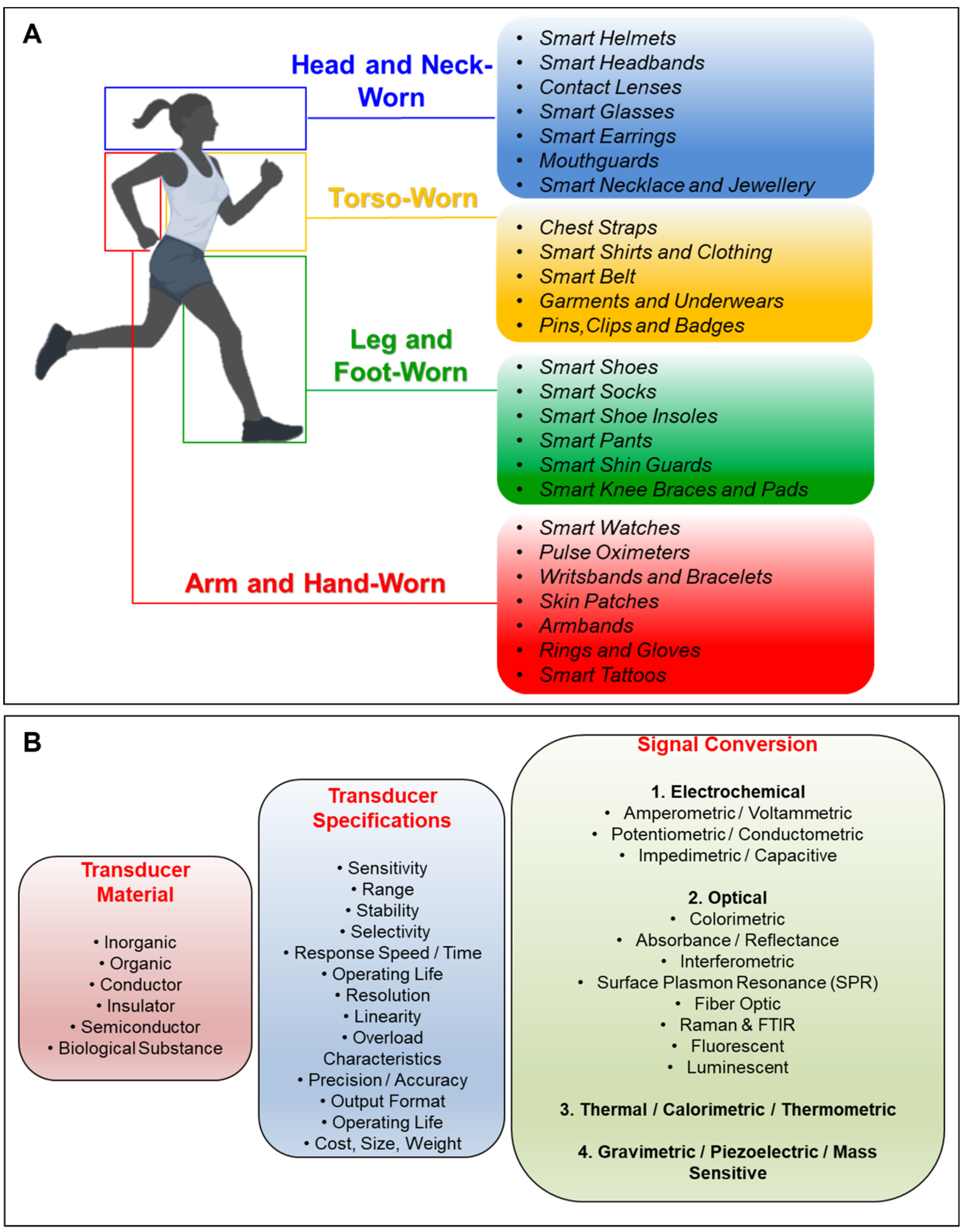
Transducers for biosensors depend on the type of material used, specifications of the sensor device, and the actual signal conversion mechanism (Figure 1B). The transducer materials are generally classified as inorganic, organic, conductor, insulator, and semiconductor, and can also be found in the form of a biological substance. While the specifications of the transducer are mostly defined by the capabilities of the active sensing material, the design of the device also plays an important role in the definition of the final specifications (Figure 1B). To that end, transducer mechanism simply defines the class of the biosensors, for instance, a biosensor is classified as an “electrochemical biosensor” if it uses an electrochemical transducer.
2. Supplementary Technologies for Wearable Biosensing
2.1. Microfluidics and Biomedical Microelectromechanical Systems (Bio-MEMS)
Microfluidics provides an efficient platform for transducers by forming the core of the sensing elements and providing advanced liquid holding and storage capabilities
[7]. Microfluidics-based point of care medical sensors are of ever-growing interest due to their versatility as wearable lab-on-the-body systems
[8][9][10]. This section is dedicated to a brief outlook on the importance and varieties of the conformal biomedical microelectromechanical (bio-MEMS) sensors as a supplementary technology to the wearable transducer-based sweat analysis. Sweat is a compatible substance with wearable medical sensor systems owing to its transparent and hypotonic properties
[8][11]. Furthermore, natural sweating induces high enough pressure (~70 kPa) to sustain pressure different for the microfluidic channels
[8][11][12]. However, sweat-based sensing is prone to false-concentration-based analyte readings due to vaporization
[8][11][13][14] and inhomogeneous local sweat gland density
[12]. It has been demonstrated that the measurement error could be as high as 114%
[9]. Furthermore, the sensory system should include microfluidics, electronics, and a power supply to collate and relay data on the collected and analyzed sweat in real-time, which results in a rather bulky system. Moreover, the said system should be conformal if desired to be employed in direct contact with the skin on different parts of the body. Despite these shortcomings and constraints, analysis of the constituents of sweat is a crucial part of medical diagnosis with an ever-growing focus owing to the variety of critical biomarkers that can be detected in tiny volumes.
Biomarkers present in the sweat that can be detected by wearable microfluidics-based medical sensors are (i) glucose, (ii) lactate, (iii) pH, (iv) chloride, (v) creatinine, (vi) tyrosine, (vii) uric acid, (viii) potassium, (ix) sodium, (x) ascorbic acid, (xi) cortisol, (xii) dopamine, and (xiii) adrenaline. The concentration of glucose in sweat is 1% of that of the plasma and its accurate measurement is very important for continuous monitoring of diabetes
[10][15]. On the other hand, lactate is an indicator of physical effort signaling the transition from aerobic to anaerobic metabolism
[11][16]. Determining the level of pH helps in ascertaining the neuromuscular condition of the subject
[11], while chloride levels are used to help with the diagnosis of cystic fibrosis
[11][12]. The level of creatinine is an important indicator in confirming the hydration status of the metabolism and renal health
[12] and determining tyrosine level from sweat is generally used for the diagnosis of liver diseases and various psychiatric disorders
[14]. Thus, the analytes found in sweat serve as crucial markers for the performance of vital organs and critical systems in the human body; therefore, using sweat as a working fluid in a wearable Bio-MEMS sensor is crucial. The reported wearable microfluidic devices that use sweat to analyze the abovementioned analytes are summarized in
Table 1.
Table 1. Biomarkers and driving mechanisms in wearable microfluidic Bio-MEMS *.
In the wearable microfluidic wearable Bio-MEMS, the sweat is collected and regulated via the natural pressure difference of sweat glands
[8][9][11][12][13][14][15][16][17][18][19]. The capillary force is usually strong enough to drive the fluid through microchannels leading to the chamber where various sensor types are employed
[8][12][15]. The flow regulation can be managed via active thermo-responsive hydrogel valves controlled by microheaters
[9], or passive capillary pressure-bursting valves
[17]. Capillary force arises due to the hydrophilicity of the wetted surface and allows liquids to travel long distances without large pressure difference when the characteristic length-scale of the ducts is in microns
[12][15]. The microchannel itself will exert a shear resistance on the flow, which will be balanced with the capillary force, and a static pressure build-up will occur when there is no flow. This pressure might be released in terms of sudden hydrodynamic pressure by changing the shape, and thus the contact angle, along the way. A bursting valve operates on the very same principle
[17]. On the other hand, an active valve, such as the thermo-responsive system
[9], does not require plugging the duct entirely but rather partially obstructs the duct, inducing substantial head loss on the flow thus depriving it of the mechanical energy necessary to overcome the shear resistance. The reported works using these main driving mechanisms for the control of sweat flow in wearable Bio-MEMS are summarized in
Table 1.
2.1.1. Energy Sources and Detection Mechanisms
Although passive effects such as the capillary force or secondary ducts do not require a power source, active valves, as well as sensory electronics along with powering the detection mechanism and achieving real-time or ad-hoc relay of data, require a power supply. Power supply in wearable Bio-MEMS sensors is based on either energy scavenging or electrochemical conversion aside from the fact that microfluidics relies on natural pressure difference of sweat
[11][12] except for thermo-responsive valves that are polymers actuated via a change in temperature
[17]. The power source exploited in such systems should be of low voltage and current for the safety and practicality of the system. Also, the source should be either easily replicable or rechargeable, avoiding the requirement for complicated reassembly procedures. To that end, the most practical common solution is to employ batteries to generate electric currents through electrochemical conversion through battery packs. On the other hand, the electronics can be fitted with energy scavenging MEMS hardware using electromagnetic fields of a certain frequency, such as the one emitted by a smartphone
[17], or the body movement of the subject wearing the system transforming mechanical vibration to electricity by means of induction and triboelectric effect during physical exercise
[18]. Furthermore, the external energy might only be required for near field communication (NFC) to pair the wearable Bio-MEMS sensor and the device employed to collect the data
[12]. Furthermore, smartphones can incorporate the wearable system in a vast IoT framework
[8]. The reported microfluidic wearables using various energy sources and the utilized detection mechanisms are summarized in
Table 2.
Table 2. The reported energy sources and utilized detection mechanisms in microfluidic wearables.
2.1.2. Data Transmission
Another important aspect for wearables to represent a mobile technology is their data transmission. To that end, a wearable system would usually exploit wireless means of communication. The wireless connection usually suffers from a limited onboard power source with wearable systems; therefore, long rage real-time radio frequency (RF) communication is not feasible, whereas short-range data transfer options are widely preferred. There are currently three main methods in the data communication of wearables, namely, wireless, i.e., Bluetooth
[9][11][13][14][18], NFC
[12][17], and visual data capture
[8][12][17]. Bluetooth communication can be real-time while NFC and visual data recording are designed to be intermittent. While visual data recording does not require either special electronics or power sources dedicated to the task, wireless and NFC need custom circuitry, an RF antenna, and a device to be paired and collate the data of interest
[9][11][12][13][14][17][18]. In addition, active flow control can be achieved by wireless communication via a mobile device
[9]. The presence of integrated electronics increases the device in size to a relative bulk, however, still practical to apply on the skin.
The transmitted sensory data are of different origins. There are simplistic systems using NFC to pair the sensor with a device such as a smartphone to start an application for image processing out of colorimetric data
[12]. Colorimetric data is obtained by the reaction of analytes with certain chemicals and enzymes that emit certain frequencies if excited by fluorescent or visible light
[8][12][17] that will reveal the spatial concentration. These systems do not necessarily require real-time data transfer and additional onboard power dedicated to the colorimetric analysis
[12]. In addition to colorimetric sensors, there are systems with hybrid sensing compartments or completely different transducing approaches, e.g., galvanic, strain, piezoresistive effect, and electrochemical. The reported devices with various transducing mechanisms are summarized in
Table 2.
Electrochemical biosensors are exclusively microfluidic and rely on custom electrodes coated with specific mediator molecules, such as carbon nanomaterials, to choose and react to a targeted analyte
[13][14][16]. The induced current by the transducer is directly related to the flux of the molecules of interest in the vicinity of the electrode
[9]. Thus, the transportation and diffusion of analytes in the microfluidic network and reservoirs are of utmost importance
[14]. Also, strain sensors could exploit swelling of bulk material, e.g., hydrogels, as sweat gets absorbed while stretching a conductive fabric changing its resistance
[19]. Similarly, the sweat rate can be detected based on the change in galvanic properties of specifically designed microchannel geometries.
2.1.3. Biocompatibility
In microfluidic wearables, the sensors and antennas, along with a printed electrical interface to the necessary PCB, are expected to be implemented on biocompatible conformal structures to seamlessly fit on any surface. Furthermore, the sensor is supposed to be attached to the skin via medical-grade adhesives. Sweat itself is supposed to be collected and stored by the carefully tailored network of microfluidic channels and chambers embedded in this elastic structure. Such a network can be implemented in PDMS
[13][15][16][18], silicone rubber
[11], and medical adhesives
[14].
Polyimide (PI)
[14][15], PDMS
[10], and polyethylene terephthalate (PET)
[14] are demonstrated as a cover to the microchannels, as well as for the printing of the sensory electrodes. Moreover, different porous materials could be used to collect sweat, such as hydrogel
[19] and Ecoflex
[15], in certain volumes. Likewise, paper and cotton can be used for sweat collection, fluid flow compartmentalization, and sensor placement
[8][10]. In addition, the thermo-responsive valves can be manufactured out of poly(
N-isopropylacrylamide)
[9].
To reach the desired wearable microfluidic device components, microchannels and reservoirs can be implemented via different fabrication techniques such as photolithography, screen printing, laser engraving, laying cotton fibers, and embossment.
Table 3 presents the list of the reported wearable fabrication techniques and biocompatible microfluidic device materials. The said techniques are utilized to manufacture tailored microchannels and electrodes meeting different production constraints. For instance, laser engraving is utilized as a faster and cheaper solution as it reduces the need for infrastructure and expertise required for photolithography
[12][13][14][16][18][20]. Also, making use of easy-to-obtain materials such as cotton or paper when feasible greatly simplifies the manufacturing procedure and reduces the overall cost
[8][10]. Likewise, the techniques of screen-printing and embossment are employed as a cost-effective solution to microchannel manufacturing while preserving the dexterity to obtain a channel network with bifurcations
[11][12][13][14]. Nevertheless, manufacturing electrodes for electrochemical or galvanic sensing or building the associated interfacial electronics entails the use of photolithography
[12][13][16][20].
Table 3. Biocompatible materials and fabrication techniques for microfluidic-based wearable biosensors.
Overall, the end product is biocompatible and flexible so that the wearable biosensor will perform without exhibiting any chemical reaction to the skin or the sweat. To that end, it is important to acknowledge that the flow rates in these microfluidic networks are usually on the order of 0.1–1 μL/min and they are designed to accommodate fluidic conditions of natural sweating.
2.2. Location and Position Services
Currently, available wearable devices are mostly equipped with location/position services for various purposes such as finding a route, counting the steps, or calculating exercise output to give extended feedback to the user. Wearables are more efficient for location services than smartphones since they are bonded to the user in various form factors. This section provides a general outlook on current advancements in the location and position systems as a supplementary technology for wearables.
The global positioning system (GPS) used in outdoor positioning is a satellite-based navigation system developed by the United States Department of Defense
[21]. Outdoor position information can be calculated when four or more GPS satellites are in the line of sight
[21]. The GPS enables critical outdoor positioning, predominantly for civil, commercial, and military applications. To that extent, Assisted-GPS (A-GPS) technology is a positioning system used in mobile devices to find the user’s location within the A-GPS address server via the base station
[22]. The accuracy provided by GPS in outdoor positioning is between 3 m and 15 m, while usually around 10 m for indoor positioning
[21][22]. On the other hand, the accuracy of the A-GPS in outdoor positioning is 15 m while limited to 50 m for indoors
[22]. Although these accuracy values allow sufficient position determination outdoors, since people spend more than 80% of their time indoors, suitable indoor geolocation systems are in high demand. Unfortunately, the use of GPS satellites in indoor localization is not sufficient enough with the weakening and loss of GPS signals due to the atmospheric delays, signal reflections (multipath), steel structures, roofs, and building walls
[21][22]. Therefore, in the last two decades, a serious amount of work has been carried out on developing new technologies that enable a reliable indoor positioning with high accuracy and low average error. When the GPS signals cannot be reached, the positioning problem is solved by using different technologies such as infrared, ultrasonic, cellular, radio frequency recognition (RFID), wireless network (Wi-Fi), Bluetooth beacon, or ultra-wideband (UWB) sensors
[23][24][25][26]. In some studies, even visible light
[27][28] and technologies that use the earth’s magnetic field have been used
[29][30]. However, new algorithms and methods are needed to improve the results.
Among the new indoor positioning methodologies, ultra-wideband (UWB) sensor technology steps forward with its ideal indoor distance estimation, indoor geolocation, indoor tracking, and navigation
[31]. The UWB sensor technology is important in providing centimeter-scale positioning accuracy indoors. UWB is a radio technology used in short-range high-bandwidth communication. UWB has higher bandwidth of 500 MHz; therefore, signals usually reach the receiver in more than one way
[32]. However, high bandwidth allows a range of frequencies to be used at different times; thus, UWB can be used to solve multipath problems and interference effects. UWB transmitters consume relatively low power compared to other indoor geolocation technologies, making them a more efficient option by providing a longer battery life for wearables. The power consumption of wearable UWB transmitters is generally less than 1 mW, while the power consumption of UWB receivers is around 400 mW
[33].
The UWB frequency range for communication applications is between 3.1 and 10.6 GHz
[34]. This frequency range makes UWB signals less affected by disturbances and prevents them from being affected by Wi-Fi and Bluetooth signals, mainly operating in the 2.4 GHz frequency band. In indoor positioning, where the number of people and objects is high, the field of view (LOS-line of sight) may be relatively blocked, which may cause delay and deviation in the received signal. It is important to note that the error rate increases if the user to be positioned is out of sight, but it is difficult to conclude that the absorbing effects of the human body will increase these errors
[35][36]. Time of flight (ToF) and time of arrival (ToA) methods are used to determine indoor location with UWB sensors.
UWB sensors with the time difference of arrival method (TDoA) draw attention in medical applications
[37][38]. Another technique, the angle of arrival (AoA), allows the signals to be compared with the signal strength of the angles received from at least two sources. In this way, the object’s location can be found from the angle of the intersection of the signals
[39]. With the ToF and TDoA methods, indoor positioning can be performed with an average error of around 20 cm
[40]. Since UWB technology requires a special transmitter and receiver infrastructure, it has not yet entered the informatics market, except for a few industrial applications
[41]. However, the current advancements and planning for the integration of UWB sensors in mobile phones show that UWB sensors will be used in a wide variety of areas in the very near future, including wearables
[42][43].