
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Scott Forbes | -- | 1958 | 2022-06-08 14:29:52 | | | |
| 2 | Lindsay Dong | -2 word(s) | 1956 | 2022-06-09 08:08:47 | | |
Video Upload Options
Creatine, a nitrogenous organic compound derived from reactions involving the amino acids arginine, glycine, and methionine, is important for resynthesizing ATP, particularly during times of increased metabolic demand (e.g., sleep deprivation, mental health conditions, or neurological diseases). Creatine supplementation (and guanidinoacetic acid; GAA) has the ability to increase brain creatine content in humans. Furthermore, creatine has shown some promise for attenuating symptoms of concussion, mild traumatic brain injury and depression but its effect on neurodegenerative diseases appears to be lacking.
1. Creatine and Guanidinoacetic Acid (GAA) Supplementation on Brain Creatine and Phophorylcreatine (PCr)
1.1. Creatine Monohdyrate Supplementation
Pioneering work in the 1990s by Harris et al. [1] and Hultman et al. [2] demonstrated increased muscle creatine levels following oral creatine monohydrate supplementation. Since the publication of these studies, it has been repeatedly shown that creatine supplementation increases muscle creatine and PCr levels using both nuclear magnetic resonance (NMR) spectroscopy and muscle biopsies (Kreider et al. [3]). It appears that the average increase in muscle creatine from creatine supplementation is about 20% with responses that could be characterized as low, medium, or high (≈40%). Intramuscular creatine levels can be further increased when creatine monohydrate ingestion is combined with exercise [1][4], insulin [5], carbohydrate [6], carbohydrate and protein [7], or lipoic acid [8].
Overall, it appears that brain creatine content can be increased with creatine supplementation. However, it is difficult to compare individual studies where brain creatine was assessed pre- and post-supplementation because the supplementation protocols are heterogeneous (2 to 20 g/d), the populations are different (e.g., patient vs. healthy), the regions of the brain assessed were dissimilar, and while some labs measure brain PCr using P31-NMR other research teams measured total creatine using H1-NMR. One factor that must be investigated in the future is the optimal dosage of creatine needed to elicit the largest increase in brain uptake in response to supplementation. Similarly, few data assessing simultaneous changes in creatine in multiple tissues (e.g., muscle and brain) are available. It is unlikely that the addition of nutrients such as carbohydrate or protein, or endocrine factors such as insulin will have any effect on brain creatine uptake. Currently, research indicates that brain creatine increases in response to creatine monohydrate supplementation. This increase is smaller than the skeletal muscle response to a similar supplementation protocol.
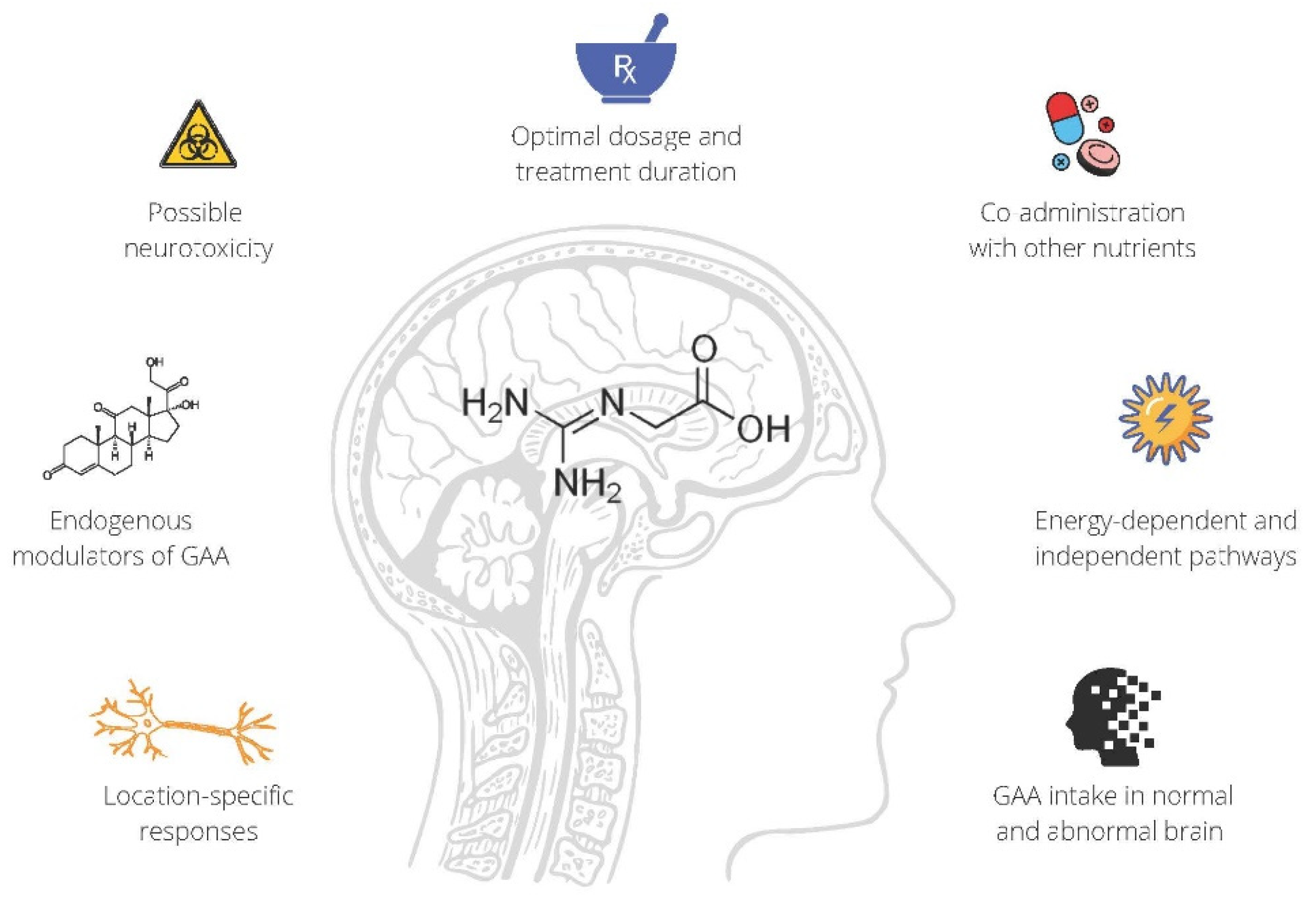
1.2. GAA Supplementation
Being a direct natural precursor of creatine, GAA (also known as glycocyamine; chemical formula: C3H7N3O2) has been used to treat neurological diseases for almost 70 years. In 1952, Henry Borsook from Caltech was arguably the first to investigate the effects of supplemental GAA (co-administered with betaine) in poliomyelitis-related disability [9]. The scholars reported beneficial effects of glycocyamine therapy in patients affected by acute anterior poliomyelitis, with the presumed therapeutic mechanism entailing enhanced creatine synthesis in target organs, including the brain and skeletal muscle. Succeeding neurological studies from the 1950s derived equivocal results in terms of GAA therapeutic potential, with some showing no clinical improvement in patients with various neurological dysfunctions (e.g., multiple sclerosis, amyotrophic lateral sclerosis, Parkinson’s disease) [10][11], while others demonstrated favorable effects of GAA on specific surrogate indicators of tissue metabolism in poliomyelitis [12], or mild patient-reported benefits from the treatment in motor-neuron disease [13]. However, these pioneering studies did not assess the effects of GAA on tissue creatine levels nor did they evaluate more brain-specific outcomes following GAA administration.
2. Creatine and Cognitive Function
Robust evidence that clearly demonstrates the importance of creatine on cognitive function comes from individuals with creatine deficient syndromes, known to deplete brain creatine stores. Creatine deficiency syndrome is characterized by mental and development disorders such as learning delays and seizures [15][16], and importantly these symptoms are reversed, at least in part, by creatine supplementation [17][18][19].
Sleep deprivation is known to impact brain bioenergetics, and it appears that the effects of creatine supplementation in combination with sleep deprivation may enhance cognitive function compared to placebo. However, presently there are only two studies that have investigated cognitive function following sleep deprivation in humans and both were combined with mild to moderate exercise [20][21].
3. Creatine for Neurodegenerative Diseases
3.1. Amyotrophic Lateral Sclerosis
3.2. Duchenne Muscular Dystrophy
3.3. Huntington’s Disease
3.4. Multiple Sclerosis
3.5. Parkinson’s Disease
4. Creatine and Mental Health
The critical role of creatine in the brain is well documented through creatine deficiency syndromes, which are characterized by intellectual disability, language delay, seizure disorders, autism spectrum disorder and various movement disorders, with the primary treatment being creatine monohydrate supplementation in an attempt to increase creatine content in the brain [39]. Many mental health disorders have also been characterized to have abnormalities in brain bioenergetics, with some of the more prevalent disorders, such as depression, being associated with low creatine levels in certain regions of the brain [40]. Based on such observations, there has been growing interest in the possible use of creatine monohydrate in various brain/neurological disorders, including mental/psychiatric disorders.
4.1. Depression
4.2. Anxiety and Post-Traumatic Stress Disorder
5. Creatine for Concussion and Traumatic Brain Injury (TBI)
6. Conclusions and Future Directions
References
- Harris, R.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374.
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle Creatine Loading in Men. J. Appl. Physiol. 1996, 81, 232–237.
- Kreider, R.; Kalman, D.; Antonio, J.; Ziegenfuss, T.; Wildman, R.; Collins, R.; Candow, D.; Kleiner, S.; Almada, A.; Lopez, H. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18.
- Robinson, T.M.; Sewell, D.A.; Hultman, E.; Greenhaff, P.L. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J. Appl. Physiol. 1999, 87, 598–604.
- Steenge, G.R.; Lambourne, J.; Casey, A.; Macdonald, I.A.; Greenhaff, P.L. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 1998, 275, E974–E979.
- Green, A.; Hultman, E.; Macdonald, I.; Sewell, D.; Greenhaff, P. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am. J. Physiol. 1996, 271, E821–E826.
- Steenge, G.R.; Simpson, E.J.; Greenhaff, P.L. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J. Appl. Physiol. 2000, 89, 1165–1171.
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Tarnopolsky, M.A.; Candow, D.G. Effect of alpha-lipoic acid combined with creatine monohydrate on human skeletal muscle creatine and phosphagen concentration. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 294–302.
- Borsook, M.; Billig, H.; Golseth, J. Betaine and glycocyamine in the treatment of disability resulting from acute anterior poliomyelitis. Ann. West. Med. Surg. 1952, 6, 423–427.
- Fallis, B.D.; Lam, R.L. Betaine and glycocyamine therapy for the chronic residuals of poliomyelitis. J. Am. Med. Assoc. 1952, 150, 851–853.
- Aldes, J. Glycocyamine betaine as an adjunct in the treatment of neuromuscular disease patients. J. Ark. Med. Soc. 1957, 54, 186–194.
- Watkins, A.L. Betaine and glycocyamine in the treatment of poliomyelitis. N. Engl. J. Med. 1953, 248, 621–623.
- Liveksedge, L.A. Glycocyamine and betaine in motor-neurone disease. Lancet 1956, 271, 1136–1138.
- Gillessen, T.; Budd, S.L.; Lipton, S.A. Excitatory amino acid neurotoxicity. Adv. Exp. Med. Biol. 2002, 513, 3–40.
- Bender, A.; Klopstock, T. Creatine for neuroprotection in neurodegenerative disease: End of story? Amin. Acids 2016, 48, 1929–1940.
- Mercimek-Andrews, S.; Salomons, G.S. Creatine Deficiency Syndromes. Inborn Metab. Dis. Diagn. Treat. 2015, 239–247.
- Salomons, G.S.; Van Dooren, S.J.M.; Verhoeven, N.M.; Marsden, D.; Schwartz, C.; Cecil, K.M.; DeGrauw, T.J.; Jakobs, C. X-linked creatine transporter defect: An overview. J. Inherit. Metab. Dis. 2003, 26, 309–318.
- Stöckler, S.; Holzbach, U.; Hanefeld, F.; Marquardt, I.; Helms, G.; Requar, T.M.; Hanicke, W.; Frahm, J. Creatine deficiency in the brain: A new, treatable inborn error of metabolism. Pediatr. Res. 1994, 36, 409–413.
- Kaldis, P.; Hemmer, W.; Zanolla, E.; Holtzman, D.; Wallimann, T. “Hot spots” of creatine kinase localization in brain: Cerebellum, hippocampus and choroid plexus. Dev. Neurosci. 1996, 18, 542–554.
- Hammett, S.T.; Wall, M.B.; Edwards, T.C.; Smith, A.T. Dietary supplementation of creatine monohydrate reduces the human fMRI BOLD signal. Neurosci. Lett. 2010, 479, 201–205.
- Ling, J.; Kritikos, M.; Tiplady, B. Cognitive effects of creatine ethyl ester supplementation. Behav. Pharmacol. 2009, 20, 673–679.
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586.
- Harmon, K.K.; Stout, J.R.; Fukuda, D.H.; Pabian, P.S.; Rawson, E.S.; Stock, M.S. The Application of Creatine Supplementation in Medical Rehabilitation. Nutrients 2021, 13, 1825.
- Gualano, B.; Artioli, G.G.; Poortmans, J.R.; Lancha Junior, A.H. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010, 38, 31–44.
- Klopstock, T.; Elstner, M.; Bender, A. Creatine in mouse models of neurodegeneration and aging. Amino Acids 2011, 40, 1297–1303.
- Xu, C.J.; Klunk, W.E.; Kanfe, J.N.; Xiong, Q.; Miller, G.; Pettegrew, J.W. Phosphocreatine-dependent glutamate uptake by synaptic vesicles. A comparison with atp-dependent glutamate uptake. J. Biol. Chem. 1996, 271, 13435–13440.
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213.
- Beal, M.F. Neuroprotective effects of creatine. Amino Acids 2011, 40, 1305–1313.
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.; Scutifero, M.; Cecio, M.R.; Torre, V.; De Luca, F.; Picillo, E.; et al. Improvement of survival in DuchenneMuscular Dystrophy: Retrospective analysisof 835 patients. Acta Myol. 2012, 31, 125.
- Louis, M.; Lebacq, J.; Poortmans, J.R.; Belpaire-Dethiou, M.-C.; Devogelaer, J.-P.; Van Hecke, P.; Goubel, F.; Francaux, M. Beneficial effects of creatine supplementation in dystrophic patients. Muscle Nerve 2003, 27, 604–610.
- Hersch, S.M.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L.; et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology 2006, 66, 250–252.
- Tabrizi, S.J.; Blamire, A.M.; Manners, D.N.; Rajagopalan, B.; Styles, P.; Schapira, A.H.V.; Warner, T.T. High-dose creatine therapy for Huntington disease: A 2-year clinical and MRS study. Neurology 2005, 64, 1655–1656.
- Tabrizi, S.J.; Blamire, A.M.; Manners, D.N.; Rajagopalan, B.; Styles, P.; Schapira, A.H.V.; Warner, T.T. Creatine therapy for Huntington’s disease: Clinical and MRS findings in a 1-year pilot study. Neurology 2003, 61, 141–142.
- Verbessem, P.; Lemiere, J.; Eijnde, B.O.; Swinnen, S.; Vanhees, L.; Van Leemputte, M.; Hespel, P.; Dom, R. Creatine supplementation in Huntington’s disease: A placebo-controlled pilot trial. Neurology 2003, 61, 925–930.
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009, 72, S1–S136.
- Yang, L.; Calingasan, N.Y.; Wille, E.J.; Cormier, K.; Smith, K.; Ferrante, R.J.; Flint Beal, M. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J. Neurochem. 2009, 109, 1427–1439.
- Bender, A.; Koch, W.; Elstner, M.; Schombacher, Y.; Bender, J.; Moeschl, M.; Gekeler, F.; Müller-Myhsok, B.; Gasser, T.; Tatsch, K.; et al. Creatine supplementation in Parkinson disease: A placebo-controlled randomized pilot trial. Neurology 2006, 67, 1262–1264.
- Hass, C.J.; Collins, M.A.; Juncos, J.L. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: A randomized trial. Neurorehabil. Neural Repair 2007, 21, 107–115.
- Fons, C.; Campistol, J. Creatine Defects and Central Nervous System. Semin. Pediatr. Neurol. 2016, 23, 285–289.
- Faulkner, P.; Paioni, S.L.; Kozhuharova, P.; Orlov, N.; Lythgoe, D.J.; Daniju, Y.; Morgenroth, E.; Barker, H.; Allen, P. Relationship between depression, prefrontal creatine and grey matter volume. J. Psychopharmacol. 2021, 35, 1464–1472.
- Bakian, A.V.; Huber, R.S.; Scholl, L.; Renshaw, P.F.; Kondo, D. Dietary creatine intake and depression risk among U.S. adults. Transl. Psychiatry 2020, 10, 52.
- Palay, J.; Taillieu, T.L.; Afifi, T.O.; Turner, S.; Bolton, J.M.; Enns, M.W.; Smith, M.; Lesage, A.; Bakal, J.A.; Rush, B.; et al. Prevalence of Mental Disorders and Suicidality in Canadian Provinces. Can. J. Psychiatry 2019, 64, 761–769.
- Watson, P. PTSD as a Public Mental Health Priority. Curr. Psychiatry Rep. 2019, 21, 61.
- Coplan, J.D.; Mathew, S.J.; Mao, X.; Smith, E.L.P.; Hof, P.R.; Coplan, P.M.; Rosenblum, L.A.; Gorman, J.M.; Shungu, D.C. Decreased choline and creatine concentrations in centrum semiovale in patients with generalized anxiety disorder: Relationship to IQ and early trauma. Psychiatry Res. 2006, 147, 27–39.
- Schuff, N.; Neylan, T.C.; Fox-Bosetti, S.; Lenoci, M.; Samuelson, K.W.; Studholme, C.; Kornak, J.; Marmar, C.R.; Weiner, M.W. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008, 162, 147–157.
- Villarreal, G.; Petropoulos, H.; Hamilton, D.A.; Rowland, L.M.; Horan, W.P.; Griego, J.A.; Moreshead, M.; Hart, B.L.; Brooks, W.M. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: Preliminary results. Can. J. Psychiatry 2002, 47, 666–670.
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14.
- Ashbaugh, A.; McGrew, C. The Role of Nutritional Supplements in Sports Concussion Treatment. Curr. Sports Med. Rep. 2016, 15, 16–19.
- Ainsley Dean, P.J.; Arikan, G.; Opitz, B.; Sterr, A. Potential for use of creatine supplementation following mild traumatic brain injury. Concussion 2017, 2, CNC34.
- Cassol, G.; Godinho, D.B.; de Zorzi, V.N.; Farinha, J.B.; Della-Pace, I.D.; de Carvalho Gonçalves, M.; Oliveira, M.S.; Furian, A.F.; Fighera, M.R.; Royes, L.F.F. Potential therapeutic implications of ergogenic compounds on pathophysiology induced by traumatic brain injury: A narrative review. Life Sci. 2019, 233, 116684.
- Pender, S.C.; Smith, A.M.; Finnoff, J.T.; Huston, J.; Stuart, M.J. Concussions in Ice Hockey—Moving Toward Objective Diagnoses and Point-of-care Treatment: A Review. Curr. Sports Med. Rep. 2020, 19, 380–386.
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447.
- Giza, C.C.; Hovda, D.A. The new neurometabolic cascade of concussion. Neurosurgery 2014, 75, S24–S33.
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The Molecular Pathophysiology of Concussive Brain Injury—An Update. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 373–393.
- Yoshino, A.; Hovda, D.A.; Kawamata, T.; Katayama, Y.; Becker, D.P. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: Evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991, 561, 106–119.
- Wang, Y.; Bartels, H.M.; Nelson, L.D. A Systematic Review of ASL Perfusion MRI in Mild TBI. Neuropsychol. Rev. 2020, 1–32.
- Signoretti, S.; Di Pietro, V.; Vagnozzi, R.; Lazzarino, G.; Amorini, A.M.; Belli, A.; D’Urso, S.; Tavazzi, B. Transient alterations of creatine, creatine phosphate, N-acetylaspartate and high-energy phosphates after mild traumatic brain injury in the rat. Mol. Cell. Biochem. 2010, 333, 269–277.
- Vagnozzi, R.; Signoretti, S.; Floris, R.; Marziali, S.; Manara, M.; Amorini, A.M.; Belli, A.; Di Pietro, V.; D’Urso, S.; Pastore, F.S.; et al. Decrease in N-acetylaspartate following concussion may be coupled to decrease in creatine. J. Head Trauma Rehabil. 2013, 28, 284–292.




