Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zorba J Hernández-Estrada | -- | 2496 | 2022-06-09 23:31:46 | | | |
| 2 | Conner Chen | Meta information modification | 2496 | 2022-06-10 03:54:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hernández-Estrada, Z.; Rojas, A.; Figueroa-Hernández, C.; González-Rios, O.; , .; González-Amaro, R.M.; Rayas-Duarte, P. Biological Activities of Chlorogenic Acids. Encyclopedia. Available online: https://encyclopedia.pub/entry/23903 (accessed on 06 March 2026).
Hernández-Estrada Z, Rojas A, Figueroa-Hernández C, González-Rios O, , González-Amaro RM, et al. Biological Activities of Chlorogenic Acids. Encyclopedia. Available at: https://encyclopedia.pub/entry/23903. Accessed March 06, 2026.
Hernández-Estrada, Zorba, Alexis Rojas, Claudia Figueroa-Hernández, Oscar González-Rios, , Rosa María González-Amaro, Patricia Rayas-Duarte. "Biological Activities of Chlorogenic Acids" Encyclopedia, https://encyclopedia.pub/entry/23903 (accessed March 06, 2026).
Hernández-Estrada, Z., Rojas, A., Figueroa-Hernández, C., González-Rios, O., , ., González-Amaro, R.M., & Rayas-Duarte, P. (2022, June 09). Biological Activities of Chlorogenic Acids. In Encyclopedia. https://encyclopedia.pub/entry/23903
Hernández-Estrada, Zorba, et al. "Biological Activities of Chlorogenic Acids." Encyclopedia. Web. 09 June, 2022.
Copy Citation
The chlorogenic acids (CGAs) are a class of phenolic compounds widely distributed in various plants sources such as fruits, vegetables, coffee beans, tea, apples, and wine. CGAs are esters of quinic acid (QA) and one trans-cinnamic acid residue such as caffeic acid (CA), p-coumaric acid (p-CoA), and ferulic acid (FA), which are known as caffeoylquinic acids (CQAs), p-coumaroylquinic acids (p-CoQAs) and feruloylquinic acid (FQAs).
chlorogenic acids
biological activity
antioxidant activity
Cardiovascular Protection Activity
Anti-Inflammatory Activity
Anticancer Activity
Antidiabetic Activity
Coffee CGAs
1. Biological Activities of CGAs
Several studies have associated CGAs with beneficial health properties, such as antioxidant, antiviral, antibacterial, anticancer, and anti-inflammatory activity [1][2][3][4]. It has also been shown that it can modulate the gene expression of antioxidant enzymes and reduce the risk of cardiovascular disease by suppressing the expression of P-selectin in platelets [4]. In addition, CGAs can reduce the relative risk of type 2 diabetes and Alzheimer’s disease [3][5][6][7][8][9]. The main biological activities attributed to CGAs are shown in Figure 1.
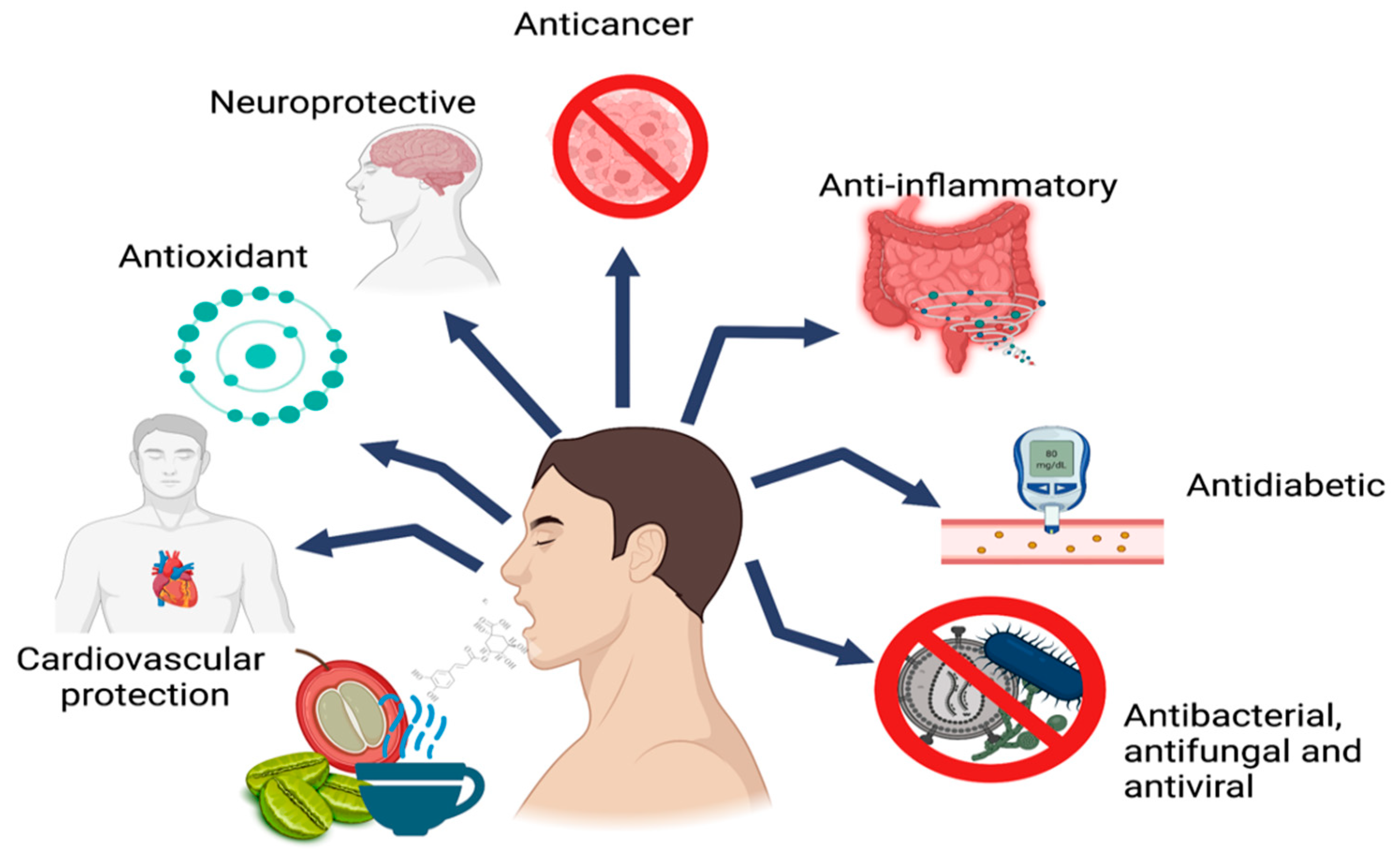
Figure 1. Main biological activities attributed to CGAs.
Some of these properties are well recognized and demonstrated by in vitro and in vivo studies, such as antioxidant activity. However, other bioactivities of interest in recent years, although not yet well demonstrated, such as the potential anti-obesity [7][10][11][12][13][14][15] or prebiotic [16][17][18] properties of CGAs. In addition, it has also been shown that CGAs can modulate the activity of glucose-6-phosphatase, an enzyme involved in glucose metabolism, and therefore it may have a positive effect on diabetes management [19].
Furthermore, it is important to highlight that these biological activities are dependent on the CGA’s stability. CGAs are particularly susceptible to environmental conditions, such as solvent type, pH, temperature, and light. These factors must be considered during the CGAs extraction. Moreover, the concentration of these compounds in plants is low. For this reason, the methodologies used for the CGAs extraction from plant sources must be efficient to guarantee the necessary concentration of CGAs to exert their biological activity.
2. Antioxidant Activity
There is a strong correlation between oxidative stress and the development of various degenerative diseases such as cancer and other aging-related diseases [20][21]. Extensive in vitro and in vivo studies have been performed to evaluate the antioxidant activity of CGAs [22]. As a result, CGAs are known to exhibit a radical scavenging effect similar to ascorbic acid [23]. In addition, CGAs can chelate transition metals such as Fe2+ to scavenge free radicals and disrupt chain reactions [24]. Studies have shown that CGAs may prevent the oxidation of low-density lipoproteins (LDL) induced by different oxidizing agents [25][26], as well as prevent DNA damage in vitro [27]. 5-CQA, which is the most important CGA in coffee, can scavenge 1,1-diphenyl-2-picrylhydrazyl radicals (DPPH), superoxide anions (O2•−), hydroxyl radicals (• OH), and peroxynitrite (ONOO−) [28][29][30], and protect DNA from damage caused by oxidative stress in different studies [22][31].
Therefore, there is enough evidence to support that CGAS can inhibit the formation of reactive oxygen species and play a beneficial role in preventing oxidative and aging-related diseases [20][21]. However, studies indicate that these compounds may also act as potent pro-oxidants. Therefore, depending on their concentration, the presence of free transition metal ions, or their redox state, the antioxidant and pro-oxidant properties of CGAs can be modified [32][33][34].
3. Anti-Inflammatory Activity
Inflammation is a complex physiological process of tissue injury caused by exogenous or endogenous sources [22]. A prolonged unregulated inflammatory process can induce tissue damage and is the cause of many chronic pathologies, such as diabetes, alcoholic liver, chronic kidney disease, and cardiovascular and neurodegenerative diseases [35][36]. CGAs, mainly 5-CQA, have been shown anti-inflammatory activity by reducing pro-inflammatory cytokines, due to modulation of key transcription factors, such as tumor necrosis factor-alpha (TNF-α) and interleukins, such as IL-8 [22][37]. Another study performed in murine RAW264.7 macrophages showed that 5-CQA decreased lipopolysaccharide (LPS)-induced cyclooxygenase (COX-2) up-regulation at both the protein and mRNA levels, suggesting that 5-CQA might exert anti-inflammatory effects through inhibition of prostaglandin E2 (PGE2) production [38]. It has also been reported that CFA can enhance the wound healing process [22]. In a study with diabetic rats, oral administration of 5-CQA increased hydroxyproline concentrations and decreased malondialdehyde/nitric oxide levels in wound tissues. In addition, it allowed elevation of reduced glutathione [39][40]. Topical administration of 5-CQA-containing hydrogels to mouse skin wounds significantly reduced the size of the wound area in the inflammatory phase, improving the healing process [41].
4. Neuroprotective Activity
Alzheimer’s disease is a neurodegenerative disease characterized by progressive deterioration of learning, memory, and other cognitive deficits, along with the extracellular deposition of β-amyloid peptides into the brain leading to neuroinflammation, synaptic loss and neuronal death [42][43]. According to Alzheimer Association [44], in 2050, the number of people aged 65 and older with Alzheimer’s disease will reach 12.7 million. Several studies found an inverse relationship between coffee consumption and the development of Alzheimer’s disease, suggesting its possible use in managing treatments [42][45][46][47][48]. The neuroprotective mechanisms of coffee are suggested to be related to the anti-inflammatory effects of caffeine and CGAs on A1 and A2 receptors. In addition, it reduces toxic deposits of β-amyloid peptides in the brain, which is a distinctive feature in Alzheimer’s patients [3][46][48][49]. Furthermore, some coffee compounds could inhibit brain acetylcholinesterase and butyrylcholinesterase (causing a delay in the degradation of acetylcholine and butyrylcholine), resulting in the prevention of oxidative stress-induced neurodegeneration due to their high antioxidant activity [3][46][50].
On the other hand, murine model trials have shown a significant association between the consumption of CGAs and the prevention of the development of degenerative diseases and aging [3][51][52][53][54]. The effect of phenolic compounds from coffee on human cognitive function has not been well studied [55]. However, the number of in vitro studies concerning the neuroprotective effects of polyphenols is rapidly increasing. It has been demonstrated that intraperitoneal injections of 5-CQA reduced oxidative damage in the cerebellum of rats exposed to methotrexate, a drug with serious side effects used to treat some types of cancer, rheumatoid arthritis, and psoriasis [56]. In the same study, these researchers also observed that application of 5-CQA decreased lipopolysaccharide (LPS)-induced IL-1β and (TNF-α) release in the substantia nigra, indicating neuroprotective effects of 5-CQA on neurodegenerative diseases caused by proinflammatory cytokines [56]. Taram et al. [57] studied the neuroprotective effects of 5-CQA and caffeic and ferulic acids on rat cerebellar granule neuron cultures. This research proposed that caffeic acid showed enhanced neuroprotection against a wide range of stressors compared to the other compounds evaluated. Thus, the authors suggest that caffeic acid could be a promising candidate in preclinical models of neurodegeneration [57].
5. Anticancer Activity
The antimutagenic properties of CGAs was demonstrated decades ago [58]. This activity is partially related to the antioxidant activity of these compounds since the overproduction of oxygen free radicals leads to oxidative DNA damage. This damage is leading cause of the proliferation of several types of cancer, such as breast, colon, bladder, pancreatic, liver, skin, and prostate cancer [59]. Dietary polyphenols, including CGAs, can protect the initiation of tumor processes by inhibiting DNA lesions caused by both free radicals and carcinogens [60]. Indeed, some epidemiological studies demonstrated an inverse relationship between coffee consumption and the risk of certain types of cancer. This effect has been associated with the intake of CGAs [61][62][63]. Several mechanisms have suggested that CGAs may have a chemopreventive effect [36]. Among those, modulation of the expression of enzymes involved as endogenous antioxidant defenses, in DNA replication, as well as in cell differentiation and aging are prominent [60][64]. Moreover, metal chelation, inactivation of reactive compounds, and changes in metabolic pathways have been proposed to impact anticancer activity significantly [65]. Boettler et al. [66] demonstrated by in vitro and in vivo assays that coffee-derived CGAs can induce a cellular and tissue protection mechanism against carcinogenesis via the Nrf2/ARE pathway. This pathway regulates the expression of S-transferases (GST), γ-glutamate-cysteine ligase (γGCL), NAD(P)H: quinone oxidoreductase 1 (NQO1), and heme oxygenase (H01). In another study by Feng et al. [64] using mouse epithelial JB6 cells, it was found that 5CQA had a protective effect against carcinogens. This effect was due to its ability to decrease the generation of free radicals and stimulate glutathione-S-transferase activity.
6. Antidiabetic Activity
According to International Diabetes Federation [67] diabetes (type 1 and 2) is one of the fastest-growing global health emergencies of the 21st century. It was estimated that 537 million adults aged 20–79 years are currently living with diabetes and type 2 diabetes mellitus (T2DM) is the most common type of diabetes, accounting for over 90% of all diabetes worldwide [67]. Several studies have demonstrated an association between moderate consumption of coffee and a lower risk of developing T2DM. This was observed in all sexes, obesity levels, and geographic locations [68][69][70][71][72][73][74][75]. This effect has been attributed to the bioactive compound 5-CQA. Through a meta-analysis, Huxley et al. [76] concluded that daily consumption of three to four cups of coffee decreased the risk of T2DM by 25%.
Furthermore, Bakuradze et al. [77] suggested that consumption of three to four cups of coffee per day could reduce oxidative damage, body fat mass, and energy/nutrient intake and that these effects were partially attributed to CGAs. Shearer et al. [78] studied the effects of regular and decaffeinated coffee (with CGAs) consumption for 28 days on insulin functions, in vivo using a rat model. They observed that the ingestion of decaffeinated coffee improves insulin-stimulated disposal in the high-fat-fed and insulin-resistant rats. Other suggested mechanisms of CGAs are related to the improvement of glucose and lipid metabolism by activating of AMP activated protein kinase (AMPK) [75], as shown in Figure 2. AMPK is a master sensor and regulator of cellular energy balance. This enzyme is activated by diverse pathological, metabolic, and pharmacological stressors such as hypoxia, exercise, thiazolidinediones, and metformin. This activation provokes the translocation of glucose transporter type 4 (GLUT4) from intracellular membranes to plasma and, therefore, the increase of glucose transport [75][79][80].
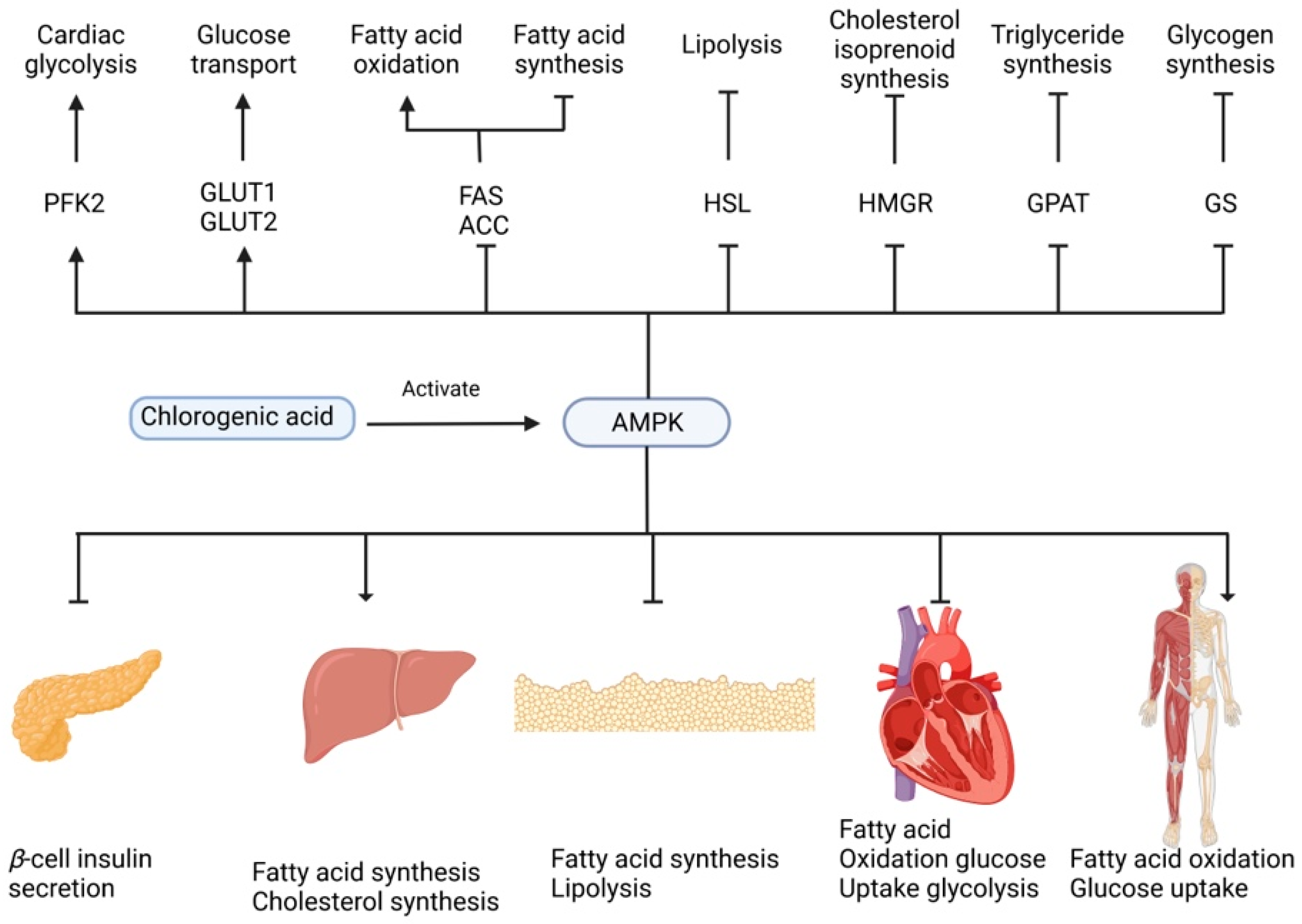
Figure 2. 5CQA-mediated regulation of glucose and lipid metabolism through activation of the AMPK pathway.
7. Cardiovascular Protection Activity
Currently, cardiovascular diseases (CVDs) comprise one of the leading causes of death and disability worldwide. The incidence of various chronic CVDs, including stroke, atherosclerosis, hypertension, ischemic heart disease, and heart failure, probably continues to increase [1]. Some risk factors, such as smoking, high blood pressure, hyperlipidemia, and hyperglycemia, have been reported to contribute, partially, to the development of CVDs [1]. According to the World Health Organization (WHO), ischemic heart disease is the leading cause of death worldwide, accounting for 16% of deaths worldwide (8.9 million people) [81]. Recently, many studies have shown that the consumption of CGAs-rich foods may be recommended to prevent CVDs [4][75][82][83][84]. The high antioxidant and anti-inflammatory activity of CGAs can improve endothelial dysfunction and reduce insulin resistance which could be critical mechanisms to enhance the cardiovascular protection attributed to these compounds, as shown in a large number of in vitro and in vivo studies [22]. Taguchi et al. [85] observed that CGAs could improve endothelial function through by releasing of vasoactive molecules such as nitric oxide. This effect was studied in streptozotocin-treated diabetic rats. On the other hand, CGAs could decrease blood pressure by the following proposed mechanisms: (i) stimulation of nitric oxide production through the endothelium-dependent pathway [86], (ii) reduction of free radicals through decreased expression and activity of NADPH oxidase [87], and (iii) through inhibition of the angiotensin-converting enzyme (ACE) [22].
8. Antibacterial, Antifungal, and Antiviral Activity
The antimicrobial (bacteriostatic and bactericidal) effects of 5-CQA and coffee extracts on various types of detrimental microorganisms that may grow in different parts of the body, from oral bacteria causative of caries to harmful intestinal bacteria, are well known. Roasted C. arabica and C. canephora extracts and brews exhibited antibacterial activity against Streptococcus mutans and other oral types of bacteria [88][89]. Furthermore, 5-CQA can have a positive affect against the adverse microbiota present in the colon. Therefore, this chlorogenic acid can be used as a preservative and food additive [90]. For this reason, CGAs, mainly 5-CQA, could be potential natural antibacterial, antifungal and antiviral agents [91]. For example, 5-CQA exhibited a broad-spectrum antimicrobial activity against Gram-positive (Streptococcus pneumoniae, Staphylococcus aureus, and Bacillus subtilis) and Gram-negative (Escherichia coli, Shigella dysenteriae, and Salmonella typhimurium) pathogenic bacteria by increasing the membrane permeability, leading to plasma membrane barrier dysfunction, as well as leakage of nucleotide [92][93]. The suggested mechanism by which 5-CQA provokes the membrane disruption could involve the perturbation of the membrane lipid bilayer, resulting in cell leakage and dissipation of the membrane electrical potential [1][93].
In addition, Sung and Lee [94] studied the antifungal properties of 5-CQA against Candida albicans, a pathogenic yeast. They suggested that this compound could exert antifungal activity by disrupting the cell membrane structure and consequently, it can be used as an option for fungal treatment. In several studies, both caffeic acid and 5-CQA have demonstrated multiviral activities against herpes simplex virus (HSV) types 1 and 2 [95], adenovirus [96], and HIV [97].
9. Other Bioactivities
9.1. Hepatoprotective Activity
The beneficial effects of coffee on liver diseases, in general, have been reported in several studies [98][99][100] for example, cirrhosis and hepatitis B and C [100]. Hepatic injury may be due to multiple factors, such as viral hepatitis, obesity, excessive alcohol consumption, and iron overload [22]. On the other hand, according to a meta-analysis of 16 human studies, coffee consumption (2 cups per day) decreased the risk of developing liver cancer by 40% compared to no coffee consumption [101][102]. The suggested mechanisms of hepatic protection were the prevention of cell apoptosis and oxidative stress damage due to the activation of natural antioxidant and anti-inflammatory systems [103][104]. These protective mechanisms have been mainly related to CGA [105] and caffeine [106], among other components of coffee.
9.2. Potential Prebiotic Activity
According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), a prebiotic definition is “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [107]. Usually, well-established prebiotics are carbohydrate-based, but other substances such as polyphenols and polyunsaturated fatty acids transformed into their respective conjugated fatty acids can potentially fit into this new prebiotic definition, provided there is sufficient evidence of their positive effect on the host [107]. The consumption of prebiotic foods or compounds selectively favors the growth of probiotic and other health-promoting microorganisms in the gut, especially Bifidobacterium and Lactobacillus [108][109][110]. Thus, indirectly, the health benefits of prebiotics are the following: (i) production of short-chain fatty acids that lower luminal pH, (ii) stimulation of the growth of beneficial intestinal bacteria and suppression of pathogenic bacteria [109][110], (iii) stimulation of the immune system [111][112], (iv) prevention of colon cancer [113], (v) decrease the prevalence to develop diabetes [114][115], and (vi) increased calcium absorption [116]. Furthermore, Kellow et al. [117] observed that dietary supplementation with prebiotics could reduce or delay the accumulation of advanced glycation end products (AGEs) formed through the Maillard reaction in individuals at high risk for type 2 diabetes and improve or restore the microbial balance within the gastrointestinal tract, potentially reducing AGE absorption.
Several studies have suggested that the non-absorbed part of 5-CQA and caffeic acid in the human gastrointestinal tract serves as a substrate for beneficial intestinal microbiota, thus stimulating their growth [118][119]. Whereas the bifidogenic effect of 5-CQA would seem consensus [16][17], the effect of 5-CQA on Lactobacillus growth remains debatable, as only selected strains can utilize it as a substrate [17][18]. Furthermore, Parkar et al. [16] reported an increase in short-chain fatty acids (butyric, acetic, and propionic acid) promoted by 5-CQA. Nevertheless, it has also been observed that 5-CQA promotes the growth of Firmicutes and Bacteroides, and Clostridium. Moreover, an inhibitory effect on the growth of E. coli has only been demonstrated in one study [93]. Therefore, more studies are needed to validate the effect of 5-CQA as a prebiotic.
References
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158.
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484.
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419.
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699.
- Amato, A.; Terzo, S.; Mulè, F. Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: A focus on Alzheimer’s disease. Antioxidants 2019, 12, 608.
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39.
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253.
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of chlorogenic acid against Diabetes Mellitus and its complications. J. Immunol. Res. 2020, 2020, 9680508.
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharmacol. 2013, 85, 1341–1351.
- Wang, Z.; Lam, K.L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588.
- He, X.; Zheng, S.; Sheng, Y.; Miao, T.; Xu, J.; Xu, W.; Huang, K.; Zhao, C. Chlorogenic acid ameliorates obesity by preventing energy balance shift in high-fat diet induced obese mice. J. Sci. Food Agric. 2021, 101, 631–637.
- Ghadieh, H.E.; Smiley, Z.N.; Kopfman, M.W.; Najjar, M.G.; Hake, M.J.; Najjar, S.M. Chlorogenic acid/chromium supplement rescues diet-induced insulin resistance and obesity in mice. Nutr. Metab. 2015, 12, 19.
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 2021, 12, 1717.
- Zheng, G.; Qiu, Y.; Zhang, Q.F.; Li, D. Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br. J. Nutr. 2014, 112, 1034–1040.
- Zhong, Y.; Ding, Y.; Li, L.; Ge, M.; Ban, G.; Yang, H.; Dai, J.; Zhang, L. Effects and mechanism of chlorogenic acid on weight loss. Curr. Pharm. Biotechnol. 2020, 21, 1099–1106.
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19.
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227.
- Sales, A.L.; Depaula, J.; Mellinger Silva, C.; Cruz, A.; Lemos Miguel, M.A.; Farah, A. Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food Funct. 2020, 11, 1410–1424.
- Hemmerle, H.; Burger, H.J.; Below, P.; Schubert, G.; Rippel, R.; Schindler, P.W.; Paulus, E.; Herling, A.W. Chlorogenic acid and synthetic chlorogenic acid derivatives: Novel inhibitors of hepatic glucose-6-phosphate translocase. J. Med. Chem. 1997, 40, 137–145.
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age- related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80.
- Akash, M.S.H.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763.
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16.
- Nakatani, N.; Kayano, S.I.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516.
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids—Occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984.
- Laranjinha, J.A.N.; Almeida, L.M.; Madeira, V.M.C. Reactivity of dietary phenolic acids with peroxyl radicals: Antioxidant activity upon low density lipoprotein peroxidation. Biochem. Pharmacol. 1994, 48, 487–494.
- Gordon, M.H.; Wishart, K. Effects of chlorogenic acid and bovine serum albumin on the oxidative stability of low density lipoproteins in vitro. J. Agric. Food Chem. 2010, 58, 5828–5833.
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on x-ray induced dna damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363.
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210.
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The polyphenol chlorogenic acid attenuates UVB-mediated oxidative stress in human HaCaT keratinocytes. Biomol. Ther. 2014, 22, 136–142.
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta Gen. Subj. 1997, 1335, 335–342.
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630.
- Du, W.Y.; Xiao, Y.; Yao, J.J.; Hao, Z.; Zhao, Y.B. Involvement of NADPH oxidase in high-dose phenolic acid-induced pro-oxidant activity on rat mesenteric venules. Exp. Ther. Med. 2017, 13, 17–22.
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczyński, P.; Łozowicka, B.; Lewandowski, W. The study of anti-/pro-oxidant, lipophilic, microbial and spectroscopic properties of new alkali metal salts of 5-O-caffeoylquinic acid. Int. J. Mol. Sci. 2018, 19, 463.
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Świderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) complex of plant phenolic chlorogenic acid: Antioxidant, antimicrobial and structural studies. Materials 2020, 13, 3745.
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubĩa, P.; Lahera, V.; Lũo, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9.
- Farah, A.; de Paula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11.
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 Cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 Mice. Food Chem. 2015, 168, 167–175.
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced Cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-ΚB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048.
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Etoz, B.C.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 1101–1116.
- Bagdas, D.; Etoz, B.C.; Gul, Z.; Ziyanok, S.; Inan, S.; Turacozen, O.; Gul, N.Y.; Topal, A.; Cinkilic, N.; Tas, S.; et al. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015, 81, 54–61.
- Affonso, R.C.L.; Voytena, A.P.L.; Fanan, S.; Pitz, H.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Varela, L.A.C.; et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (Coffea arabica L.) bean residual press cake on the skin wound healing. Oxidative Med. Cell. Longev. 2016, 2016, 1923754.
- Gardener, S.L.; Rainey-Smith, S.R.; Villemagne, V.L.; Fripp, J.; Doré, V.; Bourgeat, P.; Taddei, K.; Fowler, C.; Masters, C.L.; Maruff, P.; et al. Higher coffee consumption is associated with slower cognitive decline and less cerebral Aβ-Amyloid accumulation over 126 months: Data from the Australian imaging, biomarkers, and lifestyle study. Front. Aging Neurosci. 2021, 13, 681.
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s Disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367.
- Azheimer Association. 2022 Alzheimer’s Disease Facts and Figures; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2022.
- Basurto-Islas, G.; Blanchard, J.; Tung, Y.C.; Fernandez, J.R.; Voronkov, M.; Stock, M.; Zhang, S.; Stock, J.B.; Iqbal, K. Therapeutic benefits of a component of coffee in a rat model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2701–2712.
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain β-Amyloid production. Neuroscience 2006, 142, 941–952.
- Wu, L.; Sun, D.; He, Y. Coffee intake and the incident risk of cognitive disorders: A dose–response meta-analysis of nine prospective cohort studies. Clin. Nutr. 2017, 36, 730–736.
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and Adenosine A2a receptor antagonists prevent β-Amyloid (25–35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245.
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs 2013, 22, 863–880.
- Rosso, A.; Mossey, J.; Lippa, C.F. Caffeine: Neuroprotective functions in cognition and Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2008, 23, 417–422.
- Hao, R.; Song, X.; Li, F.; Tan, X.; Sun-Waterhouse, D.; Li, D. Caffeic acid phenethyl ester reversed cadmium-induced cell death in hippocampus and cortex and subsequent cognitive disorders in mice: Involvements of AMPK/SIRT1 pathway and amyloid-tau-neuroinflammation axis. Food Chem. Toxicol. 2020, 144, 111636.
- Cho, E.S.; Jang, Y.J.; Hwang, M.K.; Kang, N.J.; Lee, K.W.; Lee, H.J. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2009, 661, 18–24.
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Chong, C.M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343.
- Sarroca, S.; Gatius, A.; Rodríguez-Farré, E.; Vilchez, D.; Pallàs, M.; Griñán-Ferré, C.; Sanfeliu, C.; Corpas, R. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 2021, 89, 108569.
- Lakey-Beitia, J.; Berrocal, R.; Rao, K.S.; Durant, A.A. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol. Neurobiol. 2014, 51, 466–479.
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494.
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80.
- Mori, H.; Tanaka, T.; Shima, H.; Kuniyasu, T.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54.
- Toyokuni, S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch. Biochem. Biophys. 2016, 595, 46–49.
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526.
- Mishra, M.; Panta, R.; Miyares, M. Influence of coffee and its components on breast cancer: A review. Asian Pac. J. Trop. Dis. 2016, 6, 827–831.
- Li, Y.M.; Peng, J.; Li, L.Z. Coffee consumption associated with reduced risk of oral cancer: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 381–389.e1.
- Nkondjock, A. Coffee consumption and the risk of cancer: An overview. Cancer Lett. 2009, 277, 121–125.
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of activator Protein-1, NF-ΚB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005, 280, 27888–27895.
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food Chem. Toxicol. 2000, 38, 467–471.
- Boettler, U.; Volz, N.; Pahlke, G.; Teller, N.; Kotyczka, C.; Somoza, V.; Stiebitz, H.; Bytof, G.; Lantz, I.; Lang, R.; et al. Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol. Nutr. Food Res. 2011, 55, 798–802.
- International Diabetes Federation. IDF Diabetes Atlas 2021- 10th Edition|IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021.
- Salazar-Martinez, E.; Willett, W.C.; Ascherio, A.; Manson, J.A.E.; Leitzmann, M.F.; Stampfer, M.J.; Hu, F.B. Coffee consumption and risk for type 2 Diabetes Mellitus. Ann. Intern. Med. 2004, 140, 1–8.
- Agardh, E.E.; Carlsson, S.; Ahlbom, A.; Efendic, S.; Grill, V.; Hammar, N.; Hilding, A.; Östenson, C.G. Coffee consumption, type 2 Diabetes and impaired glucose tolerance in Swedish men and women. J. Intern. Med. 2004, 255, 645–652.
- Lin, W.Y.; Xaiver Pi-Sunyer, F.; Chen, C.C.; Davidson, L.E.; Liu, C.S.; Li, T.C.; Wu, M.F.; Li, C.I.; Chen, W.; Lin, C.C. Coffee consumption is inversely associated with type 2 Diabetes in Chinese. Eur. J. Clin. Investig. 2011, 41, 659–666.
- Pereira, M.A.; Parker, E.D.; Folsom, A.R. Coffee consumption and risk of type 2 Diabetes Mellitus: An 11-year prospective study of 28 812 postmenopausal women. Arch. Intern. Med. 2006, 166, 1311–1316.
- Tuomilehto, J.; Hu, G.; Bidel, S.; Lindström, J.; Jousilahti, P. Coffee consumption and risk of type 2 Diabetes Mellitus among middle-aged Finnish men and women. J. Am. Med. Assoc. 2004, 291, 1213–1219.
- van Dam, R.M. Coffee consumption and risk of type 2 Diabetes, cardiovascular diseasses, and cancer. Appl. Physiol. Nutr. Metab. 2008, 33, 1269–1283.
- van Dam, R.M.; Feskens, E.J.M. Coffee consumption and risk of type 2 Diabetes Mellitus. Lancet 2002, 360, 1477–1478.
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid.-Based Complement. Altern. 2013, 11, 801457.
- Huxley, R.; Man Ying Lee, C.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 Diabetes Mellitus a systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063.
- Bakuradze, T.; Boehm, N.; Janzowski, C.; Lang, R.; Hofmann, T.; Stockis, J.P.; Albert, F.W.; Stiebitz, H.; Bytof, G.; Lantz, I.; et al. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: Results from an intervention study. Mol. Nutr. Food Res. 2011, 55, 793–797.
- Shearer, J.; Sellars, E.A.; Farah, A.; Graham, T.E.; Wasserman, D.H. Effects of chronic coffee consumption on glucose kinetics in the conscious rat. Can. J. Physiol. Pharmacol. 2007, 85, 823–830.
- Kurth-Kraczek, E.J.; Hirshman, M.F.; Goodyear, L.J.; Winder, W.W. 5’AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 1999, 48, 1667–1671.
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabol. 2005, 1, 15–25.
- World Health Organization (WHO). The Top 10 Causes of Death. Available online: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 11 April 2022).
- Mikami, Y.; Yamazawa, T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015, 139, 69–74.
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the Metabolic Syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258.
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic acid protects against OxLDL-induced oxidative damage and mitochondrial dysfunction by modulating SIRT1 in endothelial cells. Mol. Nutr. Food Res. 2018, 62, 1700928.
- Taguchi, K.; Hida, M.; Matsumoto, T.; Ikeuchi-Takahashi, Y.; Onishi, H.; Kobayashi, T. Effect of short-term polyphenol treatment on endothelial dysfunction and thromboxane A2 levels in streptozotocin-induced diabetic mice. Biol. Pharm. Bull. 2014, 37, 1056–1061.
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138.
- Kanegae, M.P.P.; da Fonseca, L.M.; Brunetti, I.L.; de Oliveira Silva, S.; Ximenes, V.F. The reactivity of ortho-methoxy-substituted catechol radicals with sulfhydryl groups: Contribution for the comprehension of the mechanism of inhibition of NADPH oxidase by apocynin. Biochem. Pharmacol. 2007, 74, 457–464.
- Antonio, A.G.; Moraes, R.S.; Perrone, D.; Maia, L.C.; Santos, K.R.N.; Iório, N.L.P.; Farah, A. Species, roasting degree and decaffeination influence the antibacterial activity of coffee against Streptococcus mutans. Food Chem. 2010, 118, 782–788.
- Almeida, A.A.P.; Naghetini, C.C.; Santos, V.R.; Antonio, A.G.; Farah, A.; Glória, M.B.A. Influence of natural coffee compounds, coffee extracts and increased levels of caffeine on the inhibition of Streptococcus mutans. Food Res. Int. 2012, 49, 459–461.
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74.
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358.
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596.
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403.
- Sung, W.S.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010, 82, 219–226.
- Khan, M.T.H.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119.
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62.
- Tamura, H.; Akioka, T.; Ueno, K.; Chujyo, T.; Okazaki, K.I.; King, P.J.; Robinson, W.E. Anti-Human immunodeficiency virus activity of 3,4,5-tricaffeoylquinic acid in cultured cells of lettuce leaves. Mol. Nutr. Food Res. 2006, 50, 396–400.
- Honjo, S.; Kono, S.; Coleman, M.P.; Shinchi, K.; Sakurai, Y.; Todoroki, I.; Umeda, T.; Wakabayashi, K.; Imanishi, K.; Nishikawa, H.; et al. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J. Clin. Epidemiol. 2001, 54, 823–829.
- Wadhawan, M.; Anand, A.C. Coffee and liver disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46.
- La Vecchia, C. Coffee, liver enzymes, cirrhosis and liver cancer. J. Hepatol. 2005, 42, 444–446.
- Bravi, F.; Bosetti, C.; Tavani, A.; Gallus, S.; La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1413–1421.e1.
- Larsson, S.C.; Wolk, A. Coffee consumption and risk of liver cancer: A meta-analysis. Gastroenterology 2007, 132, 1740–1745.
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255.
- Ji, L.; Jiang, P.; Lu, B.; Sheng, Y.; Wang, X.; Wang, Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1919.
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373.
- Machado, S.R.; Parise, E.R.; de Carvalho, L. Coffee has hepatoprotective benefits in Brazilian patients with chronic Hepatitis C even in lower daily consumption than in American and European populations. Braz. J. Infect. Dis. 2014, 18, 170–176.
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2022, 14, 137.
- Allsopp, P.; Possemiers, S.; Campbell, D.; Oyarzábal, I.S.; Gill, C.; Rowland, I. An exploratory study into the putative prebiotic activity of fructans isolated from Agave angustifolia and the associated anticancer activity. Anaerobe 2013, 22, 38–44.
- Saha, D.C.; Reimer, R.A. long-term intake of a high prebiotic fiber diet but not high protein reduces metabolic risk after a high fat challenge and uniquely alters gut microbiota and hepatic gene expression. Nutr. Res. 2014, 34, 789–796.
- Kumar, V.P.; Prashanth, K.V.H.; Venkatesh, Y.P. Structural analyses and immunomodulatory properties of fructo-oligosaccharides from onion (Allium cepa). Carbohydr. Polym. 2015, 117, 115–122.
- Kelly-Quagliana, K.A.; Nelson, P.D.; Buddington, R.K. Dietary oligofructose and inulin modulate immune functions in mice. Nutr. Res. 2003, 23, 257–267.
- Munjal, U.; Glei, M.; Pool-Zobel, B.L.; Scharlau, D. Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Br. J. Nutr. 2009, 102, 663–671.
- Delzenne, N.M.; Cani, P.D.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut microorganisms as promising targets for the management of type 2 Diabetes. Diabetologia 2015, 58, 2206–2217.
- Cluny, N.L.; Eller, L.K.; Keenan, C.M.; Reimer, R.A.; Sharkey, K.A. Interactive effects of oligofructose and obesity predisposition on gut hormones and microbiota in diet-induced obese rats. Obesity 2015, 23, 769–778.
- Griffin, I.J.; Hicks, P.M.D.; Heaney, R.P.; Abrams, S.A. Enriched chicory inulin increases calcium absorption mainly in girls with lower calcium absorption. Nutr. Res. 2003, 23, 901–909.
- Kellow, N.J.; Coughlan, M.T.; Savige, G.S.; Reid, C.M. Effect of dietary prebiotic supplementation on advanced glycation, insulin resistance and inflammatory biomarkers in adults with pre-Diabetes: A study protocol for a double-blind placebo-controlled randomised crossover clinical trial. BMC Endocr. Disord. 2014, 14, 55.
- Rechner, A.R.; Spencer, J.P.E.; Kuhnle, G.; Hahn, U.; Rice-Evans, C.A. Novel biomarkers of the metabolism of caffeic acid derivatives in vivo. Free Radic. Biol. Med. 2001, 30, 1213–1222.
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Revisions:
2 times
(View History)
Update Date:
10 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





