Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Himanshu Batra | -- | 2123 | 2022-06-07 16:06:32 | | | |
| 2 | Catherine Yang | Meta information modification | 2123 | 2022-06-08 03:51:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Batra, H.; Taslem Mourosi, J.; , .; Jain, S. DENGUE and ZIKA Flaviviruses Nucleic Acid Vaccine. Encyclopedia. Available online: https://encyclopedia.pub/entry/23788 (accessed on 07 February 2026).
Batra H, Taslem Mourosi J, , Jain S. DENGUE and ZIKA Flaviviruses Nucleic Acid Vaccine. Encyclopedia. Available at: https://encyclopedia.pub/entry/23788. Accessed February 07, 2026.
Batra, Himanshu, Jarin Taslem Mourosi, , Swati Jain. "DENGUE and ZIKA Flaviviruses Nucleic Acid Vaccine" Encyclopedia, https://encyclopedia.pub/entry/23788 (accessed February 07, 2026).
Batra, H., Taslem Mourosi, J., , ., & Jain, S. (2022, June 07). DENGUE and ZIKA Flaviviruses Nucleic Acid Vaccine. In Encyclopedia. https://encyclopedia.pub/entry/23788
Batra, Himanshu, et al. "DENGUE and ZIKA Flaviviruses Nucleic Acid Vaccine." Encyclopedia. Web. 07 June, 2022.
Copy Citation
Dengue virus and Zika virus are mosquito-borne, single-stranded, positive-sense RNA viruses that belong to the Flaviviridae family. Both the viruses are closely related and have similarities with other flaviviruses. Dengue virus (DENV) causes a severe febrile illness with fever, joint pain, and rash leading to a life-threatening condition in severe cases. While Zika virus (ZIKV) primarily causes mild fever, it can be passed from a pregnant mother to her fetus, resulting in severe birth defect microcephaly and even causing a rare autoimmune disease—Guillain–Barre syndrome.
mRNA vaccine
DNA vaccine
Dengue and Zika
1. Dengue and Zika Virus Structure
DENV and ZIKV have single-stranded and positive-strand RNA genomes (~10.8 kilobase RNA), translated to a single polyprotein (Figure 1). Upon translation, a large polyprotein is produced having a 5′ and 3′ untranslated region. After the cleavage, polyprotein produces three structural proteins: capsid protein (C), precursor membrane protein (prM) and envelope protein (E), and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5) [1].
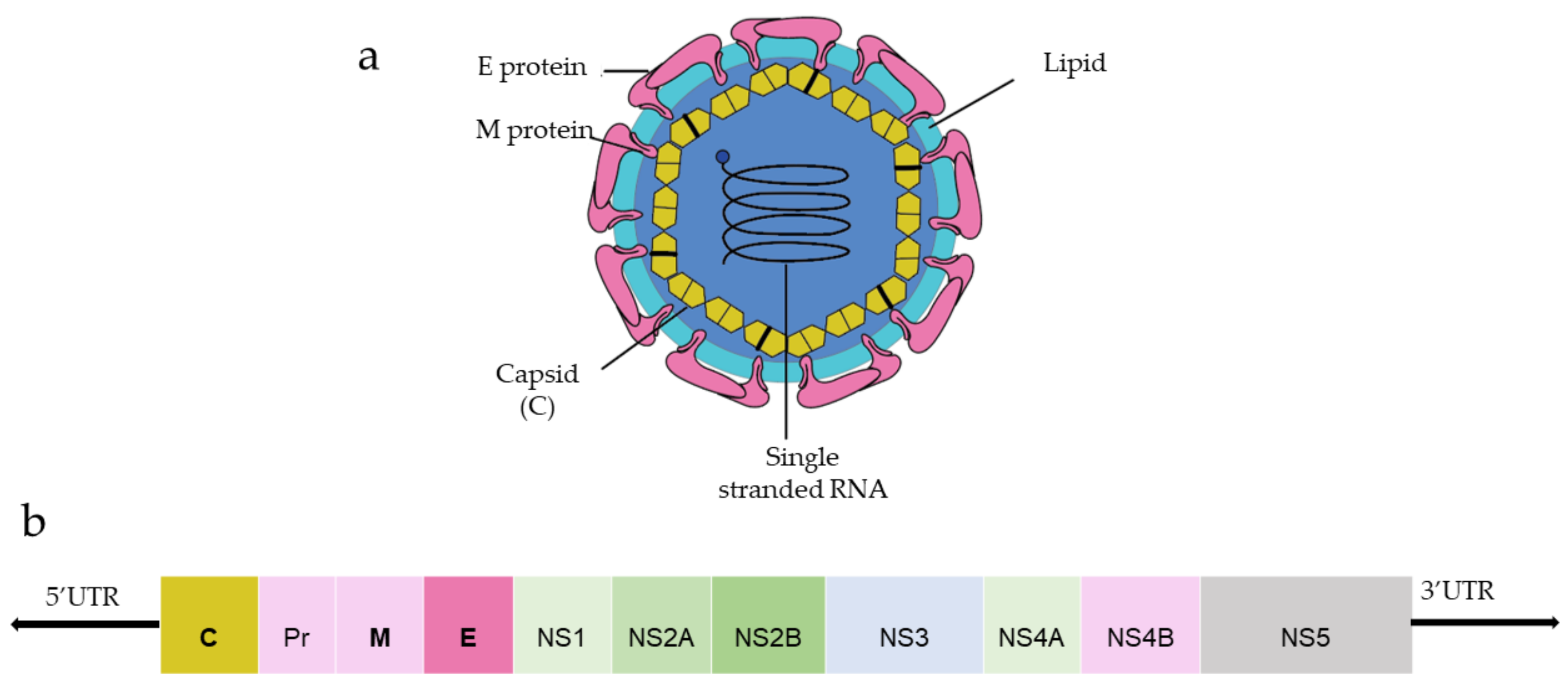
Figure 1. (a) General structure of the flavivirus; (b) genomic organization of the flavivirus (C, prM, E are structural proteins; NS1, NS2A, NS2B, NS3, NS4A, NS4B, NS5 are non-structural proteins).
Capsid protein protects the viral RNA, while envelope protein mainly interacts with the host cell receptor, and precursor membrane (prM) protects E protein from premature fusion to endosome. prM is cleaved into pre-membrane and membrane. Non-structural proteins have a role in virus replication, genome packaging, and escaping from the host immune system. NS1 helps in the replication process, NS3 has helicase and protease activity, and NS5 is a large non-structural protein that acts as a polymerase and methyltransferase.
Among three structural proteins, E protein is present outside the surface (Figure 1a), whereas M protein is found predominantly inside, both positioned parallelly. There is a total of 180 copies of E and M proteins. E protein dimer follows two-fold symmetry and has four beta-stranded domains; DI, DII, DIII encodes the viral envelope’s surface portion and stem transmembrane domain and anchors protein to the lipid membrane (Figure 2a). DI and DII domains carry the highly hydrophobic fusion loop required to initiate the infection, and DIII has the putative receptor-biding site, which interacts with the host cell membrane.
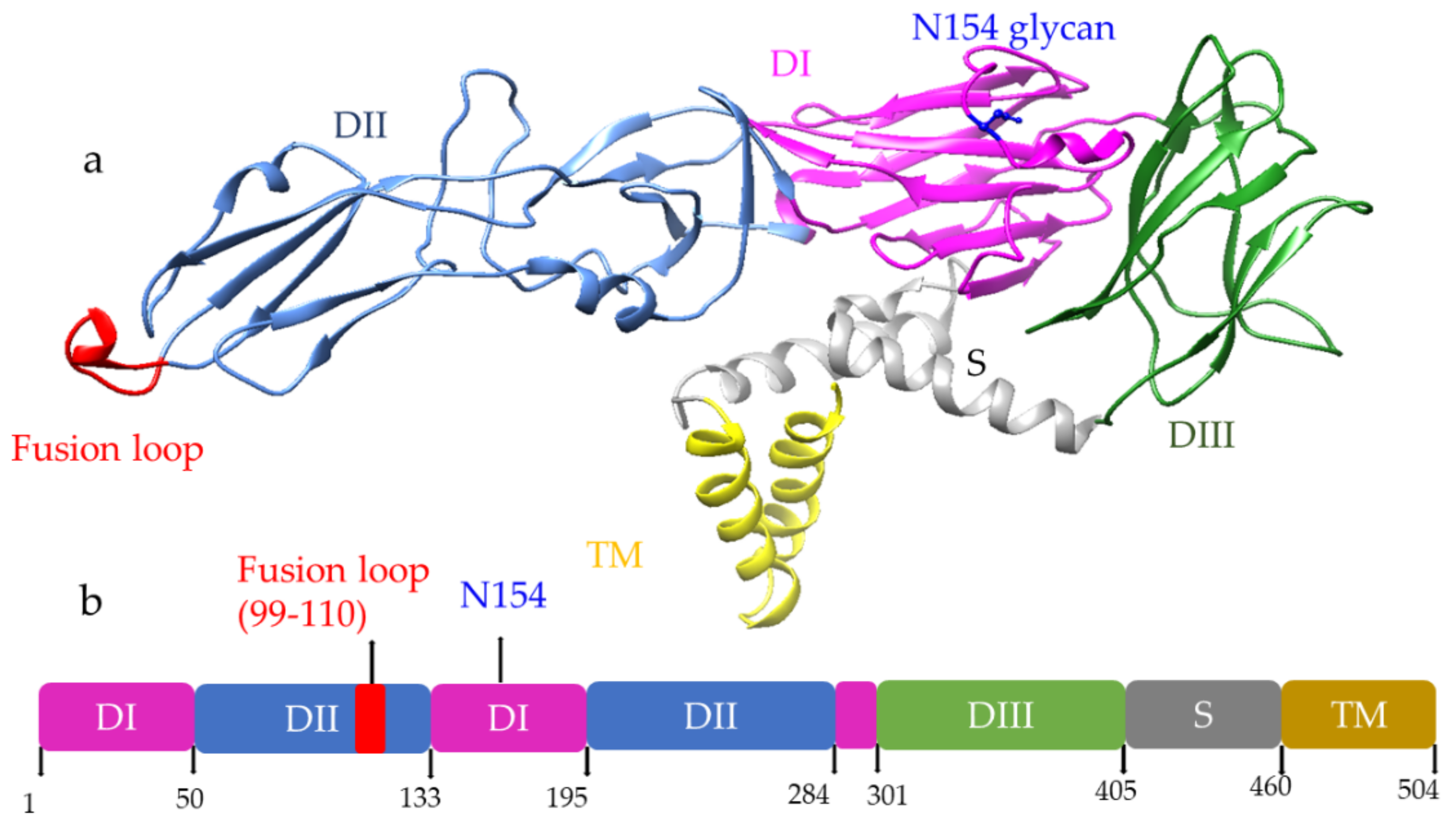
Figure 2. (a) Structure of E protein monomer (PDB ID: 5IRE) of ZIKV is shown in ribbon form. (b) Schematic of E protein showing residue positions of the all the domains. The domains are color coded as shown. Domain I (DI); Domain II (DII); Domain III (DIII); Stem (S); Transmembrane (TM) region. DI has an N-linked glycan (N154).
Both DENV and ZIKV E proteins are conserved with other flaviviruses. E protein contains a glycosylation site that plays a crucial role in virus infectivity and tropism [2]. The E protein is attached to the host receptor during viral entry, and the N-linked glycosylation creates a binding site receptor attachment. E protein of ZIKV has one glycosylation site at (Asn154) while DENV has two sites (Asn67, Asn153) [3]. The capsid protein of ZIKV forms a dimer having a two-fold symmetry, and each monomer has four alpha-helices connecting via a loop.
2. Progress of the Nucleic Acid Vaccines for DENV and ZIKV and Their Responses
2.1. DNA Vaccines for DENV
A monovalent DNA vaccine for DENV2 was constructed by cloning of the prM gene and 92% envelope gene from NGC DENV-2 strain in eukaryotic plasmid vectors, and the carboxy terminus of E protein was truncated to improve the secretion of the E protein (Figure 3a) (45). BALB/c mice immunized with this vaccine elicited anti-dengue antibodies that were able to neutralize the DENV2 strain in vitro [4]. Another study showed that the presence of the CpG motif in the plasmid improves the antibody response against the DENV2 strain, and this vaccine showed a protective effect in the mouse challenge model (Figure 3b) [5].
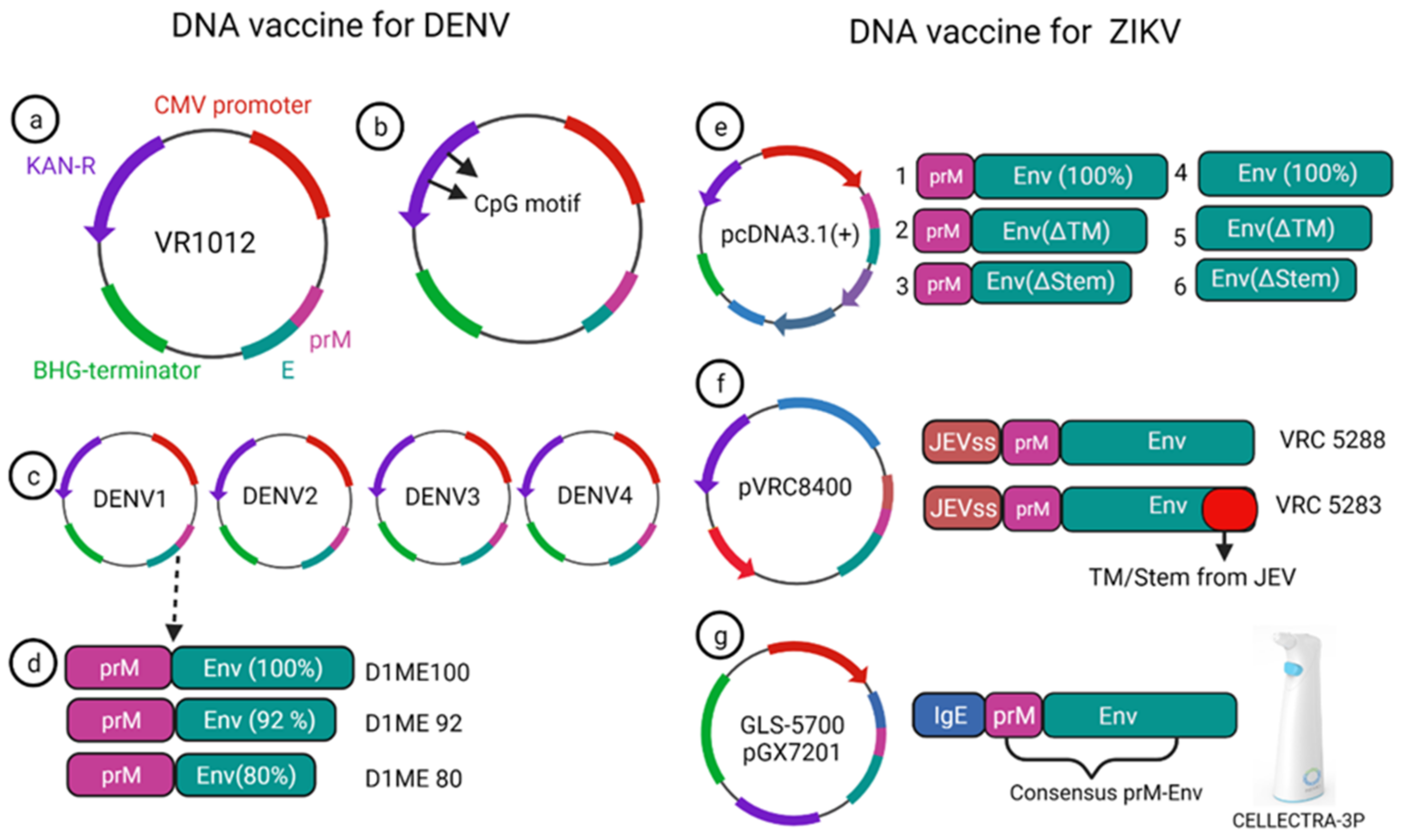
Figure 3. DNA vaccine construct designs for DENV and ZIKV. DNA vaccine for DENV(a–d); VR1012 construct is used to clone the prM and E gene under CMV promoter (a), CpG motif incorporation to the plasmid (b), tetravalent vaccine construction for all four dengue (c), DENV1 vaccine construction using prM and full length or a truncated version of E protein (d). DNA vaccine for ZIKV (e–g); prM-Env and other deletion mutant (e), JEV signal sequence containing prM and Env vaccine VRC 5288 have a WT TM/stem sequence, whereas VRC 5283 have a JEV TM/Stem (f), GLS-5700 vaccine containing consensus prM-Env with IgE sequences which delivered using a CELLECTRA-3P device (g).
A phase-I clinical trial was performed for a prototype monovalent DNA vaccine against DENV1 (D1ME), incorporating prM and E gene, the vaccine proved safe for human use; however, it was unable to provide a robust neutralizing antibody response and was found to be less immunogenic [6]. To improve the humoral response of the DNA vaccine, an adjuvanted plasmid Vaxfectin was used, which is the combination of cationic and neutral lipids; this adjuvanted vaccine showed an increased anti-dengue response as well as protection in the DENV2 challenge study [7]. Using the adjuvant, a prM and E-based tetra-valent DNA vaccine was constructed and tested for phase-I clinical trial without adjuvant, and with adjuvant, the anti-dengue antibody response improved significantly after adding the adjuvant. However, the T cell response remained unchanged compared to the non-adjuvanted vaccine [8].
The prM and E protein induce neutralizing antibodies to DENV [9]. T cell immune response from structural protein (prM and E) [10][11] and non-structural protein 3 (NS3) also elicit a cellular immune response [12][13]. Non-structural protein 1 (NS1) can alone induce a protective immune response as shown by Costa et al. They constructed NS1 and NS3 based DNA vaccines for DENV2, of which the former candidate induced a high level of NS1 specific antibody response in the BALB/c mice [14] and the latter resulted in protection via induction of interferon-γ [15].
A DNA vaccine using prM and E protein of DENV1-4 showed protection in mice from lethal dengue challenge [16][17][18][19]. A vaccine against DENV3 also showed cross-protection against the DENV1, DENV2, DENV4, which is important for tetravalent dengue vaccine design [19]. Multiple immunizations using electroporation improved the immunogenicity significantly and showed robust antigen-specific T cell response and persistent antibody response [16][17][18].
2.2. DNA Vaccines for ZIKV
Currently, there is no approved vaccine available for ZIKV. To date, 13 different vaccines have completed the phase I trial, and only one DNA vaccine has finished the phase II trial (Table 1). Larocca et al. developed several DNA vaccine constructs for Zika based on prM, E protein and their mutated versions (Figure 3e) [20]. They removed 93 amino acids from prM in one of the constructs, and also deleted transmembrane (ΔTM) and stem region (Δstem) of the envelope protein separately and cloned these constructs in a eukaryotic expression plasmid (pcDNA3.1+) vector with Kozak sequence and Japanese encephalitis virus leader sequence [20]. When 50μg of this DNA vaccine was injected in BALB/c mice via the intramuscular route, higher Env specific antibody response was elicited in the prM-Env vaccine than the other five constructs (Figure 3e [21][22][23][24][25]). Even a single dose of the prM-Env vaccine could elicit neutralizing antibody response and show protection against the ZIKV challenge [26].
Table 1. Current DNA/mRNA vaccine in clinical trial for ZIKA (As of 20 February 2022, from ClinicalTrials.gov).
| Platform | Vaccine Name | Antigen | NCT Number | Clinical Trial Stage |
|---|---|---|---|---|
| DENV DNA vaccine | D1ME100 | prM/E DENV1 | NCT00290147 | Phase I |
| TVDV | prM/E DENV1-4 | NCT01502358 | Phase I | |
| ZIKV DNA vaccine | VRC5283 | prM-E | NCT02996461 | Phase I |
| VRC5283 | prM-E | NCT03110770 | Phase II | |
| VRC5288 | prM-E | NCT02840487 | Phase 1 | |
| GLS-5700 | prM-E | NCT02809443 | Phase I | |
| ZIKV mRNA vaccine | mRNA-1325 | prM-E | NCT03014089 | Phase I |
| mRNA-1893 | prM-E | NCT04064905 | Phase I |
Dowd et al. constructed two DNA vaccines encoding full length of prM and envelope of French Polynesian ZIKV isolate, where prM signal sequence of ZIKV was exchanged with Japanese encephalitis virus (JEV) signal sequence which improved the expression level (Figure 3f) [27]. To improve the subviral particle (SVP) formation, one construct (VRC 5288) was further modified by incorporating stem and transmembrane from JEV. Single immunization of BALB/c and C57BL/6 mice with 50 μg of the DNA vaccine elicited ZIKV specific neutralizing antibodies with EC50 titer of 105 in BALB/c mice [27]. Both the vaccine candidates were immunogenic in mice and non-human primate models and competed in phase I clinical trial.
VRC5288 completed its phase I clinical trial in 2019 (Clinical trial NCT02840487, study VRC319). In a total of 80 participants (in four groups), 4 mg single doses of vaccine were administered via intramuscular injection in the deltoid muscle, with boosts at week 4, week 12, week 4 and 8, and week 4 and 20 where higher seroconversion 89% was reported on week 4, and week 12 boost with neutralizing antibody GMT titer of 120 (73–197).
VRC5283 (Clinical trial NCT02996461, study VRC 320) elicited neutralizing titer GMTs 304 (215–430) when a 4 mg single dose was posed at week 4 and 8. Further, 100% neutralizing antibody seroconversion was observed. VRC 5283 has also completed the phase II clinical trial.
A DNA vaccine for ZIKV is based on prM and envelope proteins that confer only neutralizing antibody mediated protection. However, cellular immunity is equally critical to clear ZIKV infection. NS1 is an attractive target for induction of cellular immune response as it can alone stimulate T cell mediated immune response against ZIKV [26].
2.3. mRNA Vaccines for DENV
In 2019, Roth et al. reported that the prime-boost immunization of humanized HLA class 1 transgenic mice with DENV1-NS, a vaccine that comprised of the most immunogenic portions of NS3, NS4B, and NS5, induced a strong CD8+ T cell immune response and conferred significant protection after challenge with DENV1 (Figure 4d) [28].
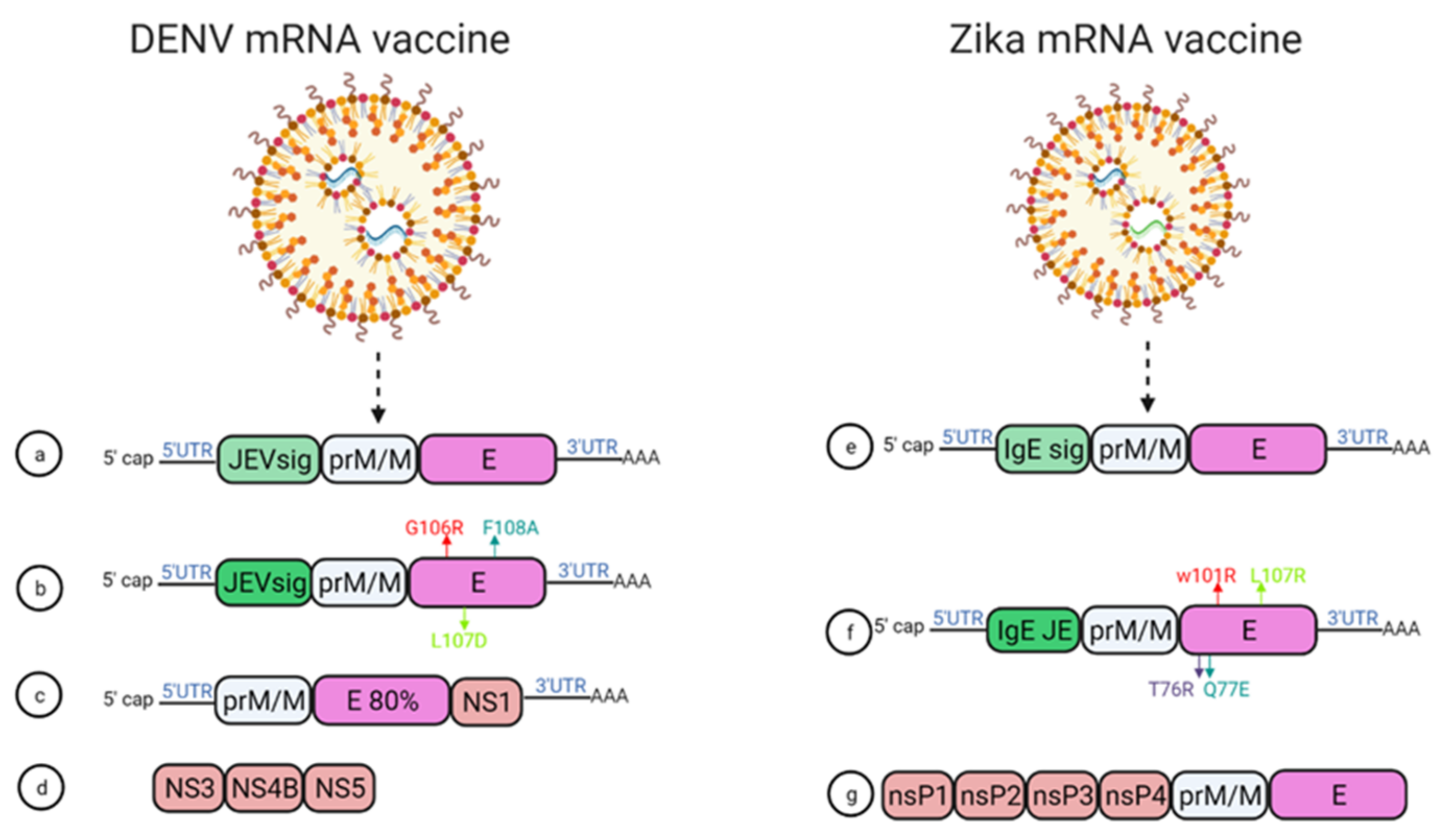
Figure 4. mRNA vaccine construction for DENV and ZIKV. DENV mRNA vaccine (a–d), Zika mRNA vaccine (e–g).
Moreover, using mRNA encapsulated by lipid nanoparticles (LNPs), Zhang et al. developed a DENV mRNA vaccine based on two structural proteins—prME and E80 (containing 80% N-terminal of the ENV protein)—and one non-structural protein (NS1) from DENV2 (Figure 4c) [29]. All mRNA elicited virion-binding antibodies; however, E80-mRNA yielded the strongest neutralizing antibody response against DENV2 with PRNT50 of 11,000 in BALB/c mice. Furthermore, vaccination with the combination of E80+NS1 mRNAs elicited antigen-specific T cell responses [29].
Wollner et al., demonstrated in immunocompromised AG129 mice that a nucleotide modified mRNA vaccine encodes DENV1 prM and E protein under JEV signal peptide elicited neutralizing antibodies, with EC50 titer of 1/400 ]. Anti-DENV1 CD4+ and CD8+ T cells were also activated. They also designed some E protein mutants, G106R, L107D, and F108A, to remove the fusion loop, and both the vaccines reduced ADE of DENV2 in K562 cells (Figure 4a,b) [30].
2.4. mRNA Vaccines for ZIKV
Two modified lipid nanoparticle packaged mRNA vaccines have been constructed (Figure 4) [31]. The first one contains (Figure 4e) a human IgE signal sequence with the full length of prM and E gene from an Asian strain (Micronesia 2007), and booster doses of this construct produced potent serum neutralizing titer and neutralizing immunity. In the second construct design, four mutations were inserted in the E protein T76R, Q77E, W101R, and L107R under JEV’s signal sequence to prevent cross-reacting antibodies (Figure 4f) [31].
In 2017, a study demonstrated that immunization with mRNA-LNP encoding the pre-membrane and envelope (prM-E) glycoproteins of ZIKV confers protection in mice and non-human primates. In this study, immunization of C57BL/6 mice with a 30 μg single dose of nucleoside-modified ZIKV prM-E mRNA-LNP vaccine-elicited E protein-specific CD4+ T cell responses. Further, vaccinated BALB/c mice and rhesus macaques (four out of five) showed no detectable viremia at day seven post ZIKV challenge [32].
Chahal et al. developed a modified dendrimer nanoparticle (MNDP) based RNA replicon vaccine [33]. This vaccine incorporated prM and E gene from an Asian lineage ZIKV isolate Z1106033, and the vaccine was able to elicit an E protein-specific IgG response in C57BL/6 mice. This study also identified an MHC I restricted 9-mer epitope IGVSNRDFV, which produces CD8+ T cell response in C57BL/6 mice (72).
Another self-replicating RNA vaccine utilizes an attenuated strain of the Venezuelan equine encephalitis (VEE) virus, TC-83. This vaccine expresses prM and E genes of ZIKV with ZIKV and Japanese encephalitis virus (JEV)’s signal sequence under a T7 promoter in the VEE replication vector, delivered through a stable nanostructured lipid carrier. A 10-ng single dose of this vaccine showed protection in mice against the lethal ZIKV challenge (Figure 4g) [34].
In another study, using a prime-boost strategy, intradermal electroporation of 1 μg of a naked self-replicating mRNA ZIKV vaccine encoding the ZIKV prM-E elicited high antibody titers in IFNAR1 knockout C57BL/6 mice, and all the mice were protected against the ZIKV challenge. The vaccine also conferred complete protection in vaccinated mice, while a four out of seven mortality was observed in the control group [35].
Luisi et al. developed another self-amplifying RNA (SA-RNA) vaccine encoding capsid, prM, and Env. A total of 1.5 μg of this SA-RNA vaccine delivered via cationic nanoemulsion was enough to produce neutralizing antibody responses in BALB/c mice [36].
References
- Kuno, G.; Chang, G.-J.J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 2007, 152, 687–696.
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218.
- Beasley, D.W.C.; Whiteman, M.C.; Zhang, S.; Huang, C.Y.-H.; Schneider, B.S.; Smith, D.R.; Gromowski, G.D.; Higgs, S.; Kinney, R.M.; Barrett, A.D.T. Envelope Protein Glycosylation Status Influences Mouse Neuroinvasion Phenotype of Genetic Lineage 1 West Nile Virus Strains. J. Virol. 2005, 79, 8339–8347.
- Kochel, T.; Wu, S.-J.; Raviprakash, K.; Hobart, P.; Hoffman, S.; Porter, K.; Hayes, C. Inoculation of plasmids expressing the dengue-2 envelope gene elicit neutralizing antibodies in mice. Vaccine 1997, 15, 547–552.
- Porter, K.R.; Kochel, T.J.; Wu, S.-J.; Raviprakash, K.; Phillips, I.; Hayes, C.G. Protective efficacy of a dengue 2 DNA vaccine in mice and the effect of CpG immuno-stimulatory motifs on antibody responses. Arch. Virol. 1998, 143, 997–1003.
- Beckett, C.G.; Tjaden, J.; Burgess, T.; Danko, J.R.; Tamminga, C.; Simmons, M.; Wu, S.-J.; Sun, P.; Kochel, T.; Raviprakash, K.; et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine 2011, 29, 960–968.
- Porter, K.R.; Ewing, D.; Chen, L.; Wu, S.-J.; Hayes, C.G.; Ferrari, M.; Teneza-Mora, N.; Raviprakash, K. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine 2012, 30, 336–341.
- Danko, J.R.; Kochel, T.; Teneza-Mora, N.; Luke, T.C.; Raviprakash, K.; Sun, P.; Simmons, M.; Moon, J.E.; De LA Barrera, R.; Martinez, L.J.; et al. Safety and immunogenicity of a tetravalent dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase 1 clinical trial. Am. J. Trop. Med. Hyg. 2018, 98, 849.
- Yeo, A.S.L.; Rathakrishnan, A.; Wang, S.M.; Ponnampalavanar, S.; Manikam, R.; Sathar, J.; Natkunam, S.K.; Sekaran, S.D. Dengue Patients Exhibit Higher Levels of PrM and E Antibodies Than Their Asymptomatic Counterparts. BioMed Res. Int. 2015, 2015, 1–10.
- Duan, Z.; Guo, J.; Huang, X.; Liu, H.; Chen, X.; Jiang, M.; Wen, J. Identification of cytotoxic T lymphocyte epitopes in dengue virus serotype 1. J. Med. Virol. 2015, 87, 1077–1089.
- Wen, J.; Shresta, S. T Cell Immunity to Zika and Dengue Viral Infections. J. Interf. Cytokine Res. 2017, 37, 475–479.
- Mathew, A.; Townsley, E.; Ennis, F.A. Elucidating the role of T cells in protection against and pathogenesis of dengue virus infections. Future Microbiol. 2014, 9, 411–425.
- Elong Ngono, A.; Chen, H.W.; Tang, W.W.; Joo, Y.; King, K.; Weiskopf, D.; Sidney, J.; Sette, A.; Shresta, S. Protective Role of Cross-Reactive CD8 T Cells Against Dengue Virus Infection. EBioMedicine 2016, 13, 284–293.
- Costa, S.M.d.; Freire, M.S.; Alves, A. DNA vaccine against the non-structural 1 protein (NS1) of dengue 2 virus. Vaccine 2006, 24, 4562–4564.
- Costa, S.M.; Yorio, A.P.; Gonçalves, A.J.S.; Vidale, M.M.; Costa, E.C.B.; Borges, R.M.; Motta, M.A.; Freire, M.S.; Alves, A.M.B. Induction of a Protective Response in Mice by the Dengue Virus NS3 Protein Using DNA Vaccines. PLoS ONE 2011, 6, e25685.
- Chen, H.; Zheng, X.; Wang, R.; Gao, N.; Sheng, Z.; Fan, D.; Feng, K.; Liao, X.; An, J. Immunization with electroporation enhances the protective effect of a DNA vaccine candidate expressing prME antigen against dengue virus serotype 2 infection. Clin. Immunol. 2016, 171, 41–49.
- Zheng, X.; Chen, H.; Wang, R.; Fan, D.; Feng, K.; Gao, N.; An, J. Effective Protection Induced by a Monovalent DNA Vaccine against Dengue Virus (DV) Serotype 1 and a Bivalent DNA Vaccine against DV1 and DV2 in Mice. Front. Cell. Infect. Microbiol. 2017, 7, 175.
- Sheng, Z.; Chen, H.; Feng, K.; Gao, N.; Wang, R.; Wang, P.; Fan, D.; An, J. Electroporation-mediated immunization of a candidate DNA vaccine expressing dengue virus serotype 4 prm-e antigen confers long-term protection in mice. Virol. Sin. 2019, 34, 88–96.
- Feng, K.; Zheng, X.; Wang, R.; Gao, N.; Fan, D.; Sheng, Z.; Zhou, H.; Chen, H.; An, J. Long-term protection elicited by a DNA vaccine candidate expressing the prM-E antigen of dengue virus serotype 3 in mice. Front. Cell. Infect. Microbiol. 2020, 10, 87.
- LaRocca, R.A.; Abbink, P.; Peron, J.P.S.; de A Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’Ang’A, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478.
- Bhatt, S.; Gething, P.; Brady, O.; Messina, J.; Farlow, A.; Moyes, C. A distribuição global e o ônus da dengue. Nature 2013, 496, 504.
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760.
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520.
- Kokernot, R.; Casaca, V.; Weinbren, M.; McIntosh, B. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of Angola. Trans. R. Soc. Trop. Med. Hyg. 1965, 59, 563–570.
- Filipe, A.R.; Martins, C.M.V.; Rocha, H. Laboratory infection with Zika virus after vaccination against yellow fever. Fish Shellfish Immunol. 1973, 43, 315–319.
- Grubor-Bauk, B.; Wijesundara, D.K.; Masavuli, M.; Abbink, P.; Peterson, R.L.; Prow, N.A.; Larocca, R.A.; Mekonnen, Z.A.; Shrestha, A.; Eyre, N.S.; et al. NS1 DNA vaccination protects against Zika infection through T cell–mediated immunity in immunocompetent mice. Sci. Adv. 2019, 5, eaax2388.
- Dowd, K.A.; Ko, S.-Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240.
- Roth, C.; Cantaert, T.; Colas, C.; Prot, M.; Casadémont, I.; Levillayer, L.; Thalmensi, J.; Langlade-Demoyen, P.; Gerke, C.; Bahl, K.; et al. A modified mRNA vaccine targeting immunodominant NS epitopes protects against dengue virus infection in HLA class I transgenic mice. Front. Immunol. 2019, 2019, 1424.
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified mRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol. Ther.-Methods Clin. Dev. 2020, 18, 702–712.
- Wollner, C.J.; Richner, M.; Hassert, M.A.; Pinto, A.K.; Brien, J.D.; Richner, J.M. A dengue virus serotype 1 mRNA-LNP vaccine elicits protective immune responses. J. Virol. 2021, 95, e02482-20.
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA vaccines protect against Zika virus infection. Cell 2017, 168, 1114–1125.e10.
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251.
- Chahal, J.S.; Fang, T.; Woodham, A.W.; Khan, O.F.; Ling, J.; Anderson, D.G.; Ploegh, H.L. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci. Rep. 2017, 7, 252.
- Erasmus, J.H.; Khandhar, A.P.; Guderian, J.; Granger, B.; Archer, J.; Archer, M.; Gage, E.; Fuerte-Stone, J.; Larson, E.; Lin, S.; et al. A Nanostructured Lipid Carrier for Delivery of a Replicating Viral RNA Provides Single, Low-Dose Protection against Zika. Mol. Ther. 2018, 26, 2507–2522.
- Zhong, Z.; Portela Catani, J.P.; Mc Cafferty, S.; Couck, L.; Van Den Broeck, W.; Gorlé, N.; Vandenvroucke, R.E.; Devriendt, B.; Ulbert, S.; Cnops, L.; et al. Immunogenicity and protection efficacy of a naked self-replicating mRNA-based Zika virus vaccine. Vaccines 2019, 7, 96.
- Luisi, K.; Morabito, K.M.; Burgomaster, K.E.; Sharma, M.; Kong, W.-P.; Foreman, B.M.; Patel, S.; Fisher, B.; Aleshnick, M.A.; Laliberte, J.; et al. Development of a potent Zika virus vaccine using self-amplifying messenger RNA. Sci. Adv. 2020, 6, eaba5068.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.0K
Revisions:
2 times
(View History)
Update Date:
08 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




