Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nitesh Kumar Nandwana | -- | 2987 | 2022-06-08 14:39:54 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2987 | 2022-06-09 03:22:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nandwana, N.; Das, B.; Nandwana, V.; , .; Almaguel, F.; Das, B. Microbiota in Neurodegenerative Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/23835 (accessed on 07 February 2026).
Nandwana N, Das B, Nandwana V, , Almaguel F, Das B. Microbiota in Neurodegenerative Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/23835. Accessed February 07, 2026.
Nandwana, Nitesh, Bhaskar Das, Varsha Nandwana, , Frankis Almaguel, Bhaskar Das. "Microbiota in Neurodegenerative Disease" Encyclopedia, https://encyclopedia.pub/entry/23835 (accessed February 07, 2026).
Nandwana, N., Das, B., Nandwana, V., , ., Almaguel, F., & Das, B. (2022, June 08). Microbiota in Neurodegenerative Disease. In Encyclopedia. https://encyclopedia.pub/entry/23835
Nandwana, Nitesh, et al. "Microbiota in Neurodegenerative Disease." Encyclopedia. Web. 08 June, 2022.
Copy Citation
Hundreds of billions of commensal microorganisms live in and on human bodies, most of which colonize the gut shortly after birth and stay there for the rest of human lives. In animal models, bidirectional communications between the central nervous system and gut microbiota (Gut–Brain Axis) have been extensively studied, and it is clear that changes in microbiota composition play a vital role in the pathogenesis of various neurodevelopmental and neurodegenerative disorders, such as Autism Spectrum Disorder, Alzheimer’s disease (AD), Parkinson’s disease (PD), Multiple Sclerosis, Amyotrophic Lateral Sclerosis, anxiety, stress, and so on.
microbiome
gut-brain axis
boron-based diet
Alzheimer’s disease
Parkinson’s disease
boron neuroprotective agent
1. Introduction
The human body is home to billions of small living creatures known collectively as the human microbiota, and their genome is referred to as the microbiome. The gut microbiota, sometimes known as the “forgotten organ,” with roughly 3 million genes, which is up to 150 times the human genome [1]. Microbes flourish on human skin, as well as in human genitourinary, gastrointestinal, and respiratory systems, with the gastrointestinal tract being the most densely infested. The colon and rectum, located at the end of the gastrointestinal (GI) tract, are thought to house the greatest number of bacteria in the human body [2]. Surprisingly, just one-third of human's gut microbiota is shared by most individuals, while the remaining two-thirds is unique to each person, similar to a personal identification card [3]. The makeup of this microbial population changes over time, and it is subject to both external and endogenous variations [4]. Diet, metabolism, age, location, stress, and antibiotic therapy may all disrupt the balance between helpful commensals and potentially pathogenic microorganisms, ‘Dysbiosis’ [5] is the word for this shattered equilibrium. The gut microbiota has been shown to have a crucial role in maintaining immune function and metabolic balance, vitamin generation, pathogen protection, stimulating angiogenesis, and maintaining the intestinal barrier. The realization that gut microbiota plays a role in maintaining homeostasis and regulating practically every major bodily system, including the central nervous system (CNS), has sparked a revolt in biomedicine during the last two decades [6]. The “gut–brain axis” (GBA) implies the existence of a two-way communication route between gut microorganisms and the CNS, is now widely accepted [7], and dysregulation of this axis is increasingly suspected of being involved in the pathophysiology of neurological disorders, such as Autism Spectrum Disorder, Alzheimer’s disease, multiple sclerosis, Parkinson’s disease, etc. [6]. Currently, microbiome-based therapies, such as prebiotics, probiotics, and synbiotics, as well as microbiota fecal transplants, aim to promote eubiosis to improve metabolic and mental health [8]. In addition, Boron (B), a kind of active bio-trace-element, has been suggested to be an essential nutrient, which imparts neuroprotective effects. Boron intake has been linked to bone, mineral, and lipid metabolism, and immunological function. As evidence mounts that B is critical for human health, it is critical to investigate probable links between B nutrient intake and brain and psychological function [9].
2. Alzheimer’s Disease
Dementia is a condition in which memory, conduct, reasoning, capacity to do daily activities, judgment, and language deteriorate. Alzheimer’s disease and other forms of dementia have been claimed to be the fifth leading cause of mortality worldwide [10]. The most important risk factor is age, with the vast majority of people with Alzheimer’s dementia being 65 or older [11]. Extracellular -amyloid (A), senile plaques (SP), and intracellular neurofibrillary tangles (NFT) are the key characteristics of Alzheimer’s disease. Increased production of reactive oxygen species (ROS) causes neuroinflammation and cell death. In addition, vascular abnormalities and mitochondrial damage have a role in the etiology of Alzheimer’s disease [12][13].
2.1. Gut Dysbiosis and Alzheimer’s Disease
The generation of signaling proteins that impact metabolic pathways relevant to AD development is affected by changes in the gut microbiota. The ageing process causes local systematic inflammation, which impairs GIT permeability and blood–brain barrier function, by modifying the GM composition, i.e., a higher abundance of pro-inflammatory bacteria than anti-inflammatory bacteria (Figure 1) [14].
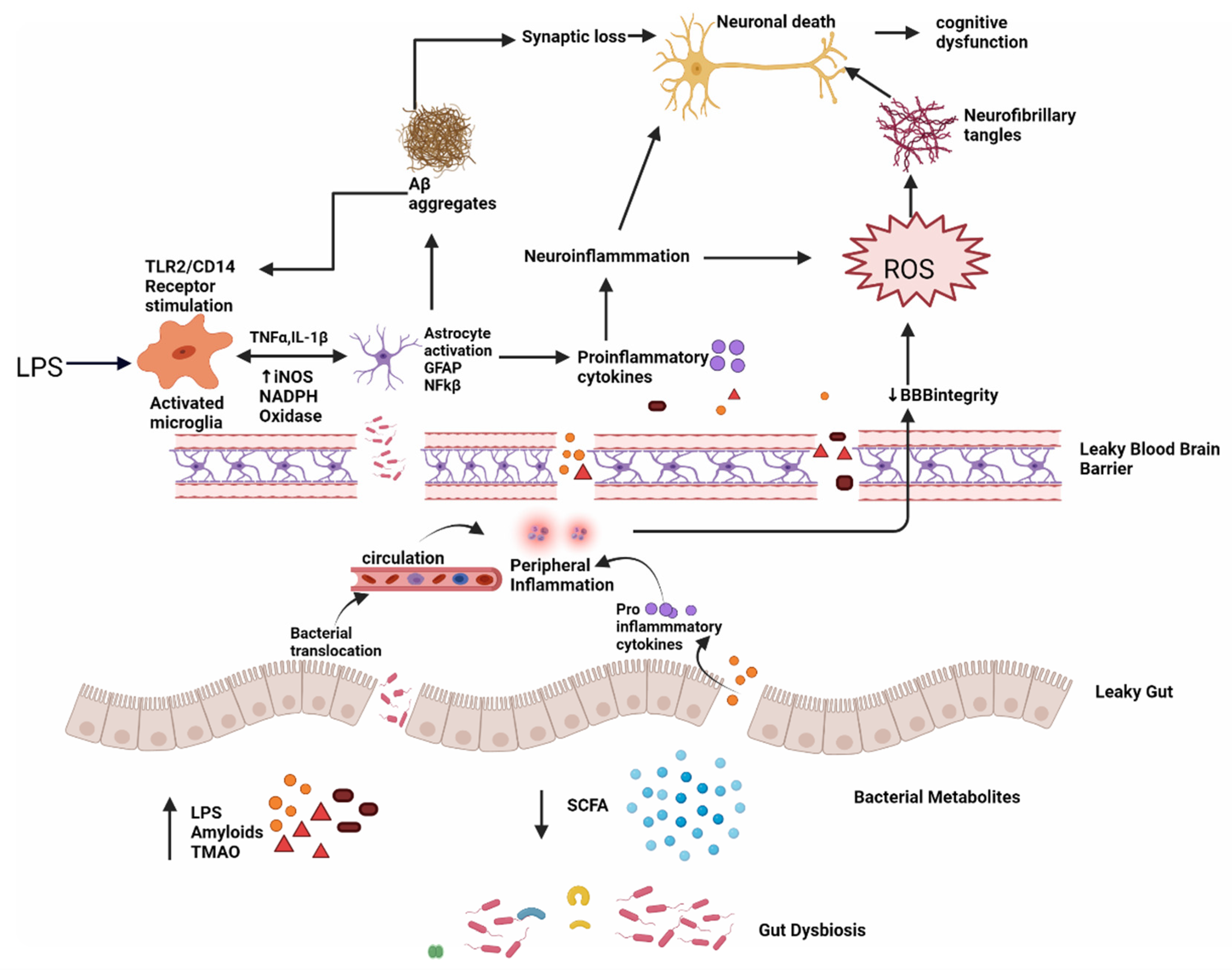
Figure 1. Dysbiosis and Alzheimer’s disease; intestinal permeability is harmed when gut equilibrium is disrupted by pro-inflammatory microorganisms that produce bacterial amyloids, LPS, TMAO and decrease beneficial bacterial metabolites, such as SCFA. Impairment in the gut and blood–brain barrier leads to the increased invasion of microbes into peripheral and CNS and increase production of pro-inflammatory cytokines and thus causing peripheral and central inflammation. This neuroinflammation leads to neuronal death directly and through ROS which leads to the formation of neurofibrillary tangles. LPS also acts on TLR2/TLR4 CD14 receptor on activated microglia, increasing TNF-α, IL-1β, iNOS, NADPH oxidase, and thus astrocyte activation and NF-kB activity which further promote Aβ aggregation. Aβ also acts as an agonist to the TLR4 receptor and thus promotes the vicious cycle of amyloid aggregation and ultimately neuronal death in AD.
2.2. Metabolites Implicated in Alzheimer’s Disease
Lipopolysaccharide (LPS)—Lipopolysaccharide (LPS) is a lipid-sugar compound that is a prominent component of Gram-negative bacteria’s cell walls [10] (50–70% in the normal gut microbiota). LPS is a valuable tool for investigating neuroinflammation in neurodegenerative diseases [15]. Tight connections between intestinal epithelial cells prevented LPS from entering the bloodstream in healthy people. LPS will enter the bloodstream and produce inflammation if the tight connections are weakened. As a result, blood LPS levels indicate not only inflammation but also a leaky gut. A slew of in vivo and in vitro investigations have revealed that LPS activates many intracellular molecules that alter the expression of several inflammatory mediators, hence contributing to or initiating neurodegeneration. LPS activates TLR4-CD14/TLR2 receptors on leukocytes and microglia, resulting in NF-kB-mediated cytokine surges that raise Aβ levels, injure oligodendrocytes, and cause myelin damage in the AD brain. Because Aβ 1–42 is also a TLR4 agonist, it may set in motion a vicious loop that accounts for AD’s persistent progression [16]. The blood–brain barrier is also disrupted by serum LPS, that can also enter the brain and reactivate microglia, astrocytes, and numerous amyloidogenic and inflammatory pathways. Increased levels of inflammatory cytokines and NF-kB promote a rise in amyloid precursor protein (APP) and Aβ protein cleavage and accumulation, resulting in neuron loss and the development of Alzheimer’s disease [10]. Zhao et al. (2019) showed that LPS administration causes illness behavior and cognitive impairment, as well as microglia activation and neuronal cell death in the hippocampus in C57BL/6J mice. The LPS treatment decreased the levels of IL-4 and IL-10 while increasing the levels of TNF, IL-1, PGE2, and nitric oxide (NO). The NF-kB signaling pathway was activated in the LPS groups, according to Western blot analysis. In addition, VIPER, a TLR-4-specific inhibitory peptide, reduced LPS-induced neuroinflammation and cognitive impairment [17]. According to Thingore et al. (2020), LPS injection elevated neuroinflammation, caused poor memory retention and exacerbated the cognitive decline, and led to oxidative stress by lowering SOD, and increasing lipid peroxidation [18].
Amyloid—Amyloids are self-aggregating proteins that can induce cellular dysfunction in patients with neurodegenerative disorders [19]. Aβ is a cleavage product of APP, a transmembrane protein implicated in neuronal growth, signaling, and intracellular transport [20]. GM-produced amyloids have been shown to cross-seed Aβ deposition in several in vitro and in vivo studies [21]. Curli is created by Escherichia coli, TasA is made by Bacillus subtilis, CsgA is produced by Salmonella Typhimurium, FapCP is produced by pseudomonas fluorescens, and so on [22]. Bacterial amyloids have a different basic structure than brain amyloids, although they have similar metabolic and structural properties [23]. In a process known as seeding, preexisting amyloid aggregates produced from the same protein can speed up the polymerization of amyloidogenic proteins into ordered fibers. These amyloids cause Aβ fibrils and oligomers to misfold, allowing bacteria to attach to one another and create biofilms that may withstand immunological or physical attack. Bacterial amyloid proteins in the gut may prime the immune system, increasing immunological responses to intrinsic neural amyloid formation in the CNS [24]. Resemblances in tertiary protein structure may play a role in the development of prion-like agents via molecular mimicry, which results in cross-seeding, in which an amyloidogenic protein induces the production of another protein, such as a host protein with a distinct structure, to adopt the pathogenic sheet structure. According to Cattaneo et al. (2017), amyloidosis-positive individuals had greater blood levels of IL-1β, IL-6, C-X-C motif chemokine ligand, and nod-like receptor protein 3, and lower levels of anti-inflammatory cytokine IL-10 [25]. Ho et al. (2018) found that the gut microbiota can help guard against Alzheimer’s disease by promoting the production of certain SCFAs that prevent the creation of harmful soluble Aß aggregates [26]. In recent work, Javed et al. (2020) found that FapCS has a catalytic ability in seeding peptide amyloidosis, poor cognitive function, and behavior pathology in vitro, in silico, and in a zebrafish AD model [27].
Calprotectin is a tiny calcium-binding protein generated by neutrophils and monocytes, a heterodimer of S100A8/A9 (a TLR4 ligand). Elevated fecal calprotectin may act as a sign of intestinal inflammation. The concentration of fecal calprotectin in 22 individuals with Alzheimer’s disease was compared to serum amounts of aromatic amino acids by Leblhuber et al. (2015). Increased fecal calprotectin concentrations are linked to impaired intestinal barrier function in Alzheimer’s patients [28].
2.3. Leaky Gut and Leaky Brain
The mucus layer, intestinal epithelium, and lamina propria [29] form the intestinal barrier, protecting the body from pathogenic germs and preventing toxic particles, chemicals, bacteria, and other health-threatening organisms from entering the bloodstream. The makeup of the microbiota influences the permeability of the mucus layer [30]. The multitude of mucin-degrading bacteria Akkermansia muciniphila improves the gut barrier function and systemic inflammation [31]. Changes in tight junctions are mediated by pathogenic E. coli strains, Salmonella, Shigella, Helicobacter pylori, Vibrio, or Clostridium [32]. Increased intestinal permeability, often known as leaky gut, is caused by problems with the tight junctions’ competence. By cleaving E-cadherin (a cell adhesion molecule), Bacteroides fragilis exotoxin disrupts adherence junctions [33]. Disruption in gut homeostasis negatively impacts gut permeability by lowering beneficial substances, such as SCFAs and H2, and increasing harmful substances, such as LPS, amyloids, and TMAO, making the intestinal mucosal barrier permeable, activating peripheral immune responses, and raising peripheral and central Oxidative Stress levels [34]. The BBB (blood–brain barrier), which is made up of specialized brain endothelial cells, astrocytes, and pericytes, is a highly selective semipermeable boundary [35]. The BBB integrity is critical for brain growth and function. According to recent research, a variety of chemicals can compromise the BBB, allowing molecules, such as protein, viruses, and even bacteria to enter the brain and endanger brain health (Welling et al., 2015) (Table 1). The BBB’s structural and functional breakdown may be an early and crucial phase in the etiology of Alzheimer’s disease [36]. Pro-inflammatory and cytotoxic events result from a deposition in the vasculature, contributing to increased BBB permeability in the AD brain (Roher et al., 2003, Carrano et al., 2011, Erickson and Banks, 2013). TJs are disrupted by Aβ1-42 oligomers, which suppress the expression of ZO-1, claudin-5, and occludin while promoting the production of matrix metalloproteases (MMP)-2 and MMP-9. It also binds to the RAGE receptor and causes the formation of ROS, which disrupts TJs and compromises BBB integrity (Carrano et al., 2012). Tau may also induce BBB degeneration, according to in vitro studies and transgenic mice tauopathy models. Both tau and Aβ may, thus, contribute to the breakdown of the BBB, exacerbating the neurodegenerative process and the inflammatory reactions that accompany it [36].
Table 1. Various studies show alteration in gut microbiota in various neurodegenerative disorders.
| Neurodegenerative Disease | Study | Experimental Subject | Control | Method | Dysbiosis/Result | Ref |
|---|---|---|---|---|---|---|
| Alzhiemer’s Disease | Liang et al. (2016) | APP/PS1 transgenic mice | C57/Bl6 wild-type (WT) | 16S rRNA sequencing | ↓Odoribacter, ↑Helicobacter | [16] |
| Vogt et al. (2017) | Fecal samples from AD (n = 25) | sex-matched Control participants (n = 25) | 16S rRNA sequencing | Firmicutes, Bifidobacterium↓, Bacteroidetes↑ | [37] | |
| Zhang et al. (2017) | APP/PS1 transgenic male mice | Age and weight-matched littermate mice wild-type (WT) | 16S rRNA sequencing | microbiota composition and diversity were perturbed and the level of SCFAs ↓in AD mice | [38] | |
| Cattaneo et al. (2017) | Cognitively impaired patients with (n = 40, Amy+) and with no brain amyloidosis (n = 33, Amy−) | Without brain amyloidosis and cognitive impairment (n = 10) |
Microbial DNA qPCR assay | Amy+—↑pro-inflammatory cytokines (IL-6, CXCL2, NLRP3, and IL-1β) ↓anti-inflammatory cytokine (IL-10) Amy+—↓E. rectale and ↑ Escherichia/Shigella |
[25] | |
| Zhuang et al. (2018) | Fecal samples- AD patients | age- and gender-matched cognitively normal controls | 16S rRNA sequencing | At family level- ↑ Ruminococcaceae and ↓ Lachnospiraceae | [39] | |
| Bauerl et al. (2018) | APP/PS1 transgenic mice | C57/B16 (WT) | 16S rRNA sequencing | ↑ Proteobacteria and Erysipelotrichaceae | [40] | |
| Honarpisheh et al. (2020) | Symptomatic Tg2576 mice | age-matched littermate WT | 16S rRNA sequencing | ↑↑Firmicutes and Bacteroidetes ↑ Lactobacillus |
[41] | |
| Parkinson Disease | Cilia et al. (2020) | Fecal samples of PD pt. (n = 39) |
16S rRNA sequencing | ↓Roseburia (Firmicutes phylum) -worse evolution of motor, non-motor and cognitive functions ↓Ruminococcaceae and Actinobacteria- rapid worsening of global cognitive functions |
[42] | |
| Tan et al. (2020) | Fecal samples of PD pt. (n = 104) |
Control (n = 96) |
16S rRNA gene sequencing | PD- ↓ SCFA (a/w poorer cognition and low BMI) and ↓ butyrate (a/w worse postural instability–gait disorder scores) | [43] | |
| Nishiwaki et al. (2020) | Patients with PD (n = 223) |
Control (n = 137) |
16S rRNA gene sequencing | PD- ↑ Akkermansia and Catabacter (genera) and Akkermansiaceae (family). ↓ Roseburia, Faecalibacterium, and Lachnospiraceae ND3007 (genera) |
[44] | |
| Heinzel et al. 2020) | Stool sample PD pt. (n = 666) |
Healthy Control | PD- ↓ Firmicutes and Faecalibacterium, ↑ Prevotella |
[45] | ||
| Shen et al. (2021) | Fifteen case–control studies | meta-analysis | PD- ↓ Prevotellaceae, Faecalibacterium, and Lachnospiraceae ↑ Bifidobacteriaceae, Ruminococcaceae, Verrucomicrobiaceae, and Christensenellaceae |
[46] | ||
| Vascellari et al. (2021) | PD patients (n = 56) (TD = Tremor Dominant-19; AR = Akinetic Rigid-23; D = Dyskinetic-14) |
16S next-generation sequencing and gas chromatography-mass spectrometry | ↓ Lachnospiraceae, Blautia, Coprococcus, Lachnospira, and ↑ in Enterobacteriaceae, Escherichia and Serratia linked to non-TD subtypes | [47] | ||
| Multiple Sclerosis | Saresella et al. (2020) | MS pt. (n = 38) |
Healthy Controls (HC) |
↓BA producers, ↑mucin-degrading, pro-inflammatory components BA/CA ratio was significantly ↓in MS (ratio: 0.9) compared to HC (ratio: 5; p < 0.0001). BA = Butyric acid CA = Caproic acid |
[48] | |
| ALS | Mazzini et al. (2018) | ALS patients (n = 50) |
Healthy controls (n = 50) |
PCR | ↑E. coli and enterobacteria ↓total yeast in patients |
[49] |
| Gioia et al. (2020) | ALS (n = 50) |
50 HC (n = 50) |
PCR 16S next-generation sequencing |
An unbalance between potentially protective microbial groups, such as Bacteroidetes, and other with potential neurotoxic or pro-inflammatory activity, such as Cyanobacteria, has been shown | [50] |
3. Parkinson’s Disease
Parkinson’s disease (PD) is the world’s second most prevalent neurodegenerative illness, characterized by an aberrant buildup of α-synuclein fibrils known as Lewy bodies (LBs) in dopaminergic neurons in the substantia nigra (SN) [51]. It has a global incidence of 10–50 per 100,000 people per year and a prevalence of 100–300 per 100,000 people, with the number of persons with PD anticipated to double by 2030 owing to global population aging [52]. Increased intestinal permeability and systemic exposure of bacterial endotoxins are caused by changes in the gut microbiota, which causes excess α-syn expression and supports its misfolding to generate LBs. The intestinal LBs will enter the CNS via the vagal nerve and eventually travel to and destroy the substantia nigra [53], resulting in the formation of clinical signs of Parkinson’s disease, such as tremors, stiffness, balance issues, and loss of spontaneous movement (akinesia). Constipation is the most prevalent premotor sign in Parkinson’s disease, involving more than 70% of individuals and advancing pathogenesis more than 10 years before clinical symptoms appear. As a result, the symptom of constipation is considered a clinical biomarker for identifying prodromal PD (Berg et al., 2015) [54]. In individuals with PD, there was a significant drop in numerous gut microbiota metabolic products, which might lead to constipation. When intestinal infection was present, a higher vulnerability to PD was reported, which might trigger PD-like symptoms. In a mouse model, PD-derived gut microbiota might exacerbate α-synuclein-mediated motor impairments and brain disease, whereas germ-free mice displayed milder α-synuclein pathology (Sampson et al., 2016) [55]. The microbiome-related changes in PD are discussed in Table 1.
4. Multiple Sclerosis (MS)
MS is a chronic autoimmune illness in which immune cells target the myelin sheath, causing demyelination and axonal loss, which leads to paralysis since myelin permits electric impulses to flow through neurons [56]. Despite multiple risk variables implicated in the development of autoimmune diseases, the gut microbiome is thought to be the most important environmental risk factor for MS [57]. MS patients had a lower number of Faecalibacterium, Eubacterium rectale, Corynebacterium, and Fusobacteria, and a higher proportion of Escherichia, Shigella, Clostridium, and Firmicutes compared to healthy controls [58][59]. The most extensively used animal model that matches the characteristics of MS in humans is EAE (Experimental Autoimmune Encephalomyelitis). EAE is not induced in GF mice, suggesting that the gut microbiota is essential for EAE induction. Oral therapy with ampicillin, vancomycin, neomycin, sulfate, and metronidazole produced a similar response, with a delay in the beginning and reduction in the severity of the illness, as well as lower levels of pro-inflammatory cytokines and higher levels of interleukin IL-10 and IL-13 [60]. Lipid 654 is expressed in considerably reduced quantities in the blood of MS patients compared to both healthy persons and those with Alzheimer’s disease, according to Farrokhi et al. (2013) [61]. Probiotics (IRT5 including Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus reuteni, Bifidobacterium bifidum, and Streptococcus thermophilus) were given before the induction of EAE, which led to a delayed start and milder duration of the disease [62]. Dysbiosis in MS is further described in Table 1.
5. Amyotrophic Lateral Sclerosis (ALS)
ALS is a deadly neurodegenerative disease that affects the neurons of the brain and spinal cord, resulting in the premature death of motor neurons [63]. Because of respiratory paralysis, the majority of ALS patients die within 3 to 5 years [64]. A number of studies have discovered indications of abnormalities in the gut microbiota in people with amyotrophic lateral sclerosis. Using an ALS animal model, Wu et al. (2015) discovered that tight junction structure was disrupted and intestinal permeability was enhanced. Gut dysbiosis has also been seen in ALS mice, with lower numbers of butyrate-producing bacteria, such as Butyrivibrio fibrisolvens and E. coli [65]. Fang et al. (2016) discovered a decreased Firmicutes/Bacteroidetes ratio, a large reduction in the genera Anaerostipes, Oscillibacter, and Lachnospiraceae (beneficial bacteria), and a significant rise in glucose metabolizing Dorea in ALS patients [66]. Using an ALS mouse model and a diet supplemented with 2% butyrate in drinking water, Zhang et al. found that intestinal microbial equilibrium was restored, gut integrity was enhanced, and life duration was extended as compared to control mice [67]. Further studies related to microbiota and ALS are discussed in Table 1.
References
- Parashar, A.; Udayabanu, M. Gut microbiota: Implications in Parkinson’s disease. Parkinsonism Relat. Disord. 2017, 38, 1–7.
- Wu, W.; Kong, Q.; Tian, P.; Zhai, Q.; Wang, G.; Liu, X.; Zhao, J.; Zhang, H.; Lee, Y.K.; Chen, W. Targeting gut microbiota dysbiosis: Potential intervention strategies for neurological disorders. Engineering 2020, 6, 415–423.
- Larroya-García, A.; Navas-Carrillo, D.; Orenes-Piñero, E. Impact of gut microbiota on neurological diseases: Diet composition and novel treatments. Crit. Rev. Food Sci. Nutr. 2019, 59, 3102–3116.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904.
- Hughes, H.K.; Rose, D.; Ashwood, P. The gut microbiota and dysbiosis in autism spectrum disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81.
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194.
- Nandwana, V.; Debbarma, S. Fecal Microbiota Transplantation: A Microbiome Modulation Technique for Alzheimer’s Disease. Cureus 2021, 13, e16503.
- Moos, W.H.; Faller, D.V.; Harpp, D.N.; Kanara, I.; Pernokas, J.; Powers, W.R.; Steliou, K. Microbiota and neurological disorders: A gut feeling. BioResearch Open Access 2016, 5, 137–145.
- Penland, J.G. The importance of boron nutrition for brain and psychological function. Biol. Trace Elem. Res. 1998, 66, 299–317.
- Giacomeli, R.; Izoton, J.C.; Dos Santos, R.B.; Boeira, S.P.; Jesse, C.R.; Haas, S.E. Neuroprotective effects of curcumin lipid-core nanocapsules in a model Alzheimer’s disease induced by β-amyloid 1-42 peptide in aged female mice. Brain Res. 2019, 1721, 146325.
- Agrawal, I.; Jha, S. Mitochondrial dysfunction and Alzheimer’s disease: Role of microglia. Front. Aging Neurosci. 2020, 12, 252.
- Nandwana, V.; Kaur, J.; Singh, R.; Jaka, S.; Kaur, G.; Rawal, E.; Mathialagan, K.; Williams, O.C.A. Predictors of Hospitalization for Manic Episode in Alzheimer’s Dementia: Inputs From an Inpatient Case-Control Study. Cureus 2021, 13, e17333.
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792.
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; De Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293.
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: A review. Front. Aging Neurosci. 2018, 10, 42.
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790.
- Thingore, C.; Kshirsagar, V.; Juvekar, A. Amelioration of oxidative stress and neuroinflammation in lipopolysaccharide-induced memory impairment using Rosmarinic acid in mice. Metab. Brain Dis. 2021, 36, 299–313.
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. eLife 2020, 9, e53111.
- Chen, G.-f.; Xu, T.-h.; Yan, Y.; Zhou, Y.-r.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235.
- Friedland, R.P.; McMillan, J.D.; Kurlawala, Z. What are the molecular mechanisms by which functional bacterial amyloids influence amyloid beta deposition and neuroinflammation in neurodegenerative disorders? Int. J. Mol. Sci. 2020, 21, 1652.
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654.
- Zhao, Y.; Lukiw, W.J. Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer’s disease (AD). J. Nat. Sci. 2015, 1, e138.
- Zhou, Y.; Smith, D.; Leong, B.J.; Brännström, K.; Almqvist, F.; Chapman, M.R. Promiscuous cross-seeding between bacterial amyloids promotes interspecies biofilms. J. Biol. Chem. 2012, 287, 35092–35103.
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68.
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2018, 18, 83–90.
- Javed, I.; Zhang, Z.; Adamcik, J.; Andrikopoulos, N.; Li, Y.; Otzen, D.E.; Lin, S.; Mezzenga, R.; Davis, T.P.; Ding, F. Accelerated amyloid beta pathogenesis by bacterial amyloid FapC. Adv. Sci. 2020, 7, 2001299.
- Leblhuber, F.; Geisler, S.; Steiner, K.; Fuchs, D.; Schütz, B. Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J. Neural Transm. 2015, 122, 1319–1322.
- Kowalski, K.; Mulak, A. Brain-gut-microbiota axis in Alzheimer’s disease. J. Neurogastroenterol. Motil. 2019, 25, 48.
- Jakobsson, H.E.; Rodríguez-Piñeiro, A.M.; Schütte, A.; Ermund, A.; Boysen, P.; Bemark, M.; Sommer, F.; Bäckhed, F.; Hansson, G.C.; Johansson, M.E. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015, 16, 164–177.
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071.
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human intestinal barrier function in health and disease. Clin. Transl. Gastroenterol. 2016, 7, e196.
- Choi, V.M.; Herrou, J.; Hecht, A.L.; Teoh, W.P.; Turner, J.R.; Crosson, S.; Wardenburg, J.B. Activation of Bacteroides fragilis toxin by a novel bacterial protease contributes to anaerobic sepsis in mice. Nat. Med. 2016, 22, 563–567.
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut microbiota and dysbiosis in Alzheimer’s disease: Implications for pathogenesis and treatment. Mol. Neurobiol. 2020, 57, 5026–5043.
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13.
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56.
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537.
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.-F.; Huang, L. Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 60, 1241–1257.
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346.
- Bäuerl, C.; Collado, M.C.; Diaz Cuevas, A.; Viña, J.; Pérez Martínez, G. Shifts in Gut Microbiota Composition in an APP/PSS 1 Transgenic Mouse Model of Alzheimer’s Disease during Lifespan; Wiley Online Library: New York, NY, USA, 2018.
- Honarpisheh, P.; Reynolds, C.R.; Blasco Conesa, M.P.; Moruno Manchon, J.F.; Putluri, N.; Bhattacharjee, M.B.; Urayama, A.; McCullough, L.D.; Ganesh, B.P. Dysregulated Gut Homeostasis Observed Prior to the Accumulation of the Brain Amyloid-β in Tg2576 Mice. Int. J. Mol. Sci. 2020, 21, 1711.
- Cilia, R.; Piatti, M.; Cereda, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cassani, E.; Bonvegna, S.; Ferrarese, C.; Zecchinelli, A.L. Does Gut Microbiota Influence the Course of Parkinson’s Disease? A 3-Year Prospective Exploratory Study in de novo Patients. J. Parkinson’s Dis. 2021, 11, 159–170.
- Tan, A.H.; Chong, C.W.; Lim, S.Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.L.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q. Gut microbial ecosystem in Parkinson’s disease: New clinico-biological insights from multi-omics. Ann. Neurol. 2021, 89, 546–559.
- Hiroshi, N.; Mikako, I.; Tomohiro, I.; Tomonari, H.; Tetsuya, M.; Kenichi, K.; Yoshio, T.; Jun, U.; Hiroshi, M.; Ken, K. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1626–1635.
- Heinzel, S.; Aho, V.T.; Suenkel, U.; von Thaler, A.K.; Schulte, C.; Deuschle, C.; Paulin, L.; Hantunen, S.; Brockmann, K.; Eschweiler, G.W. Gut microbiome signatures of risk and prodromal markers of Parkinson disease. Ann. Neurol. 2020, 88, 320–331.
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.-Y. The association between the gut microbiota and Parkinson’s disease, a meta-analysis. Front. Aging Neurosci. 2021, 13, 40.
- Vascellari, S.; Melis, M.; Palmas, V.; Pisanu, S.; Serra, A.; Perra, D.; Santoru, M.L.; Oppo, V.; Cusano, R.; Uva, P. Clinical Phenotypes of Parkinson’s Disease Associate with Distinct Gut Microbiota and Metabolome Enterotypes. Biomolecules 2021, 11, 144.
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated With Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390.
- Mazzini, L.; Mogna, L.; De Marchi, F.; Amoruso, A.; Pane, M.; Aloisio, I.; Cionci, N.B.; Gaggìa, F.; Lucenti, A.; Bersano, E.; et al. Potential Role of Gut Microbiota in ALS Pathogenesis and Possible Novel Therapeutic Strategies. J. Clin. Gastroenterol. 2018, 52, S68–S70.
- Di Gioia, D.; Bozzi Cionci, N.; Baffoni, L.; Amoruso, A.; Pane, M.; Mogna, L.; Gaggìa, F.; Lucenti, M.A.; Bersano, E.; Cantello, R.; et al. A prospective longitudinal study on themicrobiota composition in amyotrophic lateral sclerosis. BMC Med. 2020, 18, 153.
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinson’s Dis. 2021, 7, 27.
- Liu, J.; Xu, F.; Nie, Z.; Shao, L. Gut Microbiota Approach—A New Strategy to Treat Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2020, 10, 648.
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1600–1611.
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12.
- Colpitts, S.L.; Kasper, E.J.; Keever, A.; Liljenberg, C.; Kirby, T.; Magori, K.; Kasper, L.H.; Ochoa-Repáraz, J. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 2017, 8, 561–573.
- Brown, J.; Quattrochi, B.; Everett, C.; Hong, B.-Y.; Cervantes, J. Gut commensals, dysbiosis, and immune response imbalance in the pathogenesis of multiple sclerosis. Mult. Scler. J. 2021, 27, 807–811.
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734.
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E.; US Network of Pediatric MS Centers. Gut microbiota in early pediatric multiple sclerosis: A case−control study. Eur. J. Neurol. 2016, 23, 1308–1321.
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050.
- Farrokhi, V.; Nemati, R.; Nichols, F.C.; Yao, X.; Anstadt, E.; Fujiwara, M.; Grady, J.; Wakefield, D.; Castro, W.; Donaldson, J. Bacterial lipodipeptide, Lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin. Transl. Immunol. 2013, 2, e8.
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 2016, 7, 1081.
- Ojeda, J.; Ávila, A.; Vidal, P.M. Gut Microbiota Interaction with the Central Nervous System throughout Life. J. Clin. Med. 2021, 10, 1299.
- Alonso, A.; Logroscino, G.; Jick, S.S.; Hernán, M.A. Incidence and lifetime risk of motor neuron disease in the United Kingdom: A population-based study. Eur. J. Neurol. 2009, 16, 745–751.
- Wu, S.; Yi, J.; Zhang, Y.G.; Zhou, J.; Sun, J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 2015, 3, e12356.
- Fang, X.; Wang, X.; Yang, S.; Meng, F.; Wang, X.; Wei, H.; Chen, T. Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front. Microbiol. 2016, 7, 1479.
- Zhang, Y.-g.; Wu, S.; Yi, J.; Xia, Y.; Jin, D.; Zhou, J.; Sun, J. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 2017, 39, 322–336.
- Balanzá-Martínez, V. Nutritional supplements in psychotic disorders. Actas Esp. De Psiquiatr. 2017, 45, 16–25.
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
09 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




