J.S. Liquid Phase Furfural Hydrotreatment to 2-Methylfuran on Carbon Supported Nickel Catalyst - Effect of Process Conditions. ChemistrySelect 2016, 1, 5363–5373.
- Yoshida, A. Binder Composition for Mold Making Purposes. WO/2012/081577, 21 June 2012.
- Kanekawa, H.; Kawakatsu, Y.; Sakai, M. Binder for Casting Mold. JP22385883A, 21 June 1983.
- Smith, S.B. Method of Making a Low-Griability, Thermosetting Foam. US23846781A, 21 September 1982.
- Hamermesh, C.L.; Hogenson, P.A.; Tung, C.M. Intumescent Flame-Resistant Coating. US19690580A, 1 December 1980.
- Katada, H.; Kobayashi, S.; Ogasawara, S.; Tsujimoto, N. Method for Producing Furfuryl Alcohol-Formaldehyde Copolymer. JP2012238393A, 6 December 2012.
- Matsumoto, Y.; Murai, Y. Method for Producing Spherical Furfuryl Alcohol Resin Particle, Spherical Furfuryl Alcohol Resin Particle Produced Thereby, Spherical Carbon Particle, and Spherical Activated Carbon Particle. JP2011059103A, 11 October 2011.
- Chen, M.C.; Everett, G.S.; Maclennan, G.R. Resinous Binder Compositions. CN94191466A, 27 March 1996.
- Kozinski, A.A. Furfuryl Alcohol Production Process. US4185022A, 22 January 1980.
- Swadesh, S. Catalytic Production of Furfuryl Alcohol and Catalyst Therefor. US2754304A, 10 July 1956.
- Lillwitz, L.D. Manufacture of Furfuryl Alcohol. US4089871A, 16 May 1978.
- Kangyi, L. Method for Preparing Furfuryl Alcohol by Catalyzing Furfural. CN105418551A, 23rd March 2016.
- Zhao, H.; Liu, C.; Jing, M.; Yin, C.; Chai, Y.; Zhao, R.; Zhang, K.; Liu, Y. Method for Preparing Furfuryl Alcohol through Furfural Liquid-Phase Catalytic Hydrogenation. CN102603681A, 25 July 2012.
- Liu, C.; Liu, X.; Wang, H.; Xia, Y.; Xie, Y.; Xu, G. Preparation Method of 2-Zirconium Hydroxyphosphinyl Acetate and Application of 2-Zironiumhydroxyphosphinyl Acetate in Synthesis of Furfuryl Alcohol. CN201910077968A, 16 April 2019.
- Liu, Y.; Qi, Z.; Zhang, J. Method for Preparing Furfuryl Alcohol through Transfer Hydrogenation of Furfural and Low-Grade Alkanol. CN201910664083A, 12 November 2019.
- André Fraga, M.; Farias Perez, R. Process for Obtaining Furfuryl Alcohol by Multifunctional Catalysts. BR102013025330A2, 18 August 2015.
- Perez, R.F.; Fraga, M.A. Hemicellulose-derived chemicals: One-step production of furfuryl alcohol from xylose. Green Chem. 2014, 16, 3942–3950.
- Perez, R.F.; Canhaci, S.J.; Borges, L.E.P.; Fraga, M.A. One-step conversion of xylose to furfuryl alcohol on sulfated zirconia-supported Pt catalyst—Balance between acid and metal sites. Catal. Today 2017, 289, 273–279.
- Paulino, P.N.; Perez, R.F.; Figueiredo, N.G.; Fraga, M.A. Tandem dehydration-transfer hydrogenation reactions of xylose to furfuryl alcohol over zeolite catalysts. Green Chem. 2017, 19, 3759–3763.
- Perez, R.F.; Soares, O.S.G.P.; de Farias, A.M.D.; Pereira, M.F.R.; Fraga, M.A. Conversion of hemicellulose-derived pentoses over noble metal supported on 1D multiwalled carbon nanotubes. Appl. Catal. B 2018, 232, 101–107.
- Perez, R.F.; Albuquerque, E.M.; Borges, L.E.P.; Hardacre, C.; Fraga, M.A. Aqueous-phase tandem catalytic conversion of xylose to furfuryl alcohol over [Al]-SBA-15 molecular sieves. Catal. Sci. Technol. 2019, 9, 5350–5358.
- Canhaci, S.J.; Perez, R.F.; Borges, L.E.P.; Fraga, M.A. Direct conversion of xylose to furfuryl alcohol on single organic–inorganic hybrid mesoporous silica-supported catalysts. Appl. Catal. B 2017, 207, 279–285.
- Cui, J.; Tan, J.; Cui, X.; Zhu, Y.; Deng, T.; Ding, G.; Li, Y. Conversion of Xylose to Furfuryl Alcohol and 2-Methylfuran in a Continuous Fixed-Bed Reactor. ChemSusChem 2016, 9, 1259–1262.
- Zheng, H.; Zhu, Y.; Teng, B.; Bai, Z.; Zhang, C.; Xiang, H.; Li, Y. Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran. J. Mol. Catal. A Chem. 2006, 246, 18–23.
- Zheng, H.; Zhu, Y.; Bai, Z.; Huang, L.; Xiang, H.; Li, Y. An environmentally benign process for the efficient synthesis of cyclohexanone and 2-methylfuran. Green Chem. 2006, 8, 107–109.
- Xu, L.; Nie, R.; Xu, H.; Chen, X.; Li, Y.; Lu, X. One-Pot Tandem Dehydration-Hydrogenation of Xylose with Formic Acid over Co Catalysts. Ind. Eng. Chem. Res. 2020, 59, 2754–2760.
- Ordomsky, V.V.; Schouten, J.C.; van der Schaaf, J.; Nijhuis, T.A. Biphasic single-reactor process for dehydration of xylose and hydrogenation of produced furfural. Appl. Catal. A 2013, 451, 6–13.




