| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Merlin Raud | + 2117 word(s) | 2117 | 2020-09-24 08:39:34 | | | |
| 2 | Vivi Li | -25 word(s) | 2092 | 2020-10-10 08:09:27 | | |
Video Upload Options
This entry gives overview of the lignin separation methods from lignocellulosic biomass using various methods but the main attention has been paid on lignin separation using ionic liquids. Lignin is a natural polymer, one that has an abundant and renewable resource in biomass. Due to a tendency towards the use of biochemicals, the efficient utilization of lignin has gained wide attention. The delignification of lignocellulosic biomass makes its fractions (cellulose, hemicellulose, and lignin) susceptible to easier transformation to many different commodities like energy, chemicals, and materials that could be produced using the biorefinery concept.
1. Introduction
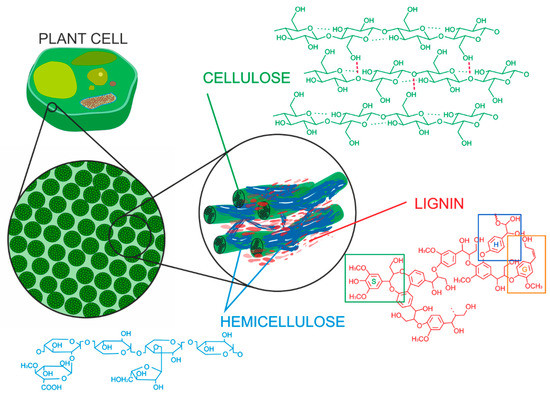
Lignocellulosic (LC) biomass is the most abundant plant material for the production and sustainable supply of liquid biofuels at a relatively low cost [1]. It mainly consists of sugar polymers (cellulose and hemicellulose) and lignin [2]. These three biopolymers, depicted in Figure 1, are the main constituents of plant cell walls [3]. The lignin and hemicelluloses forms a cross-linked matrix which covers the cellulose fibers and thereby forms a tight and, compact structure [4]. Lignin is one of the three primary components of LC biomass which, protects cellulose and hemicellulose by providing mechanical strength but also hydrophobic and indigestible properties to plant cell walls [5][6].
However, the large volumes of lignin resources never been cost efficiently used [6]. It is clear that the chemical pulp industries globally produce about 50 million tons of lignin every year [9]. Despite that, the commercially utilized lignin is only about 2% of total, while the rest is used for combustion in order to produce heat and energy [10]. The use of cheap and renewable bioresources, like lignin, has been attractive since the increasing depletion of fossil fuel resources is concerning level [10]. Besides of economic value, the use of renewable bioresources has potential to decrease the negative effects of the production and use of fuels and chemicals on environment and health [10]. Lignin is the only natural biopolymer containing aromatic phenolpropanoid monomers. Therefore, it can be utilized to produce value-added end products, for example fuel components, via a process of depolymerization [11].
An efficient low-cost technique is required to remove and recover the lignin from biomass to gain enable easier access to the biomass polysaccharides in order to produce different products from lignin [12]. Due to the complexed structure of cell wall microfibrils in LC biomass, the immaculate separation of celluloses and lignin resulting minimal degradation of polymers is a great challenge [11]. Among the various reported biomass pretreatment technologies, pretreatment with ionic liquids (ILs) has shown the most feasible results, especially in a sense of lignin separation [11]. The properties such as no measurable vapor pressure, wide electrochemical window, high thermal stability, nonflammability, excellent solvent power for both organic and inorganic substances, etc.—makes ILs unique and versatile among its kind [13]. More importantly, the ILs properties where they relate to hydrophobicity, polarity, and solvent power can be modified by combining or modifying the cations and anions [14].
2. LC Pretreatment for Lignin Isolation
Lignin separation from the LC biomass is carried out by applying various pretreatment processes [15][16]. As a result of complicated structure and molecular interactions with other components like hemicellulose, dissolution of the natural lignin in wood is very complicated and money consuming procedure. The required methods to separate lignin from the plant cell walls and cellulose, are quite harsh and this partially changes its native conformation [17].
Technical lignins are lignins which are separated from various biomasses during one of a number of available technical processes [18]. Among these are kraft lignin, soda lignin, organosolv lignin, ionic liquid lignin, and hydrolysis lignin. On the other hand, native lignin is lignin in its original form present in LC biomasses, which are, wood lignin (MWL), cellulolytic enzymatic lignin (CEL), and enzymatic mild acidolysis lignin (EMAL).
MWL is mostly used as standard lignin because of its closeness to the native lignin structure. Chang et al. reported the CEL treatment procedure, to further reduction for the isolation-induced modifications [19]. Contrary to MWL, the CEL was obtained in higher yields, in the same milling duration, therefore, it was less degraded. Due to that, CEL is considered more similar to the total lignin fraction in wood. Enzymatic mild acidolysis lignin (EMAL) extraction was studied by Guerra et al. which is more complex procedure that combines milling, enzymatic treatment, and an additional acidolysis step [20]. The aim of the study was the further maximize the yield of the isolated lignin. Moreover, to the researches focused on simplifying the methods for lignin isolation in order to make them more environmentally clean, as well as to obtain lignin closer to its native structure [21].
Usually, Kraft lignin, alkaline, or organosolv lignin are used to test the dissolution rate of lignin. The choices are made considering their commercial availability, that they are products of the chemical pulping process [22]. Kraft lignin refers to the use of the alkaline degradation of LC, while sulfonated lignin arises from the use of a sulfite pulping process. In addition, the pulp and paper industries, extract Kraft lignin from black liquor.
Hydrolysis lignin (HL) is a by-product of enzymatic hydrolysis and fermentation processes in cellulosic ethanol plants. It is primarily consist of lignin (up to 60%) balanced with unreacted cellulose and mono- and oligosaccharides. Further modifications are required for the utilization of HL which are too expensive at the moment otherwise the HL-derived materials do not function well. As a result of this, the majority of HL is disposed without any valorization [23].
The structure of isolated lignin, cellulose, and hemicellulose depends upon the pretreatment method being used. For the separation of lignin from the LC biomass the used methods should focus on; increasing of the accessibility surface area, and cellulose decrystallization, partial depolymerization of cellulose and hemicellulose, maximizing the enzymatic digestibility of the pretreated material, minimizing the sugar-losses, and minimizing capital plus operating costs [24].
The used lignin extraction methods affect the dissolution behavior of lignin. For example, lignosulphonate, Kraft, and soda lignin are soluble in aqueous solvent, while alkali and organosolv lignins are soluble in a wide range of organic solvents [25].
For obtaining the separation of lignin from LC biomasses, there are four broad categories of pretreatment processes, which are; physical pretreatment, chemical pretreatment, physiochemical pretreatment, and native lignin separation (Figure 2).
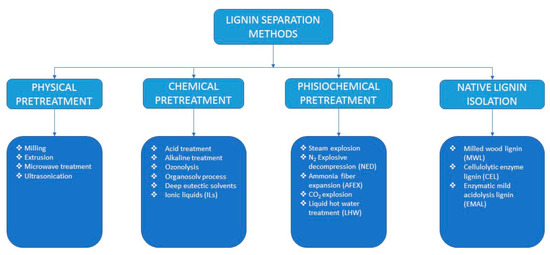
Figure 2. Classification of different lignin separation methods.
Lignin Separation with ILs
Several studies have presented that different solvents like ethylene glycol, DMSO [26], ILs [27], g-valerolactone [28] and Tween-80/H2O are able to dissolve up to 70 wt% lignin [29]. Among these solvents, ILs have acquired especial levels of interest due to their particular chemo-physical properties when compared to traditional molecular solvents.
ILs are molten salts which are also named as being room temperature salts because they are in a liquid state at, or close to liquid state, ambient conditions [30]. The IL-s dissolve a wide range of materials including hydrophobic, hydrophilic, and polymeric compounds [31]. The ability of ILs to be an effective form of disruption targeting the non-covalent interactions between these polymers [32].
In essence, the ILs are formed by a combination of organic cations with organic or inorganic anions, and the desired properties of IL were precursor on the choice of ions [33]. Ion pairs are the parts of all ILs, and the possible combinations of cations and anions are endless. Generally, lignin solubility is high for five groups of ILs containing imidazolium, pyridinium, pyrrolidinium, ammonium, and phosphonium cations. The anions of IL usually include BF4−, FAP−, Tf2N−, OTF−, Cl−, PF6−, CH3COO−, and N(CN)2−. Large non-coordinating anions, such as [BF4]− and [PF6]−, showed low lignin dissolution ability [34].
ILs attract attentions due to their appealing properties. These properties include a wide electrochemical range, low vapor pressure, high thermal stability, and outstanding ionic conductivity even under anhydrous conditions. The dissolution properties of ILs are unique and can be tuned by choosing suitable cations and/or anions. One of the most desirable advantages of ILs in comparison with traditional molecular solvents is its extremely low vapor pressure [35].
Recent studies report that certain ILs have turned up to be very effective in terms of LC biomass delignification. Due to this, they have been considered as fractionation solvents for LC materials. ILs intensify the access to lignin by dissolving LC biomass, which in response leads to the possible further valorization of lignin. Different strategies can be applied during or after IL-aided fractionation in order to produce fuels, chemicals, and materials. These include the extraction of lignin from lignocelluloses, catalytic delignification to acquire separated lignin components from biomass, and the saccharification of polysaccharides that are attached to lignin fragments [28]. Delignification causes disruption of lignin structure, resulting in biomass swelling, which is not feasible in a sense of native lignin.
Hydrogen bond acceptability in IL anions is necessary for efficient biomass dissolution because it contributes to the formation of the hydrogen bonds with biomass components. Considering that, cations should contain strong acidic protons and short side chains to attain the reduction of the inhibition between the IL and biomass during dissolution [36]. As an exception, Muhammad et al. presented their findings which stated that the [Ch][PrOO] IL has a poor dissolution ability, despite of a high hydrogen basicity [32]. This behavior was explained by the internal interaction between the hydroxyl end group of IL cation and its anion.
The dissolution of lignin has been investigated in several aprotic and protic ILs (PILs) (see Table 1). The ILs with moderate hydrogen-bonding anions represents better ability to dissolve lignin [37].
Table 1. The dissolution of lignin with ionic liquids (ILs) and protic ionic liquids (PILs).
| Sample | Solvent | Temp (°C) | Time (h) | Lignin (%) | Cellulose (%) | References |
|---|---|---|---|---|---|---|
| Kraft lignin | Py | 90 | 24 | >50 | 0.10 ± 0.00 | [38] |
| Kraft lignin | Mim | 90 | 24 | >50 | 0.24 ± 0.02 | [38] |
| Kraft lignin | Pyrr | 90 | 24 | 7.98 ± 0.10 | 0.63 ± 0.00 | [38] |
| Kraft lignin | HAc | 90 | 24 | 0.72 ± 0.04 | 0.07 ± 0.01 | [38] |
| Kraft lignin | [Py] [For] | 75 | 1 | 70 | <1 | [39] |
| Kraft lignin | [Py] [Pro] | 75 | 1 | 55 | [40] | |
| Kraft lignin | [Py] [Ac] | 75 | 1 | 64 | [41] | |
| Kraft lignin | [Mmim] [MeSO4] | 80 | 24 | 50 | [41] | |
| Kraft lignin | [Bmim] [CF3SO3] | 80 | 24 | 50 | [38] | |
| Kraft lignin | [Py] [Ac] | 90 | 24 | >50 | 0.12 ± 0.03 | [38] |
| Kraft lignin | [Mim] [Ac] | 90 | 24 | >50 | 0.20 ± 0.05 | [38] |
| Kraft lignin | [Pyrr] [Ac] | 90 | 24 | >50 | 0.79 ± 0.04 | [42] |
| Kraft lignin | GVL/[Bmim]Ac | 30 | 20.9 | [42] | ||
| Kraft lignin | GVL/[Bmim]Ac | 60 | 28.0 | [42] | ||
| Kraft lignin | GVL/[Amim]Cl | 30 | 13.4 | [42] | ||
| Kraft lignin | GVL/[Amim]Cl | 60 | 43 | [43] | ||
| Kraft lignin | [Emim]Ac/water | 60 | 38 | [21] | ||
| Pinus radiata | [C2mim] Ace | 100 | 2 | 58 | [21] | |
| Pinus radiata | [C4mim] Ace | 100 | 2 | 63 | [21] | |
| Pinus radiata | [C4mim]Ace/DMSO | 100 | 2 | 51 | [44] | |
| Maple | [Emim]Ac | 110 | 1.5 | 9 | [44] | |
| Maple | [Emim]Ac | 130 | 24 | 6 | [44] | |
| Maple | [Mmim] [MeSO4] | 80 | 24 | 43.4a | [44] | |
| Maple | [Bmim] [CF3SO3] | 80 | 24 | 5.8b | [40] | |
| Birch | [Emim] [OAc] | 110 | 16 | 97c | [45] | |
| Polar wood | [Emim] [OAc] | 110 | 16 | 85.3c | [46] | |
| Bagasse | [Emim] [ABS] | 190 | 1–1.5 | 60.0c | [47] | |
| Bamboo | [Emim] [Gly] | 120 | 8 | 13.7a | [28] | |
| Corn stover | [Emim] [OAc]/NMP | 140 | 1 | 10.51a | [28] | |
| Cotton stalk | [Amim] [Cl]/DMSO | 130 | 4 | 74.4c | [28] | |
| Bagasse | [Bmim] [Cl] + NaOH | 110 | 12 | 12.73b | [28] | |
| Polar wood | [Emim] [OAc] + NaOH | 110 | 12 | 8.72b | ||
| Bamboo | [Amim] [Cl] + NaOH | 100 | 5 | 4.04b | ||
| Corncob | [Emim] [OAc]/H2O + NaOH | 110 | 9.78b | |||
| Corncob | [Emim] [OAc]/DMF + NaOH | 7.24b | ||||
| Corncob | [Emim] [OAc]/DMSO + NaOH | 19.5a | [48] | |||
| Corncob | [Emim] [OAc]/DMAc + NaOH | 32a | ||||
| Eucalyptus | [Bmim] [Ace] + NaOH | 120 | 3 | 37.3a | ||
| Eucalyptus | [Bmim] [Ace]/DMAc + NaOH | 25.8a | ||||
| Eucalyptus | [Bmim] [Ace]/Dioxane + NaOH | 29.9a | ||||
| Eucalyptus | [Bmim] [Ace]/Ethylacetate + NaOH | 70 | [28] | |||
| Eucalyptus | [Bmim] [Ace]/Toluene + NaOH | 74 | [28] | |||
| Eucalyptus | [Ch] [Lys] | 21 | [28] | |||
| Switchgrass | [Ch] [Lys] | 140 | 1 | |||
| Pine | [Ch] [Lys] |
a Based on dry biomass; b based on Klason lignin; c based on the original lignin content in raw biomass.
3. Conclusions and Future Remarks
The exploitation of ILs in LC biomass treatment shows the tremendous potential in this research filed, especially within the context of the separation of lignin from LC biomass. The successful dissolution of lignin in ILs has broadened the sight when it comes to utilizing biomass for the production of biofuels, chemicals, and materials in a sense of technical and economic growth. The dependence of the ILs physical properties and their ability to solubilize LC biomass is considered important in the production of biochemicals. Due to its easily synthesizing process, various ILs can be used to design solvents with desired physicochemical properties that focus on the specific subcomponents of LC biomass.
To take into account the possible use in biorefinery scale is necessary for the practical considerations of pretreatment with ILs techniques. The results of experiments that have been carried out in relation to regeneration and the potential for recycling when it comes to pyridinium and pyrrolidinium-based PILs showed that the present dissolution processes that are using them could open the door to the diverse production of high-value products from lignin, including, binders, carbon fibers, phenolic compounds lignin-based polymers, and sorbents.
In all likelihood, lignin is a promising resource when it comes to replacing petroleum products in the chemical industry. Numerous studies have also shown there is undeniable potential for the depolymerization of lignin for the production of chemicals. However, these studies have mostly focused on experiments at a laboratory scale. The product characterization from the upgraded scale of lignin depolymerization can improve utilization of the lignin depolymerization process in the production of chemicals. Such an advance could afford what would be considered the foundation of biomass utilization and a green future.
References
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries—Industrial processes and products. In Ullmann’s Biotechnology and Biochemical Engineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; Volume 2, pp. 785–817.
- De Gonzalo, G.; Colpa, D.I.; Habib, M.H.M.; Fraaije, M.W. Bacterial enzymes involved in lignin degradation. J. Biotechnol. 2016, 236, 110–119.
- Kikas, T.; Tutt, M.; Raud, M.; Alaru, M.; Lauk, R.; Olt, J. Basis of Energy Crop Selection for Biofuel Production: Cellulose vs. Lignin. Int. J. Green Energy 2016, 13, 49–54.
- Rocha-Meneses, L.; Ferreira, J.A.; Mushtaq, M.; Karimi, S.; Orupõld, K.; Kikas, T. Genetic modification of cereal plants: A strategy to enhance bioethanol yields from agricultural waste. Ind. Crops Prod. 2020, 150, 112408.
- Raud, M.; Mitt, M.; Oja, T.; Olt, J.; Orupõld, K.; Kikas, T. The utilisation potential of urban greening waste: Tartu case study. Urban For. Urban Green. 2017, 21, 96–101.
- Xu, A.; Guo, X.; Zhang, Y.; Li, Z.; Wang, J. Efficient and sustainable solvents for lignin dissolution: Aqueous choline carboxylate solutions. Green Chem. 2017, 19, 4067–4073.
- Wang, B.; Sun, Y.-C.; Sun, R.-C. Fractionational and structural characterization of lignin and its modification as biosorbents for efficient removal of chromium from wastewater: A review. J. Leather Sci. Eng. 2019, 1, 5.
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 631013.
- Ahmad, E.; Pant, K.K. Chapter 14—Lignin Conversion: A Key to the Concept of Lignocellulosic Biomass-Based Integrated Biorefinery. In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–444. ISBN 978-0-444-63992-9.
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Top. Issue Biomater. 2014, 39, 1266–1290.
- Moniruzzaman, M.; Moniruzzaman, M.; Goto, M.; Goto, M. Ionic Liquid Pretreatment of Lignocellulosic Biomass for Enhanced Enzymatic Delignification. Adv. Biochem. Eng. Biotechnol. 2019, 168, 61–77.
- Raud, M.; Rooni, V.; Kikas, T. Explosive decompression pretreatment: Nitrogen vs. compressed air. Agron. Res. 2016, 14, 569–578.
- Earle, M.J.; Seddon, K.R. Ionic Liquids: Green Solvents for the Future. In Clean Solvents; Martin, A.A., Luc, M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 819, pp. 10–25. ISBN 978-0-8412-3779-7.
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183.
- Chen, H.; Junying, Z. Clean production technology of integrated pretreatment for Lignocellulose. Afr. J. Agric. Res. 2013, 8, 339–348.
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729.
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular self-assembled chaos: Polyphenolic lignin’s barrier to cost-effective lignocellulosic biofuels. Mol. Basel Switz. 2010, 15, 8641–8688.
- Erdtman, H. Lignins: Occurrence, formation, structure and reactions, K.V. Sarkanen and C. H. Ludwig, Eds., John Wiley & Sons, Inc., New York, 1971. 916 pp. $35.00. J. Polym. Sci. B 1972, 10, 228–230.
- Chang, H.; Cowling, E.B.; Brown, W. Comparative Studies on Cellulolytic Enzyme Lignin and Milled Wood Lignin of Sweetgum and Spruce. J. Biol. Chem. Phys. Technol. Wood 1975, 29, 153–159.
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Saquing, C.; Baumberger, S.; Argyropoulos, D.S. Toward a better understanding of the lignin isolation process from wood. J. Agric. Food Chem. 2006, 54, 5939–5947.
- Pinkert, A.; Goeke, D.F.; Marsh, K.N.; Pang, S. Extracting wood lignin without dissolving or degrading cellulose: Investigations on the use of food additive-derived ionic liquids. Green Chem. 2011, 13, 3124–3136.
- Nasrullah, A.; Bhat, A.H.; Sada Khan, A.; Ajab, H. Chapter 9—Comprehensive approach on the structure, production, processing, and application of lignin. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Jawaid, M., Md Tahir, P., Saba, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 165–178. ISBN 978-0-08-100959-8.
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419.
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609.
- Krigstin, S.; Sameni, J.; Sain, M. Solubility of lignin and acetylated lignin in organic solvents. BioResources 2017, 12, 1548–1565.
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Valorizing Recalcitrant Cellulolytic Enzyme Lignin via Lignin Nanoparticles Fabrication in an Integrated Biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710.
- Merino, O.; Fundora-Galano, G.; Luque, R.; Martínez-Palou, R. Understanding Microwave-Assisted Lignin Solubilization in Protic Ionic Liquids with Multiaromatic Imidazolium Cations. ACS Sustain. Chem. Eng. 2018, 6, 4122–4129.
- Zhu, X.; Peng, C.; Chen, H.; Chen, Q.; Zhao, Z.; Zheng, Q.; Xie, H. Opportunities of Ionic Liquids for Lignin Utilization from Biorefinery. ChemistrySelect 2018, 3, 7945–7962.
- Xu, A.; Li, W.; Zhang, Y.; Xu, H. Eco-friendly polysorbate aqueous solvents for efficient dissolution of lignin. RSC Adv. 2016, 6, 8377–8379.
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583.
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881.
- Da Costa Lopes, A.M.; João, K.G.; Morais, A.R.C.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquids as a tool for lignocellulosic biomass fractionation. Sustain. Chem. Process. 2013, 1, 3.
- Álvarez, V.H.; Dosil, N.; Gonzalez-Cabaleiro, R.; Mattedi, S.; Martin-Pastor, M.; Iglesias, M.; Navaza, J.M. Brønsted Ionic Liquids for Sustainable Processes: Synthesis and Physical Properties. J. Chem. Eng. Data 2010, 55, 625–632.
- Melro, E.; Alves, L.; Antunes, F.; Medronho, B. A brief overview on lignin dissolution. J. Mol. Liq. 2018, 265.
- Anugwom, I.; Eta, V.; Virtanen, P.; Mäki-Arvela, P.; Hedenström, M.; Hummel, M.; Sixta, H.; Mikkola, J.-P. Switchable Ionic Liquids as Delignification Solvents for Lignocellulosic Materials. ChemSusChem 2014, 7, 1170–1176.
- Moyer, P.; Smith, M.D.; Abdoulmoumine, N.; Chmely, S.C.; Smith, J.C.; Petridis, L.; Labbé, N. Relationship between lignocellulosic biomass dissolution and physicochemical properties of ionic liquids composed of 3-methylimidazolium cations and carboxylate anions. Phys. Chem. Chem. Phys. 2018, 20, 2508–2516.
- Hou, Q.; Ju, M.; Li, W.; Liu, L.; Chen, Y.; Yang, Q. Pretreatment of Lignocellulosic Biomass with Ionic Liquids and Ionic Liquid-Based Solvent Systems. Molecules 2017, 22, 490.
- Achinivu, E. Protic Ionic Liquids for Lignin Extraction—A Lignin Characterization Study. Int. J. Mol. Sci. 2018, 19, 428.
- Miranda, R.d.C.M.; Neta, J.V.; Ferreira, L.F.R.; Gomes, W.A.J.; do Nascimento, C.S.; Gomes, E.d.B.; Mattedi, S.; Soares, C.M.F.; Lima, A.S. Pineapple crown delignification using low-cost ionic liquid based on ethanolamine and organic acids. Carbohydr. Polym. 2019, 206, 302–308.
- Rashid, T.; Kait, C.F.; Regupathi, I.; Murugesan, T. Dissolution of kraft lignin using Protic Ionic Liquids and characterization. Ind. Crops Prod. 2016, 84, 284–293.
- Scriven, E.F.V.; Toomey, J.E., Jr.; Murugan, R. Pyridine and Pyridine Derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology; American Cancer Society: Oklahoma City, OK, USA, 2000; ISBN 978-0-471-23896-6.
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734.
- Tolesa, L.; Gupta, B.; Lee, M.-J. Degradation of Lignin with Aqueous Ammonium-Based Ionic Liquid Solutions under Milder Condition. New J. Chem. 2019, 43.
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376.
- Achinivu, E.C.; Howard, R.M.; Li, G.; Gracz, H.; Henderson, W.A. Lignin extraction from biomass with protic ionic liquids. Green Chem. 2014, 16, 1114–1119.
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic Liquid as a Green Solvent for Lignin. J. Wood Chem. Technol. 2007, 27, 23–33.
- Xue, Z.; Zhao, X.; Sun, R.; Mu, T. Biomass-Derived γ-Valerolactone-Based Solvent Systems for Highly Efficient Dissolution of Various Lignins: Dissolution Behavior and Mechanism Study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870.
- Wang, Y.; Wei, L.; Li, K.; Ma, Y.; Ma, N.; Ding, S.; Wang, L.; Zhao, D.; Yan, B.; Wan, W.; et al. Lignin dissolution in dialkylimidazolium-based ionic liquid-water mixtures. Bioresour. Technol. 2014, 170C, 499–505.