
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lotte Elisabeth Ruudje Kleimeier | -- | 2695 | 2022-06-08 14:41:08 | | | |
| 2 | Peter Tang | -388 word(s) | 2307 | 2022-06-09 03:12:07 | | | | |
| 3 | Peter Tang | Meta information modification | 2307 | 2022-06-09 03:12:33 | | |
Video Upload Options
Noonan Syndrome (NS) is a multisystem disorder, caused by dysregulation of the Ras/MAPK signaling pathway. Pathogenic gene variants in the Ras/MAPK pathway can therefore lead to various lymphatic diseases such as lymphedema, chylothorax and protein losing enteropathy.
1. Introduction
2. Imaging the Lymphatic System
3. Clinical Manifestation Compared to Radiological Findings in Patients with NS
DCMRL and T2 imaging were performed in seven patients with NS and lymphatic diseases. Informed consent for publication was acquired from all parents and, when appropriate, patients aged 12 years or older. This research has been approved by the Medical Ethics Committee at Radboud University Medical Center Nijmegen (file number 2020-6852).
Patient 1: 11-year-old female was diagnosed with NS and a de novo pathogenic variant in SOS2 (c.800T>A p.(Met267Lys)). Prenatal ultrasounds showed polyhydramnios. She had no signs of the lymphatic disorders until six years of age, when she presented with lower extremity lymphedema. The T2 imaging showed a partially developed thoracic duct, pulmonary (interstitial) and periportal edema and lymphangiectasia in the retroperitoneal space and mesentery. DCMRL demonstrated retrograde lymphatic flow in the lung interstitium, mesenteric and periportal lymphatic vessels, findings that are consistent with CCLA.
Patient 2: 17-year-old female who was diagnosed with NS, with a pathogenic variant in SOS2 (c.800T>A p.(Met267Lys)). Prenatal ultrasounds showed an increased nuchal translucency (>3 mm) without polyhydramnios. She had no signs of the lymphatic disorders until the age of nine years, when she developed lymphedema of the lower extremities and abdominal wall. In addition, she had neuropathic pain in both feet. The T2 images suggested a partial aplasia of the thoracic duct, an enlarged cisterna chyli, peritoneal lymphatic cysts, lymphangiectasia in the retroperitoneal space, and subcutaneous body wall as well as signs of pulmonary interstitial edema were also noted. DCMRL showed no contrast opacification of the thoracic duct, and retrograde flow in the peribronchial, mesenteric and periportal lymphatic vessels, as well as dermal backflow in the abdominal wall, findings that are consistent with CCLA.
Patient 3: 32-year-old female was diagnosed with NS, and a de novo pathogenic variant in SOS2 (c.800T>C p.(Met267Thr)). Prenatal ultrasounds showed polyhydramnios. She presented with lymphedema in the extremities during infancy, which improved within the first few years of her life. At 16 years she again developed severe lymphedema of the upper and lower extremities. DCMRL showed a dilated, and in intermittently duplicated, thoracic duct, without cisterna chyli. However there was no failure to empty into the thoracic duct or the subclavian vein. In addition, there was no abnormal retrograde lymphatic flow or extravasation of contrast. Therefore, these findings were not consistent with CCLA, according to the definition of Ricci, K.W. et al. (2021)
Patient 4: 20-year-old male was diagnosed with NS and a pathogenic variant in RIT1 (c.280G>A (p.(Ala77Thr)). At the age of 6 years, he developed chylous ascites, followed by chylothorax, lymphedema of the lower extremities and scrotal area, and scrotal chylous leakage. The T2 imaging showed left-sided pleural fluids and mesenteric edema. DCMRL images showed partial aplasia of the thoracic duct, retrograde contrast flow into the scrotum as well as dermal backflow and lymphangiectasia behind the cecum and throughout the retroperitoneum, findings that are consistent with CCLA Figure 1.
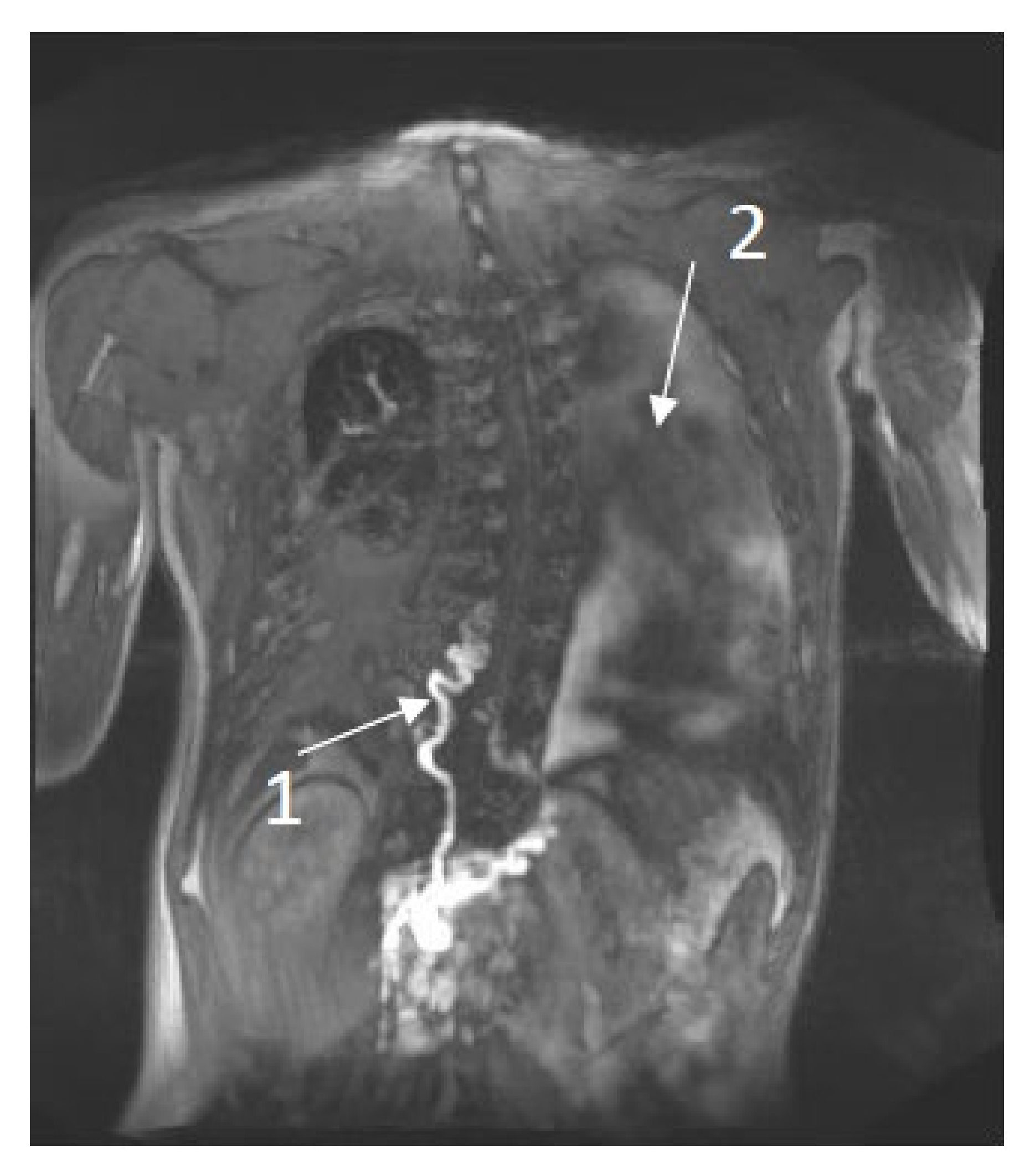
Figure 1. DCMRL results of a 20-year-old male with NS. 1. Abnormal tortuous thoracic duct and partial aplasia. 2. Left-sided pleural fluid.
Patient 5: 31-year-old female was diagnosed with NS and pathogenic variant in PTPN11 (c.181G>A p.(Asp61Asn)). Prenatal ultrasound information was not available. At the age of one year she developed chylothorax after cardiac surgery for repair pulmonary valve stenosis. At the age of ten years she developed lymphedema of the lower extremities as well as vulvar and labial lymphangiectasia, and genital lymphorrhea. At the age of 26 years, she underwent surgical resection of the vulvar lymphangiectasia, which has been reported by Winters et al. [24] T2 imaging shows a dilation of the thoracic duct and lymphangiectasia behind the cecum and throughout the retroperitoneum. DCMRL showed dermal backflow towards the labia, lower extremities, and the abdominal wall, findings that are consistent with CCLA.
Patient 6: 27-year-old male was diagnosed with NS and a pathogenic variant in SOS1 (c.1277A>C p.(Gln426Pro)). During adulthood, he was diagnosed with protein losing enteropathy. T2 images showed a normally developed thoracic duct. DCMRL demonstrated normal antegrade flow in the thoracic duct however retrograde flow into the mesentery; which can potentially explain the protein losing enteropathy, findings that are consistent with CCLA.
Patient 7: 34-year-old male was diagnosed with NS and pathogenic variant in PTPN11 (c.182A>G (p.Asp61Gly)). He was diagnosed with pericardial effusion at the age of 18 years, and chylothorax at the age of 32. T2 imaging visualized bilateral pleural and pericardial fluid as well as ascites, mesenterial and subcutaneous edema and pelvic cysts. DCMRL showed a tortuous, dilated and partially duplicated thoracic duct, without cisterna chyli, as well as retrograde flow into the periportal lymphatic vessels and dermal backflow in the abdominal wall, findings that are consistent with CCLA.
The clinical lymphatic diseases of CCLA seen in these patients include lymphedema, chylothorax, genital lymph leakage and protein losing enteropathy. In all patients the thoracic duct was abnormal. In six patients the retrograde flow in the peribronchial, periportal or mesenteric lymphatic vessels, or dermal backflow was demonstrated.
|
Nr. |
Age (y) (m/f) |
Clinical Manifestation of Lymphatic Disease |
Radiological Findings |
Reference |
||
|---|---|---|---|---|---|---|
|
TD |
Flow abnormalities |
Other findings |
||||
|
1 |
14 (f) |
CT, PLE, MLA, RLA |
ND |
Retrograde mesenteric and pulmonary flow |
Leak of contrast into duodenal lumen, abnormal CLS |
Dori, Y [13] |
|
2 |
13 (m) |
LE, PLE, HT |
ND |
Pleural fluids ascites |
oedematous intestine |
Keberle, M [17] |
|
3 |
17 (f) |
PLE |
absent |
ND |
abdominal collateral lymphatics and bilateral iliac lymphangiectasia |
Matsumoto, T [16] |
|
4 |
61 (m) |
LE, SLE |
Occlusion at the neck |
Increased pelvic and retroperitoneal flow |
PLA, abdominal ascites |
Othman, S [15] |
|
5 |
0.9 (f) |
CT |
Dilated |
Bilateral perfusion |
- |
Biko [1] |
|
6 |
0.6 (m) |
CT |
Double duct, central TD not present |
Bilateral perfusion |
Body wall edema |
Biko [1] |
|
7 |
0.1 (m) |
CT |
ND |
Bilateral pleural effusions |
Body wall edema, ascites |
Biko [1] |
|
8 |
0.8 (m) |
CT, ascites |
absent |
Bilateral perfusion |
Body wall edema, ascites |
Biko [1] |
|
9 |
7 (f) |
CT |
absent |
Bilateral perfusion |
Pericardial effusion, ascites |
Biko [1] |
|
10 |
0.2 (m) |
CT |
absent |
Bilateral perfusion |
Body wall edema |
Biko [1] |
|
11 |
0.1 (f) |
CT |
rudimentary |
Bilateral perfusion |
ascites |
Biko [1] |
|
12 |
0.1 (f) |
CT |
Double duct |
Perfusion right lung |
- |
Biko [1] |
|
13 |
0.1 (m) |
CT, anasarca |
Dilated |
Bilateral perfusion |
Network of lymphatic collaterals in left neck, body wall edema, ascites |
Biko [1] |
|
14 |
5 (m) |
ascites |
absent |
Peritoneum perfusion |
Ascites |
Biko [1] |
CLS: central lymphatic system, CT: chylo-thorax, f: female, HT: hydrocele testis, LE: lymphedema, m: male, MLA: mesenteric lymphangiectasia, ND: no data, PLA: pulmonary lymphangiectasia, PLE: protein losing enteropathy, RLA: retroperitoneal lymphangiectasia, SLE: scrotal lymphedema.
4. Possible Implications for Therapeutic Options
References
- David M. Biko; Breanne Reisen; Hansel J. Otero; Chitra Ravishankar; Teresa Victoria; Andrew C. Glatz; Jonathan J. Rome; Yoav Dori; Imaging of central lymphatic abnormalities in Noonan syndrome. Pediatric Radiology 2019, 49, 586-592, 10.1007/s00247-018-04337-6.
- Karim Kouz; Christina Lissewski; Stephanie Spranger; Diana Mitter; Angelika Riess; Vanesa Lopez-Gonzalez; Sabine Lüttgen; Hatip Aydin; Florian von Deimling; Christina Evers; et al.Andreas HahnMaja HempelUlrike IssaAnne-Karin KahlertAdrian LiebPablo Villavicencio-LoriniMaria Juliana Ballesta-MartinezSheela NampoothiriAngela Ovens-RaederAlena PuchmajerováRobin SatanovskijHeide SeidelStephan UnkelbachBernhard ZabelKerstin KutscheMartin Zenker Genotype and phenotype in patients with Noonan syndrome and a RIT1 mutation. Genetics in Medicine 2016, 18, 1226-1234, 10.1038/gim.2016.32.
- William E. Tidyman; Katherine A. Rauen; Expansion of the RASopathies. Current Genetic Medicine Reports 2016, 4, 57-64, 10.1007/s40142-016-0100-7.
- Andrew Grant; Brandon Cushman; Helene Cave; Mitchell W. Dillon; Bruce D. Gelb; Karen W. Gripp; Jennifer A. Lee; Heather Mason-Suares; Katherine A. Rauen; Lisa M. Vincent; et al.Martin Zenker Assessing the Gene-Disease Association of 19 Genes with the RASopathies using the ClinGen Gene Curation Framework. Hum Mutat. 2018, 11, 323303, 10.1101/323303.
- Amy E Roberts; Judith E Allanson; Marco Tartaglia; Bruce D Gelb; Noonan syndrome. The Lancet 2013, 381, 333-342, 10.1016/s0140-6736(12)61023-x.
- Marco Tartaglia; Bruce D. Gelb; Martin Zenker; Noonan syndrome and clinically related disorders. Best Practice & Research Clinical Endocrinology & Metabolism 2011, 25, 161-179, 10.1016/j.beem.2010.09.002.
- Yong Deng; Michael Simons; Lymphatic fate determination: playing RAF with ERK.. Cell Cycle 2013, 12, 1157-8, 10.4161/cc.24491.
- Taija Mäkinen; Laurence M. Boon; Miikka Vikkula; Kari Alitalo; Lymphatic Malformations: Genetics, Mechanisms and Therapeutic Strategies. Circulation Research 2021, 129, 136-154, 10.1161/circresaha.121.318142.
- Angela Myers; Jonathan A. Bernstein; Marie-Luise Brennan; Cynthia Curry; Edward Esplin; Jamie Fisher; Margaret Homeyer; Melanie A. Manning; Eric A. Muller; Anna-Kaisa Niemi; et al.Laurie H. SeaverSusan R. HintzLouanne Hudgins Perinatal features of the RASopathies: Noonan syndrome, Cardiofaciocutaneous syndrome and Costello syndrome. American Journal of Medical Genetics Part A 2014, 164, 2814-2821, 10.1002/ajmg.a.36737.
- Swarts J KL, Leenders E, Klein WM, Draaisma JMT.; Lymphatic anomalies during lifetime in patients with Noonan Syndrome. Article in preparation 2021, 0, 0.
- Christina Lissewski; Valérie Chune; Francesca Pantaleoni; Alessandro De Luca; Yline Capri; Julia Brinkmann; Francesca Lepri; Paola Daniele; Erika Leenders; Laura Mazzanti; et al.Emanuela ScaranoFrancesca Clementina RadioKerstin KutscheAlma KuechlerMarion GérardKara RanguinMarine LegendreYoann VialIneke van der BurgtTuula RinneElena AndreucciGioia MastromoroMaria Cristina DigilioHélène CaveMarco TartagliaMartin Zenker Variants of SOS2 are a rare cause of Noonan syndrome with particular predisposition for lymphatic complications. European Journal of Human Genetics 2020, 29, 51-60, 10.1038/s41431-020-00708-6.
- Julia Sleutjes; Lotte Kleimeier; Erika Leenders; Willemijn Klein; Jos Draaisma; Lymphatic Abnormalities in Noonan Syndrome Spectrum Disorders: A Systematic Review. Molecular Syndromology 2021, 13, 1-11, 10.1159/000517605.
- Yoav Dori; Chris Smith; Erin Pinto; Kristen Snyder; Michael E. March; Hakon Hakonarson; Jean Belasco; Severe Lymphatic Disorder Resolved With MEK Inhibition in a Patient With Noonan Syndrome and SOS1 Mutation. Pediatrics 2020, 146, 6, 10.1542/peds.2020-0167.
- Dong Li; Michael March; Alvaro Gutierrez-Uzquiza; Charlly Kao; Christoph Seiler; Erin Pinto; Leticia S. Matsuoka; Mark R. Battig; Elizabeth J. Bhoj; Tara L. Wenger; et al.Lifeng TianNora RobinsonTiancheng WangYichuan LiuBrant M. WeinsteinMatthew SwiftHyun Min JungCourtney N. KaminskiRosetta ChiavacciJonathan A. PerkinsMichael LevinePatrick M. A. SleimanPatricia J. HicksJanet T. StrausbaughJean B. BelascoYoav DoriHakon Hakonarson ARAF recurrent mutation causes central conducting lymphatic anomaly treatable with a MEK inhibitor. Nature Medicine 2019, 25, 1116-1122, 10.1038/s41591-019-0479-2.
- Sammy Othman; Saïd C. Azoury; David DiBardino; Denise M. Adams; Maxim Itkin; Stephen J. Kovach; Respiratory Failure in Noonan Syndrome Treated by Microsurgical Thoracic Duct-Venous Anastomosis. The Annals of Thoracic Surgery 2021, 113, e219-e221, 10.1016/j.athoracsur.2021.05.039.
- Tomohiro Matsumoto; Takahiro Kudo; Jun Endo; Kazunobu Hashida; Nao Tachibana; Takatsugu Murakoshi; Terumitsu Hasebe; Transnodal lymphangiography and post-CT for protein-losing enteropathy in Noonan syndrome. Minimally Invasive Therapy & Allied Technologies 2014, 24, 1-4, 10.3109/13645706.2014.996162.
- M. Keberle; H. Mörk; M. Jenett; D. Hahn; M. Scheurlen; Computed tomography after lymphangiography in the diagnosis of intestinal lymphangiectasia with protein-losing enteropathy in Noonan's syndrome. European Radiology 2000, 10, 1591-1593, 10.1007/s003300000384.
- Robert I. Hilliard; James B.J. Mckendry; M. James Phillips; Congenital Abnormalities of the Lymphatic System: A New Clinical Classification. Pediatrics 1990, 86, 988-994, 10.1542/peds.86.6.988.
- Michel Wassef; Francine Blei; Denise Adams; Ahmad Alomari; Eulalia Baselga; Alejandro Berenstein; Patricia Burrows; Ilona J. Frieden; Maria C. Garzon; Juan-Carlos Lopez-Gutierrez; et al.David J.E. LordSally MitchelJulie PowellJulie PrendivilleMiikka Vikkula Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203-e214, 10.1542/peds.2014-3673.
- Govind B. Chavhan; Joao G. Amaral; Michael Temple; Maxim Itkin; MR Lymphangiography in Children: Technique and Potential Applications. RadioGraphics 2017, 37, 1775-1790, 10.1148/rg.2017170014.
- Caroline J. Schaik; Lucas L. Boer; Jos M. T. Draaisma; Carine J. M. van der Vleuten; Jan Jaap Janssen; Jurgen J. Fütterer; Leo J. Schultze Kool; Willemijn M. Klein; The lymphatic system throughout history: from hieroglyphic translations to state of the art radiological techniques. Clinical Anatomy 2022, 0, 0, 10.1002/ca.23867.
- Lymphoscintigraphy of the Lower Extremities Haas de M. Richtlijnendatabase (2022)
- Maxim Itkin; Aaron Chidekel; Kelly A. Ryan; Deborah Rabinowitz; Abnormal pulmonary lymphatic flow in patients with paediatric pulmonary lymphatic disorders: Diagnosis and treatment. Paediatric Respiratory Reviews 2020, 36, 15-24, 10.1016/j.prrv.2020.07.001.
- Harm Winters; Hanneke J.P. Tielemans; Dietmar J.O. Ulrich; Lymphovenous Anastomosis and Secondary Resection for Noonan Syndrome with Vulvar Lymphangiectasia. Plastic and Reconstructive Surgery - Global Open 2016, 4, e1007, 10.1097/gox.0000000000001007.
- Robert J. Damstra; Anne-Berth Halk; B. Halk; J.P. Van Den Berg; Y. Born; E.S.F.A. Butter; E.B.L. van Dorst; J.J.E. van Everdingen; C. Feenstra; P. Gielink; et al.M.J. de HaasG. van der Heide-SchoonA. HendrickxJ.T. HiddingJ.B. Storm van LeeuwenS. NijlandE. OskamI. PaulusmaN. RussellK. Rutgers-Van WijlenI. SissinghL.J.A. StrobbeC.J.A. Verhoeff- BraatR. VerwaardC. van der VleutenH.G.J.M. VoestenA. HartogM.C. Urgert The Dutch lymphedema guidelines based on the International Classification of Functioning, Disability, and Health and the chronic care model. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2017, 5, 756-765, 10.1016/j.jvsv.2017.04.012.
- J. Strehl; M. Schepke; E. Wardelmann; W. H. Caselmann; T. Sauerbruch; Chronische Diarrhö bei einem 43-jährigen Patienten. Der Internist 2003, 44, 626-630, 10.1007/s00108-003-0856-1.
- Durga Prasad; Anshu Srivastava; Anil Tambe; Surender Kumar Yachha; Moinak Sen Sarma; Ujjal Poddar; Clinical Profile, Response to Therapy, and Outcome of Children with Primary Intestinal Lymphangiectasia. Digestive Diseases 2019, 37, 458-466, 10.1159/000499450.
- Lilian Downie; Arun Sasi; Atul Malhotra; Congenital chylothorax: Associations and neonatal outcomes. Journal of Paediatrics and Child Health 2013, 50, 234-238, 10.1111/jpc.12477.
- Kaitlin Mitchell; Angela Weiner; Patricia Ramsay; Mitali Sahni; Use of Propranolol in the Treatment of Chylous Effusions in Infants. Pediatrics 2021, 148, 0, 10.1542/peds.2020-049699.
- Gregory J. Nadolski; Maxim Itkin; Thoracic Duct Embolization for Nontraumatic Chylous Effusion. Chest 2013, 143, 158-163, 10.1378/chest.12-0526.
- Tamer A Othman; Tali Azenkot; Benjamin N Moskoff; Matthew E Tenold; Brian A Jonas; Venetoclax-based combinations for the treatment of newly diagnosed acute myeloid leukemia. Future Oncology 2021, 17, 2989-3005, 10.2217/fon-2021-0262.
- Eugenia Manevitz-Mendelson; Gil S. Leichner; Ortal Barel; Inbal Davidi-Avrahami; Limor Ziv-Strasser; Eran Eyal; Itai Pessach; Uri Rimon; Aviv Barzilai; Abraham Hirshberg; et al.Keren ChechekesNinette AmariglioGideon RechaviKarina YanivShoshana Greenberger Somatic NRAS mutation in patient with generalized lymphatic anomaly. Angiogenesis 2018, 21, 287-298, 10.1007/s10456-018-9595-8.
- Sarah F. Barclay; Kyle W. Inman; Valerie L. Luks; John B. McIntyre; Alyaa Al-Ibraheemi; Alanna J. Church; Antonio R. Perez-Atayde; Shamlal Mangray; Michael Jeng; Sara R. Kreimer; et al.Lori WalkerSteven J. FishmanAhmad I. AlomariGulraiz ChaudryCameron C. TrenorDenise AdamsHarry P. W. KozakewichKyle C. Kurek A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genetics in Medicine 2018, 21, 1517-1524, 10.1038/s41436-018-0390-0.
- Masabumi Shibuya; VEGFR and Type-V RTK Activation and Signaling. Cold Spring Harbor Perspectives in Biology 2013, 5, a009092-a009092, 10.1101/cshperspect.a009092.




