Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giulia Mattei | -- | 4010 | 2022-06-07 11:38:29 | | | |
| 2 | Jason Zhu | -21 word(s) | 3989 | 2022-06-08 05:19:52 | | | | |
| 3 | Jason Zhu | Meta information modification | 3989 | 2022-06-08 05:21:28 | | | | |
| 4 | Jason Zhu | -1 word(s) | 3988 | 2022-06-10 11:01:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mattei, G.; Di Tucci, C.; Galati, G.; Chinè, A.; Fracassi, A.; Muzii, L. Fertility after Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/23772 (accessed on 07 February 2026).
Mattei G, Di Tucci C, Galati G, Chinè A, Fracassi A, Muzii L. Fertility after Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/23772. Accessed February 07, 2026.
Mattei, Giulia, Chiara Di Tucci, Giulia Galati, Alessandra Chinè, Alice Fracassi, Ludovico Muzii. "Fertility after Cancer" Encyclopedia, https://encyclopedia.pub/entry/23772 (accessed February 07, 2026).
Mattei, G., Di Tucci, C., Galati, G., Chinè, A., Fracassi, A., & Muzii, L. (2022, June 07). Fertility after Cancer. In Encyclopedia. https://encyclopedia.pub/entry/23772
Mattei, Giulia, et al. "Fertility after Cancer." Encyclopedia. Web. 07 June, 2022.
Copy Citation
Approximately one million new cases of cancer are diagnosed in women of reproductive age every year. In the last few decades, advances in early diagnosis and treatment have improved the survival rate. However, the adverse effects of anticancer therapy on the ovaries and uterus have a significant impact on future fertility and may affect the quality of life of cancer survivors. Impaired fertility in cancer survivors is a growing issue that is complicated by an increasing number of women delaying childbearing.
infertility
gynecologic cancers
fertility sparing treatments
ovarian damage
1. Introduction
The incidence of any type of cancer in 15–39 year old women is 52.3 rate per 100,000 [1].
In Italy, 3% of cancer cases are diagnosed in women under the age of 40. The most common types of cancer in women under 40 are breast cancer, thyroid cancer, melanoma, cervical cancer, endometrial cancer, ovarian cancer, leukemia/lymphomas, and colorectal cancer [2].
Breast cancer (BC) is the most common cancer in women under 49 (40% of all cancer cases). Despite the increased incidence of BC with age, approximately 7–10% of women diagnosed with BC are under 40 [3]. The incidence trend in Italy is slightly increasing (0.3% per year), whereas mortality continues to decline (−0.8% per year). The 5-year survival rate in young women (15–44 years) is 91% [4].
Thyroid cancer is common between the ages of 0–49 years (15% of all cancer cases). The most important prognostic factor is represented by the following histotype: the 20-year survival rate is 98–99% for papillary carcinomas and 80–90% for follicular ones (together they constitute 90% of thyroid cancer with papillary/follicular ratio 10:1), whereas it drops to 50–75% at 10 years for medullary carcinomas [5].
Melanoma is the third most frequent cancer in women aged between 18 and 39 years, with an increasing incidence trend (3.1% per year) [6].
Cervical cancer is the second for incidence in women. Its incidence increases with age up to 45 years with a peak between 45–55 years. The incidence rate is rising in developing countries but is decreasing in high resource countries [7].
Endometrial cancer has a very low incidence in reproductive age. Only 20% of endometrial cancers affect premenopausal women, and of these, no more than 5–8% affect women under 40 [8].
As for ovarian cancer, 80–90% occurs amongst women between the ages of 20 and 65 years old, and 90% of malignant tumors are diagnosed in women over the age of 40. A total of 5–10% of ovarian cancers are of intermediate malignancy (borderline) and approximately 30% of them affect women under 40. However, the diagnosis of epithelial ovarian cancer in women of reproductive age has become more frequent with increasing gynecological physical checkups [2].
Colorectal cancer’s (CRC) incidence is increasing in women of reproductive age. Fortunately, the 5-year survival rate from CRC is improving, with a survival rate of 65% [9].
Considering pediatric cancer patients, a recent study of over 3500 young women who survived childhood cancer such as leukemia, central nervous system cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone tumor, shows a significantly higher risk of infertility than in the control group (RR: 1.48) [10][11]. There are still limited modalities available to preserve prepubertal fertility. Ovarian tissue reimplantation is the only fertility preservation technique that can be used to preserve prepubertal fertility, whereas mature oocyte cryopreservation is the main technique used for fertility preservation in post-menarche adolescents. However, a growing awareness and competence on the subject is beginning to emerge, especially amongst oncology pediatricians in Northern Europe [12][13].
In the United States, the 5-year overall survival rate for all invasive cancers between ages 15–39 is about 82.5% [14]. Despite the increased incidence of cancer cases, advances in early diagnosis and treatment have increased the survival rate [15].
Furthermore, in the last four decades, there has been a rising trend of delaying childbearing [16]. Hence, there is an increasing number of couples referred to Reproductive Medicine Centers for infertility problems after one partner has been treated for cancer. In these cases, the main cause of reduced fertility derives from the gonadotoxic effects of chemo/radiotherapy treatment [17]. As far as young women are concerned, there are two main concerns: the possible harmful effects of previous anticancer treatments on a future pregnancy, and the consequences pregnancy may have on the patient, particularly in the case of endocrine-sensitive neoplasms, even if to this day, there is no evidence of such possible adverse effects [18][19][20][21][22][23]. All reproductive-aged cancer patients must therefore be adequately informed of the risk of fertility loss/reduction as a consequence of anticancer treatments, and at the same time the strategies available to reduce this risk.
2. Influence of Cancer on Ovarian Function
If the cancer “per se” may induce alterations of ovarian functions, these should be investigated.
Several studies have examined the fertility of women with cancer before chemo/radiotherapy and show mixed results. Different studies found a negative interference of cancer on ovarian function even before starting cancer treatment [24][25]. Pal et al. were the first that found an adverse effect of the tumor on ovarian function [26]. An observational study described how women with hormone-dependent cancer have a poorer response to controlled ovarian stimulation, with fewer oocytes retrieved than those with non-hormone-dependent cancer [27].
Alvarez and Ramanathan found significantly fewer oocytes in breast cancer patients than in patients with hematological cancer [28]. In a retrospective cohort study that included 155 women, Volodarsky-Perel et al. showed that women with grade G3 and stage III-IV breast cancer had significantly fewer numbers of mature oocytes than patients with grade G1-2 and at stage I-II of the disease. Also, the number of cryopreserved embryos was lower in women with grade G3. The same researchers demonstrated that patients with high-grade cancer have fewer oocytes and embryos retrieved than those with low-grade cancer [29].
Similarly, Decanter et al. found fewer oocytes in women with cancer than in the age-matched control group [30]. Moria et al. showed that breast cancer patients had fewer retrieved oocytes than the control group [31].
However, most of the studies have not demonstrated relevant differences in ovarian reserve and oocytes retrieved between women with cancer and the healthy group. Almog et al., in their study with 81 women, stated that the ovarian function was not affected by the type of cancer [32][33][34][35][36].
These observations are thought to be linked to the systemic effect of cancer, causing higher catabolic status, increased stress hormones levels, and impaired function of the granulosa cells. Furthermore, invasive cancer infiltrates and destroys the surrounding tissue causing immune system reactions involving distant organs [37][38]. This systemic response conditions folliculogenesis, follicular cell proliferation, and oocyte maturation through the release of metalloproteases and growth factors [39][40].
Due to the close relationship between cancer and BRCA1 and 2 mutations, it is important to evaluate whether the cancer itself or the BRCA mutations underlie the reduced ovarian reserve in these women [41][42]. Porcu et al. demonstrated that BRCA 1 patients have a higher risk of premature ovarian failure compared to non-BRCA-mutated women with breast cancer and the healthy controls. BRCA1 groups have lower AMH levels and a significantly lower rate of mature oocytes. This effect seems to be independent of the probable interference of cancer. Therefore, the ovarian response after ovarian stimulation may be influenced by the presence of cancer [43].
More studies are needed to understand if there is a negative effect of cancer on ovarian function even before cancer treatment is started and if the type of cancer influences the ovarian response.
3. Effects of Cancer Treatment on Female Reproductive Function
The adverse effects of cancer treatment on female reproductive function are an increasing problem that affects the quality of life in survivors of childhood, adolescent, and young adult cancer.
The three principal anti-cancer treatments are surgery, radiotherapy, and chemotherapy.
In most cases, primary ovarian or uterine cancers are surgically removed together with these organs, leading to sterility. For these women, using donated eggs or surrogacy are the only chance to have a child [44].
Chemotherapy and radiotherapy may damage the reproductive system by destroying the hypothalamic–pituitary axis, the uterus, or the primordial and growing follicles within the ovaries.
The effects of chemotherapy and radiotherapy on the ovaries and uterus have a significant impact on the future fertility of childhood cancer patients as well as women up to the age of menopause.
3.1. Ovarian Damage
Cancer therapy can involve the administration of a wide variety of therapeutic protocols [45].
The American Society of Clinical Oncology published a useful classification of cancer treatments based on their level of gonadotoxicity and their consequent risk of permanent amenorrhea [46].
The level of gonadotoxicity depends on chemotherapeutic classes, dose, method of administration (oral versus intravenous), and combination of drugs; moreover, the toxicity changes with the type of disease, the woman’s age at the time of treatment, and the woman’s pre-treatment fertility.
“Highly gonadotoxic treatments” cause permanent amenorrhea in at least an 80% of cases; the most common of them are, for example, chemotherapy used to treat breast cancer in women over 40 (combinations of cyclophosphamide, methotrexate, fluorouracil, doxorubicin, epirubicin), external beam radiation to a field that includes the ovaries, or myeloablative conditioning for hematopoietic stem cell transplantation with high-dose alkylating agents (cyclophosphamide/busulfan) in combination with total body irradiation [46][47]. Different studies have reported that the risk of gonadal insufficiency after stem cell transplantation is related to a woman’s age; the risk of infertility is 65–95% in adult women, higher than in prepubertal girls (around 50%) because of higher ovarian reserve [48][49].
Treatments classified as “intermediate gonadotoxicity” involve a 40–60% risk of amenorrhoea. These include adjuvant chemotherapy for breast cancer in women aged 30–39 (combinations of cyclophosphamide, methotrexate, fluorouracil, doxorubicin, epirubicin) and escalated (second-line) chemotherapy used for Hodgkin’s lymphoma [27].
“Low” gonadotoxic cancer treatments, with a risk of amenorrhea < 20%, include first-line treatment for Hodgkin’s lymphoma (ABVD therapy) and treatment for acute lymphoblastic and myeloid leukemia [44].
Treatments considered “very low risk” or “no risk” are antimetabolites (such as methotrexate, cytarabine) and vinca alkaloids (vincristine, vinblastine), which do not cause damage to human follicles [44][47].
3.1.1. Impact of Chemotherapy
Many studies have investigated the type of damage of each chemotherapeutic agent on different cell types of the ovary.
These findings have reported that ovarian damage can occur via several mechanisms [50].
Apoptotic death of primordial follicle and growing follicles are caused by direct DNA damage (via DNA double-strand breaks and inter-strand crosslinking) [47][51].
Direct damage to the ovarian stroma causes fibrosis and hyalinisation of small blood vessels, resulting in ischemia and necrosis due to a reduction in ovarian blood volume and consequently indirect damage to follicles growth [47][52].
Indirect damage to primordial follicles is due to increased follicle activation. Meirow’s group explained this mechanism of enhanced follicular demise owing to accelerated folliculogenesis by proposing the “burnout theory” [53][54]. In particular, the destruction of growing follicles and thus the local reduction in AMH concentrations causes an upregulation in the PI3K/PTEN/Akt signaling pathway.
3.1.2. Impact of Radiotherapy
Radiation therapy is one treatment modality for various types of malignancies, but unfortunately, exposure to ionizing radiation can lead to acute and long-term damage.
The effects of radiation to the abdominopelvic region depends on the dose intensity, fractionation, field of irradiation, and the age of the patient [44][55][56]. Women in the prepubertal period have ovaries relatively more resistant to gonadotoxicity [57].
The radiosensitivity of oocytes is high and differs according to their growth phase. In particular, dividing granular cells appear to be the main target of radiation-related gonadotoxicity.
Radiotherapy-induced ovarian injury also involves the stroma with vascular damage, leading to tissue atrophy and fibrosis [58].
The underlying mechanism induced by radiotherapy is both direct and indirect. The radiation induces a direct ionization of the cellular macromolecules, such as gonadal DNA causing multiple lesions within the helical turns of the DNA, which is referred to as “direct” damage. Radiotherapy also leads to the generation of reactive oxygen species (ROS) in cells, increasing oxidative stress and diminishing antioxidant defense mechanisms.
This imbalance may play a role in the etiology of radiotherapy-induced gonadotoxicity, which is defined as “indirect” damage [59].
Through these pathways, radiotherapy can affect healthy normal tissues in the ovaries and can influence the length of a women’s fertile lifespan and the timing of menopause.
3.2. Uterine Damage
Recent trials have reported that anti-cancer treatments can cause permanent injury to the uterus and compromise its ability to allow and sustain a healthy pregnancy [60].
3.2.1. Impact of Chemotherapy
The percentage of pregnancies obtained through egg donation or using one’s own eggs before anticancer treatment is lower than in the control group. Even though patients underwent assisted reproductive technologies (ART), the implantation rate and clinical pregnancy rate (4.9% and 9.5%, respectively) were statistically significantly lower, underlining that cancer therapy-induced damage to the uterus may contribute to infertility [60][61].
A small number of studies have demonstrated that chemotherapy exposure during childhood (especially to alkylating agents) is associated with a smaller uterine size and volume [62][63].
Despite this indirect evidence of uterine damage induced by chemotherapy, there is a paucity of data regarding the specific pathological mechanism through which these drugs can act. Currently, the damage to endometrial epithelium, myometrium, uterine vasculature, and the endometrial stem cell niche can only be extrapolated from animal models or laboratory and clinical findings on the analogous cell line of other human tissue (for example intestinal stem cells, cardiomyocites, skeletal muscle) [60].
3.2.2. Impact of Radiotherapy
Radiation to the uterus can impair reproductive function. Evidence has reported that radiotherapy can cause microvascular injury with endothelial damage and myometrial fibrosis compromising uterine growth and distensibility. Radiation may also damage muscle fibers and decrease pelvic floor muscle function [47]; when the woman’s exposure happens before puberty, stunted uterine growth and fibrosis can only be partially rescued by hormone replacement therapy [37]. Critchley et al. reported a shorter uterine length and a rare uterine blood flow in patients who received radiation during childhood compared to women with a history of POF (Primary Ovarian Failure) without radiation exposure.
The radiation consequences on the uterus are greater in the case of exposure at a young age, leading to greater risks for future pregnancy. The radiation dose that poses the greatest risk of reproductive failure is >45 Gy in adults or >25 Gy in childhood [47].
4. Oncofertility
Approximately one million new cases of cancer are diagnosed in reproductive-aged women every year [60][64].
In the last few decades, life expectancy of these patients has increased thanks to anticancer treatments. The increased survival rate, combined with increased age for childbearing, has led to the occurrence of side effects such as fertility problems [65].
Diagnosis and treatment of tumors can often cause fertility problems in women. It has been estimated that 70–75% of cancer survivors are interested in parenthood and that 80% of them are affected by reduced fertility [66].
Despite patients’ interest in parenthood, the percentage of patients who receive correct information varies from 51% to 95%, and the percentage of patients who access fertility preservation techniques is low [67][68].
Fertility loss can cause devastating emotional reactions in women impacting their plans for the future. Various studies demonstrate that discussing fertility preservation options can improve quality of life and can contribute to psychological health [69][70].
The creation of an oncofertility team around the patient would allow this conversation to happen at the appropriate time and would reduce fertility loss in cancer patients [71].
Oncologists should inform patients about impaired fertility risk and should provide information on strategies available to preserve it.
Fertility preservation strategies in females depend on age, type of treatment planned, diagnosis, presence of a partner, time available before starting treatment, and potential for cancer to metastasize to the ovaries [46].
After consultation with a hematologist, oncologist, and specialist in reproductive medicine, an adequate consult can be carried out to evaluate ovarian reserve and gonadotoxicity of the therapies and to propose an appropriate fertility preservation technique [64].
4.1. Fertility Preservation Options
Different fertility preservation strategies can be proposed (Figure 1):
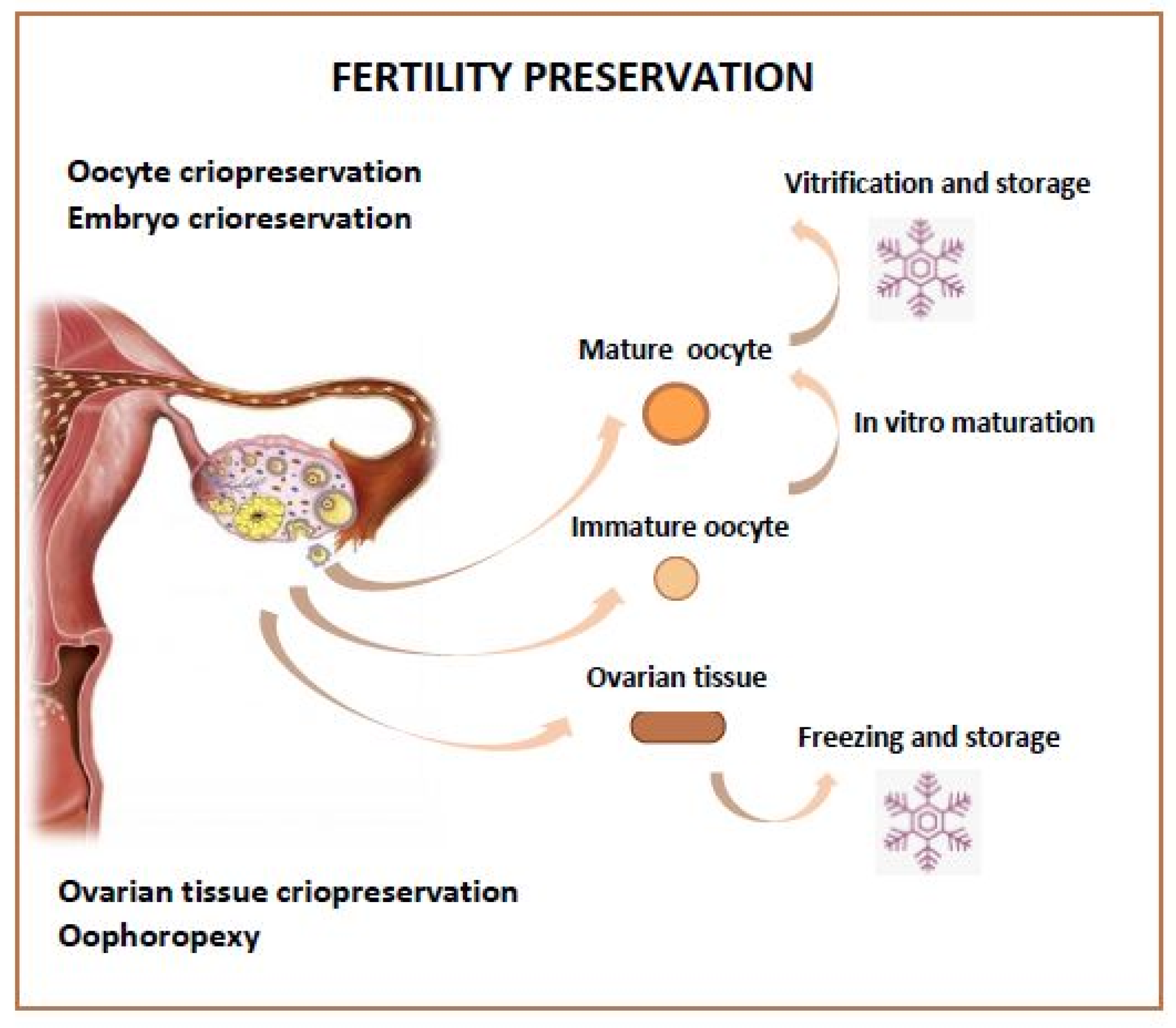
Figure 1. Fertility Preservation Options.
-
Hormone Protection by Suppressing Ovaries
-
Oophoropexy
-
Embryo Storage, Oocyte Storage
-
Ovarian Tissue Storage
-
Fertility Sparing Surgery
4.1.1. GnRH-Analogues
In cancer patients who are candidates for chemotherapy, the use of GnRH analogues should be proposed but not considered as an alternative to cryopreservation [72].
GnRH analogues are used as chemoprotectors; used during chemotherapy they induce menopause, suppressing the ovarian cycle.
In 2018 and 2020, ASCO Guidelines and the European Society for Medical Oncology, respectively, recommended that GnRHa use should be offered to all cancer patients who desire to preserve fertility. [73]
In 2018, the British Fertility Society affirmed that GnRHa should be started immediately before chemotherapy and continued for the duration of therapy. [73]
GnRH analogues arrest ovarian cells in the G0 phase inducing cellular quiescence and making these cells less responsive to chemotherapy [74][75]; this treatment has shown effects in reducing primary ovarian insufficiency (POI) risk, increasing pregnancy rates, and having no negative effects on the cancer’s outcome.
The use of GnRH analogues can be also proposed in patients with hormone receptor-positive disease with safety [76].
Several studies have shown that the use of Goserelin has preserved fertility in a high percentage of patients affected by breast cancer [77][78].
The use of Goserelin reduces premature ovarian failure risk as well as prevalence of amenorrhoea and also improves disease-free survival and overall survival [79][80].
AMH can be used to evaluate the GnRHa protective effect on fertility [73].
4.1.2. Oophoropexy
Ovarian transposition (oophoropexy) consists of surgical removal of the ovaries from the irradiation site.
This strategy can be proposed to patients who are candidates for pelvic radiotherapy (children and pre-menopausal women who desire to preserve fertility and prevent early menopause), in cases of gynecological or hematological cancers, such as cervical cancer and Hodgkin’s lymphomas, and in cases of medulloblastoma, urogenital rhabdomyosarcoma, pelvic sarcomas, Wilm’s tumor, and rectal cancer [75][81][82].
Before the procedure, a pelvic MRI should be performed to ensure that the tumor does not involve the ovarian region.
Ovarian transposition can be performed by laparoscopy or laparotomy (in case of concomitant resection of the tumor). One or both ovaries are relocated, either medially (behind the uterus in the case of Hodgkin’s Lymphoma), laterally, near the inguinal ring, in the paracolic gutters, or near the lower kidney pole (in the case of urogenital tumors, medulloblastoma, and rhabdomyosarcoma), or to any distant site.
At the end of the procedure, two metal clips should be applied to the transposed ovaries to make them visible on imaging.
Possible complications of oophoropexy are: torsion of the ovarian blood vessels, development of benign ovarian cysts, and subsequent chronic pain and ovarian and abdominal wall metastases at the trocar site [81][82][83].
Success rate of this treatment, in terms of preserved ovarian function, varies between 60% and 83% and several spontaneous pregnancies after this procedure are described. Nevertheless, some researchers claim that these patients could require assisted reproductive technologies because of increased distance between the ovary and the fallopian tube.
This increased distance compromises oocyte migration through the Fallopian tube and impairs fertility [82].
4.1.3. Embryo and Oocyte Cryopreservation
Embryo cryopreservation is one of the options to preserve fertility.
An oocyte and a sperm cell (obtained from a male partner or sperm donor) are needed to create an embryo [75].
Embryo cryopreservation is a good choice for patients with a stable relationship because of better pregnancy outcomes [84]. However, not all women have a stable relationship at the time of diagnosis; in this case, oocyte cryopreservation is a valid alternative, giving women an opportunity to procreate with a chosen partner in the future, without the need to fertilize the oocyte after retrieval [85][86].
Ovaries are stimulated with daily injections of follicle-stimulating hormone and stimulation can be started at any point in the menstrual cycle.
Follicle growth is monitored by ultrasounds and blood tests (serum estradiol and progesterone levels). Ovulation is induced with an HCG injection when appropriate and oocytes are collected by transvaginal aspiration (with ultrasound guidance). The oocytes retrieved can be cryopreserved or fertilized in vitro to obtain embryos [46][88].
Oocyte and embryo cryopreservation, performed prior to anti-cancer therapies, are defined by the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) as the most appropriate procedures to ensure motherhood in cancer survivors [89].
Among reproductive-aged women, breast cancer is one of the most frequent. In some cases, this is a hormone-sensitive cancer because of the expression of estrogen receptors.
In patients with these tumors, exposure to high estrogen levels can be risky. In order to avoid this exposure, oocytes can be recovered from a natural cycle or from ovarian stimulation protocol with aromatase inhibitor or tamoxifen (chemoprotective agents that have ovulation-inducing properties) [74][90].
4.1.4. New Strategies
Among new strategies, immature oocytes retrieval is an experimental technique that involves cryopreservation of immature oocytes or matured in vitro.
These oocytes can be used for vitrification or to obtain embryos by ICSI with partner sperm. This strategy allows for a reduction in the time needed for preservation and avoids exposure to hyperestrogenism caused by stimulation [91].
Mature oocytes can be obtained by in vitro maturation of immature oocytes (IVM) or in vitro activation of dormant follicles (IVA) [89].
Achieving pregnancy is possible using oocytes maturated in vitro [92] but pregnancy rates are lower in patients who have used embryos obtained from immature oocytes or oocytes matured in vitro than those who have used embryos obtained from mature oocytes [91].
After treatment, patients should be informed about their ovarian function using different parameters (AMH, FSH, estradiol) in order to decide whether to use cryopreserved oocytes or to start a new cycle [92].
The discovery of ovarian stem cells in the ovarian cortex, first found in mammals and then also in women, opened new chances to preserve fertility.
Despite these findings, in vitro maturation of ovarian stem cells (OSC)s to oocyte-like cells (OLCs) still needs to be investigated for future clinical use in female cancer survivors [89].
4.1.5. Ovarian Tissue Cryopreservation
Ovarian tissue cryopreservation (OTC) is the only fertility preservation strategy available in prepubertal patients and those who cannot postpone treatment [87][93].
The entire ovary or part of this can be collected laparoscopically at any period of menstrual cycle. Obtained tissue is sliced and cryopreserved.
When patients are declared free from cancer with a good prognosis, the ovarian tissue is thawed, tested to assess the absence of cancer cells, and reimplanted orthotopically or heterotopically [94].
Although the use of this technique has shown good results in adult patients, ex vivo maturation of ovarian tissue taken in childhood and the subsequent auto-transplantation is still considered experimental [95].
One of the problems of ovarian tissue transplantation is that revascularization occurs after a few days from the time of the procedure. This causes tissue ischemia and a loss of more than 60% of the primordial follicles [74].
Local administration of antiapoptotic and angiogenic factors can improve the revascularization of ovarian tissue [96].
Another problem with ovarian tissue transplantation is the possibility of transferring cancer cells in patients. Even though the tissue is controlled before freezing and before transplantation, the risk of tumor cell transmission remains.
Therefore, this treatment is only proposed to patients with a low risk of ovarian metastasis [75].
Tumor cell transmission can be reduced by transferring primordial follicles onto an artificial tissue to replace native organs [99].
Depending on the number of follicles in cryopreserved tissue, ovarian function resumes after transplantation for 4–5 years on average and in some cases up to 7 years [86][100].
The first pregnancy after this fertility preservation technique was obtained in 2004; pregnancy and live birth rates are growing exponentially over the years. [87][101].
Patients who have undergone this fertility preservation strategy often have undetectable AMH levels, but spontaneous pregnancies after orthotopic transplantation have been reported [73].
AMH level is not associated with the duration of ovarian graft function or the possibility to achieve pregnancy in these women [102].
4.2. Success Rates
Cryopreservation of embryos, oocytes, ovarian tissue, or fertility preservation does not guarantee achieving a pregnancy in the future [71].
Among survivors, pregnancy rates are about 40% lower than the general population [16].
Several studies have described success rates after fertility preservation techniques:
References
- You, L.; Lv, Z.; Li, C.; Ye, W.; Zhou, Y.; Jin, J.; Han, Q. Worldwide cancer statistics of adolescents and young adults in 2019: A systematic analysis of the Global Burden of Disease Study 2019. ESMO Open 2021, 6, 100255.
- Mahajan, N. Fertility preservation in female cancer patients: An overview. J. Hum. Reprod. Sci. 2015, 8, 3–13.
- Rossi, L.; Mazzara, C.; Pagani, O. Diagnosis and Treatment of Breast Cancer in Young Women. Curr. Treat. Options Oncol. 2019, 20, 86.
- Francis, P.A.; Regan, M.M.; Fleming, G.F.; Láng, I.; Ciruelos, E.; Bellet, M.; Bonnefoi, H.R.; Climent, M.A.; Da Prada, G.A.; Burstein, H.J.; et al. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 2015, 372, 436–446.
- Smallridge, R.C.; Copland, J.A. Anaplastic thyroid carcinoma: Pathogenesis and emerging therapies. Clin. Oncol. 2010, 22, 486–497.
- DiSano, J.A.; Schaefer, E.W.; Kjerulff, K.; Hollenbeak, C.S.; Pameijer, C.R. Pregnancy after a melanoma diagnosis in women in the United States. J. Surg. Res. 2018, 231, 133–139.
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203.
- Guillon, S.; Popescu, N.; Phelippeau, J.; Koskas, M. A systematic review and meta-analysis of prognostic factors for remission in fertility-sparing management of endometrial atypical hyperplasia and adenocarcinoma. Int. J. Gynaecol. Obstet. 2019, 146, 277–288.
- Shandley, L.M.; McKenzie, L.J. Recent Advances in Fertility Preservation and Counseling for Reproductive-Aged Women with Colorectal Cancer: A Systematic Review. Dis. Colon Rectum 2019, 62, 762–771.
- Salama, M.; Anazodo, A.; Woodruff, T.K. Preserving fertility in female patients with hematological malignancies: A multidisciplinary oncofertility approach. Ann. Oncol. 2019, 30, 1760–1775.
- Barton, S.E.; Najita, J.S.; Ginsburg, E.S.; Leisenring, W.M.; Stovall, M.; Weathers, R.E.; Sklar, C.A.; Robison, L.L.; Diller, L. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013, 14, 873–881.
- Overbeek, A.; van den Berg, M.; Louwé, L.; Wendel, E.; Ter Kuile, M.; Kaspers, G.; Stiggelbout, A.; van Dulmen-Den Broeder, E.; Hilders, C. Practice, attitude and knowledge of Dutch paediatric oncologists regarding female fertility. Neth. J. Med. 2014, 72, 264–270.
- De Lambert, G.; Poirot, C.; Guérin, F.; Brugières, L.; Martelli, H. Preservation of fertility in children with cancer. Bull. Cancer 2015, 102, 436–442.
- Keegan, T.H.; Ries, L.A.; Barr, R.D.; Geiger, A.M.; Dahlke, D.V.; Pollock, B.H.; Bleyer, W.A.; National Cancer Institute Next Steps for Adolescent and Young Adult Oncology Epidemiology Working Group. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer 2016, 122, 1009–1016.
- De Melo, A.S.; de Paula, C.T.V.; Rufato, M.A.F.; Rufato, M.C.A.C.; Rodrigues, J.K.; Ferriani, R.A.; Barreto, J. Fertility optimization in women with cancer: From preservation to contraception. JBRA Assist. Reprod. 2019, 23, 418–429.
- Peccatori, F.A.; Azim, H.A., Jr.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G.; ESMO Guidelines Working Group. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, 160–170.
- Barton, S.E.; Missmer, S.A.; Berry, K.F.; Ginsburg, E.S. Female cancer survivors are low responders and have reduced success compared with other patients undergoing assisted reproductive technologies. Fertil. Steril. 2012, 97, 381–386.
- Boyle, K.E.; Vlahos, N.; Jarow, J.P. Assisted reproductive technology in the new millennium: Part II. Urology 2004, 63, 217–224.
- Howell, S.; Shalet, S. Gonadal damage from chemotherapy and radiotherapy. Endocrinol. Metab. Clin. N. Am. 1998, 27, 927–943.
- Gelber, S.; Coates, A.S.; Goldhirsch, A.; Castiglione-Gertsch, M.; Marini, G.; Lindtner, J.; Edelmann, D.Z.; Gudgeon, A.; Harvey, V.; Gelber, R.D. International Breast Cancer Study Group. Effect of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J. Clin. Oncol. 2001, 19, 1671–1675.
- Córdoba, O.; Bellet, M.; Vidal, X.; Cortés, J.; Llurba, E.; Rubio, I.T.; Xercavins, J. Pregnancy after treatment of breast cancer in young women does not adversely affect the prognosis. Breast 2012, 21, 272–275.
- Azim Jr, H.A.; Santoro, L.; Pavlidis, N.; Gelber, S.; Kroman, N.; Azim, H.; Peccatori, F.A. Safety of pregnancy following breast cancer diagnosis: A meta-analysis of 14 studies. Eur. J. Cancer 2011, 47, 74–83.
- Azim Jr, H.A.; Kroman, N.; Paesmans, M.; Gelber, S.; Rotmensz, N.; Ameye, L.; De Mattos-Arruda, L.; Pistilli, B.; Pinto, A.; Jensen, M.B. Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: A multicenter retrospective study. J. Clin. Oncol. 2013, 31, 73–79.
- Friedler, S.; Koc, O.; Gidoni, Y.; Raziel, A.; Ron-El, R. Ovarian response to stimulation for fertility preservation in women with malignant disease: A systematic review and meta-analysis. Fertil. Steril. 2012, 97, 125–133.
- Garcia-Velasco, J.A.; Domingo, J.; Cobo, A.; Martinez, M.; Carmona, L.; Pellicer, A. Five years’ experience using oocyte vitrification to pre- serve fertility for medical and nonmedical indications. Fertil. Steril. 2013, 99, 1994–1999.
- Pal, L.; Leykin, L.; Schifren, J.L.; Isaacson, K.B.; Chang, Y.C.; Nikruil, N.; Chen, Z.; Toth, T.L. Malignancy may adversely influence the quality and behaviour of oocytes. Hum. Reprod. 1998, 13, 1837–1840.
- Domingo, J.; Guillen, V.; Ayllon, Y.; Martınez, M.; Munoz, E.; Pellicer, A.; Garcia-Velasco, J.A. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil. Steril. 2012, 97, 930–934.
- Alvarez, R.M.; Ramanathan, P. Fertility preservation in female oncology patients: The influence of the type of cancer on ovarian stimulation response. Hum. Reprod. 2018, 33, 2051–2059.
- Volodarsky-Perel, A.; Cohen, Y.; Arab, S.; Son, W.Y.; Suarthana, E.; Dahan, M.H.; Tulandi, T.; Buckett, W. Effects of cancer stage and grade on fertility preservation outcome and ovarian stimulation response. Hum. Reprod. 2019, 34, 530–538.
- Decanter, C.; Robin, G.; Mailliez, A.; Sigala, J.; Morschhauser, F.; Ramdane, N.; Devos, P.; Dewailly, D.; Leroy-Martin, B.; Keller, L. Prospective assessment of follicular growth and the oocyte cohort after ovarian stimulation for fertility preservation in 90 cancer patients versus 180 matched controls. Reprod. Biomed. Online 2018, 36, 543–551.
- Moria, A.; Das, M.; Shehata, F.; Holzer, H.; Son, W.Y.; Tulandi, T. Ovarian reserve and oocyte maturity in women with malignancy undergoing in vitro maturation treatment. Fertil. Steril. 2011, 95, 1621–1623.
- Johnson, L.N.; Dillon, K.E.; Sammel, M.D.; Efymow, B.L.; Mainigi, M.A.; Dokras, A.; Gracia, C.R. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod. Biomed. Online 2013, 26, 337–344.
- Levin, I.; Almog, B. Effect of cancer on ovarian function in patients undergoing in vitro fertilization for fertility preservation: A reappraisal. Curr. Oncol. 2013, 20, e1–e33.
- Nurudeen, S.K.; Douglas, N.C.; Mahany, E.L.; Sauer, M.V.; Choi, J.M. Fertility preservation decisions among newly diagnosed oncology patients: A single-center experience. Am. J. Clin. Oncol. 2016, 39, 154–159.
- Cardozo, E.R.; Thomson, A.P.; Karmon, A.E.; Dickinson, K.A.; Wright, D.L.; Sabatini, M.E. Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: A 17-year experience. J. Assist. Reprod. Genet. 2015, 32, 587–596.
- Almog, B.; Azem, F.; Gordon, D.; Pauzner, D.; Amit, A.; Barkan, G.; Levin, I. Effects of cancer on ovarian response in controlled ovarian stimulation for fertility preservation. Fertil. Steril. 2012, 98, 957–960.
- Liu, D.; Yan, J.; Qiao, J. Effects of malignancies on fertility preservation outcomes and relevant cryobiological advances. Sci. China Life Sci. 2020, 63, 217–227.
- Roxburgh, C.S.; McMillan, D.C. Cancer and systemic inflammation: Treat the tumour and treat the host. Br. J. Cancer 2014, 110, 1409–1412.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Ingman, W.V.; Robertson, S.A. Defining the actions of transforming growth factor beta in reproduction. Bioessays 2002, 24, 904–914.
- Derks-Smeets, I.A.P.; Van Tilborg, T.C.; Van Montfoort, A.; Smits, L.; Torrance, H.L.; Meijer-Hoogeveen, M.; Broekmans, F.; Dreesen, J.C.F.M.; Paulussen, A.D.C.; Tjan-Heijnen, V.C.G. BRCA1 mutation carriers have a lower number of mature oocytes after ovarian stimulation for IVF/PGD. J. Assist. Reprod. Genet. 2017, 34, 1475–1482.
- Lekovich, J.; Lobel, A.L.S.; Stewart, J.D.; Pereira, N.; Kligman, I.; Rosenwaks, Z. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J. Assist. Reprod. Genet. 2016, 33, 657–662.
- Porcu, E.; Cillo, G.M.; Cipriani, L.; Sacilotto, F.; Notarangelo, L.; Damiano, G.; Dirodi, M.; Roncarati, I. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J. Assist. Reprod. Genet. 2020, 37, 709–715.
- Balachandren, N.; Davies, M. Fertility, ovarian reserve and cancer. Maturitas 2017, 105, 64–68.
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693.
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931.
- Jayasinghe, J.L.; Wallace, W.H.B.; Anderson, R.A. Expert Review of Endocrinology & Metabolism Ovarian function, fertility and reproductive lifespan in cancer patients. Expert Rev. Endocrinol. Metab. 2018, 13, 125–136.
- Tauchmanova, L.; Selleri, C.; De Rosa, G.; Esposito, M.; Orio, J.F.; Palomba, S.; Bifulco, G.; Nappi, C.; Lombardi, G.; Rotoli, B.; et al. Gonadal status in reproductive age women after haematopoietic stem cell transplantation for haematological malignancies. Hum. Reprod. 2003, 18, 1410–1416.
- Vatanen, A.; Wilhelmsson, M.; Borgström, B.; Gustafsson, B.; Taskinen, M.; Saarinen-Pihkala, U.M.; Winiarski, J.; Jahnukainen, K. Ovarian function after allogeneic hematopoietic stem cell transplantation in childhood and adolescence. Eur. J. Endocrinol. 2014, 170, 211–218.
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566.
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344.
- Cho, H.W.; Lee, S.; Min, K.J.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W.; Kim, T. Advances in the Treatment and Prevention of Chemotherapy-Induced Ovarian Toxicity. Int. J. Mol. Sci. 2020, 21, 7792.
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra162.
- Gavish, Z.; Peer, G.; Roness, H.; Cohen, Y.; Meirow, D. Follicle activation and ‘burn-out’ contribute to post-transplantation follicle loss in ovarian tissue grafts: The effect of graft thickness. Hum. Reprod. 2014, 29, 989–996.
- Wallace, W.H.; Shalet, S.M.; Hendry, J.H.; Morris-Jones, P.H.; Gattamaneni, H.R. Ovarian failure following abdominal irradiation in childhood: The radiosensitivity of the human oocyte. Br. Inst. Radiol. 2014, 62, 995–998.
- Kim, S.; Kim, S.W.; Han, S.J.; Lee, S.; Park, H.T.; Song, J.Y.; Kim, T. Molecular Mechanism and Prevention Strategy of Chemotherapy-and Radiotherapy-Induced Ovarian Damage. Int. J. Mol. Sci. 2021, 22, 7484.
- Beerendonk, C.C.M.; Braat, D.D.M. Present and future options for the preservation of fertility in female adolescents with cancer. Endocr. Dev. 2005, 8, 166–175.
- Stroud, J.S.; Mutch, D.; Rader, J.; Powell, M.; Thaker, P.H.; Grigsby, P.W. Effects of cancer treatment on ovarian function. Fertil. Steril. 2009, 92, 417–427.
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 27.
- Griffiths, M.J.; Winship, A.L.; Hutt, K.J. Do cancer therapies damage the uterus and compromise fertility? Hum. Reprod. Update 2020, 26, 161–173.
- Fujimoto, A.; Ichinose, M.; Harada, M.; Hirata, T.; Osuga, Y.; Fujii, T. The outcome of infertility treatment in patients undergoing assisted reproductive technology after conservative therapy. J. Assist. Reprod. Genet. 2014, 31, 1189–1194.
- Beneventi, F.; Locatelli, E.; Giorgiani, G.; Zecca, M.; Mina, T.; Simonetta, M.; Cavagnoli, C.; Albanese, M.; Spinillo, A. Adolescent and adult uterine volume and uterine artery Doppler blood flow among subjects treated with bone marrow transplantation or chemotherapy in pediatric age: A case-control study. Fertil. Steril. 2015, 103, 455–461.
- Van de Loo, L.; Van den Berg, M.H.; Overbeek, A.; van Dijk, M.; Damen, L.; Lambalk, C.B.; Ronckers, C.M.; van den Heuvel-Eibrink, M.M.; Kremer, L.; van der Pal, H.J.; et al. Uterine function, pregnancy complications, and pregnancy outcomes among female childhood cancer survivors. Fertil. Steril. 2019, 111, 372–380.
- Massarotti, C.; Scaruffi, P.; Lambertini, M.; Sozzi, F.; Remorgida, V.; Anserini, P. Beyond fertility preservation: Role of the oncofertility unit in the reproductive and gynecological follow-up of young cancer patients. Hum. Reprod. 2019, 34, 1462–1469.
- Lambertini, M.; Del Mastro, L.; Pescio, M.C.; Andersen, C.Y.; Azim, H.A.; Peccatori, F.A.; Costa, M.; Revelli, A.; Salvagno, F.; Gennari, A.; et al. Cancer and fertility preservation: International recommendations from an expert meeting. BMC Med. 2016, 14, 1.
- Linkeviciute, A.; Boniolo, G.; Chiavari, L.; Peccatori, F.A. Fertility preservation in cancer patients: The global framework. Cancer Treat. Rev. 2014, 40, 1019–1027.
- Van den Berg, M.; Baysal, Ö.; Nelen, W.L.D.M.; Braat, D.D.M.; Beerendonk, C.C.M.; Hermens, R.P.M.G. Professionals’ barriers in female oncofertility care and strategies for improvement. Hum. Reprod. 2019, 34, 1074–1082.
- Goodman, L.R.; Balthazar, U.; Kim, J.; Mersereau, J.E. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Hum. Reprod. 2012, 27, 2076–2081.
- Vindrola-Padros, C.; Dyer, K.E.; Cyrus, J.; Lubker, I.M. Healthcare professionals’ views on discussing fertility preservation with young cancer patients: A mixed method systematic review of the literature. Psychooncology 2017, 26, 4–14.
- Lewin, J.; Ma, J.M.Z.; Mitchell, L.; Tam, S.; Puri, N.; Stephens, D.; Srikanthan, A.; Bedard, P.; Razak, A.; Crump, M.; et al. The positive effect of a dedicated adolescent and young adult fertility program on the rates of documentation of therapy-associated infertility risk and fertility preservation options. Support. Care Cancer 2017, 25, 1915–1922.
- Dolmans, M.M.; Lambertini, M.; Macklon, K.T.; Almeida Santos, T.; Ruiz-Casado, A.; Borini, A.; Bordes, V.; Frith, L.; Van Moer, E.; Germeyer, A. EUropean REcommendations for female FERtility preservation (EU-REFER): A joint collaboration between oncologists and fertility specialists. Crit. Rev. Oncol. Hematol. 2019, 138, 233–240.
- Lambertini, M.; Cinquini, M.; Moschetti, I.; Peccatori, F.A.; Anserini, P.; Menada, M.V.; Tomirotti, M.; Del Mastro, L. Temporary ovarian suppression during chemotherapy to preserve ovarian function and fertility in breast cancer patients: A GRADE approach for evidence evaluation and recommendations by the Italian Association of Medical Oncology. Eur. J. Cancer 2017, 71, 25–33.
- Lee, J.H.; Choi, Y.S. The role of gonadotropin-releasing hormone agonists in female fertility preservation. Clin. Exp. Reprod. Med. 2021, 48, 11–16.
- Roberts, J.E.; Oktay, K. Fertility preservation: A comprehensive approach to the young woman with cancer. JNCI Monogr. 2005, 2005, 57–59.
- Ajala, T.; Rafi, J.; Larsen-Disney, P.; Howell, R. Fertility preservation for cancer patients: A review. Obstet. Gynecol. Int. 2010, 2010, 160386.
- Lambertini, M.; Boni, L.; Michelotti, A.; Magnolfi, E.; Cogoni, A.A.; Mosconi, A.M.; Giordano, M.; Garrone, O.; Arpino, G.; Poggio, F.; et al. Long-term outcomes with pharmacological ovarian suppression during chemotherapy in premenopausal early breast cancer patients. J. Natl. Cancer. Inst. 2021, 114, 400–408.
- Lambertini, M.; Moore, H.C.; Leonard, R.C.; Loibl, S.; Munster, P.; Bruzzone, M.; Boni, L.; Unger, J.M.; Anderson, R.A.; Mehta, K.; et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: A systematic review and meta-analysis of individual patient–level data. J. Clin. Oncol. 2018, 36, 1981–1990.
- Urruticoechea, A.; Arnedos, M.; Walsh, G.; Dowsett, M.; Smith, I.E. Ovarian protection with goserelin during adjuvant chemotherapy for pre-menopausal women with early breast cancer (EBC). Breast Cancer Res. Treat. 2008, 110, 411–416.
- Moore, H.C.; Unger, J.M.; Albain, K.S. Ovatian protection during adjuvant chemotherapy. N. Eng. J. Med. 2015, 372, 2268–2269.
- Leonard, R.C.F.; Adamson, D.J.A.; Bertelli, G.; Mansi, J.; Yellowlees, A.; Dunlop, J.; Thomas, A.; Coleman, R.E.; Anderson, R.A. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: The Anglo Celtic Group OPTION trial. Ann. Oncol. 2017, 28, 1811–1816.
- Huang, K.G.; Lee, C.L.; Tsai, C.S.; Han, C.M.; Hwang, L.L. A new approach for laparoscopic ovarian transposition before pelvic irradiation. Gynecol. Oncol. 2007, 105, 234–237.
- Irtan, S.; Orbach, D.; Helfre, S.; Sarnacki, S. Ovarian transposition in prepubescent and adolescent girls with cancer. Lancet Oncol. 2013, 14, e601–e608.
- Laios, A.; Portela, S.D.; Papadopoulou, A.; Gallos, I.D.; Otify, M.; Ind, T. Ovarian transposition and cervical cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 75, 37–53.
- Corney, R.H.; Swinglehurst, A.J. Young childless women with breast cancer in the UK: A qualitative study of their fertility-related experiences, options, and the information given by health professionals. Psychooncology 2014, 23, 20–26.
- Jones, G.; Hughes, J.; Mahmoodi, N.; Smith, E.; Skull, J.; Ledger, W. What factors hinder the decision-making process for women with cancer and contemplating fertility preservation treatment? Hum. Reprod. Update 2017, 23, 433–457.
- Dolmans, M.M.; Donnez, J. Fertility preservation in women for medical and social reasons: Oocytes vs ovarian tissue. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 63–80.
- Donnez, J.; Dolmans, M.M. Fertility preservation in women. N. Engl. J. Med. 2017, 377, 1657–1665.
- Vu, J.V.; Llarena, N.C.; Estevez, S.L.; Moravek, M.B.; Jeruss, J.S. Oncofertility program implementation increases access to fertility preservation options and assisted reproductive procedures for breast cancer patients. J. Surg. Oncol. 2017, 115, 116–121.
- Silvestris, E.; De Palma, G.; Canosa, S.; Palini, S.; Dellino, M.; Revelli, A.; Paradiso, A.V. Human ovarian cortex biobanking: A fascinating resource for fertility preservation in cancer. Int. J. Mol. Sci. 2020, 21, 3245.
- Kasum, M.; Beketić-Orešković, L.; Peddi, P.F.; Orešković, S.; Johnson, R.H. Fertility after breast cancer treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 173, 13–18.
- Creux, H.; Monnier, P.; Son, W.Y.; Buckett, W. Thirteen years’ experience in fertility preservation for cancer patients after in vitro fertilization and in vitro maturation treatments. J. Assist. Reprod. Genet. 2018, 35, 583–592.
- Segers, I.; Bardhi, E.; Mateizel, I.; Van Moer, E.; Schots, R.; Verheyen, G.; Tournaye, H.; De Vos, M. Live births following fertility preservation using in-vitro maturation of ovarian tissue oocytes. Hum. Reprod. 2020, 35, 2026–2036.
- Wallace, W.H.B.; Smith, A.G.; Kelsey, T.W.; Edgar, A.E.; Anderson, R.A. Fertility preservation for girls and young women with cancer: Population-based validation of criteria for ovarian tissue cryopreservation. Lancet Oncol. 2014, 15, 1129–1136.
- Levine, J.; Stern, C.J. Fertility preservation in adolescents and young adults with cancer. J. Clin. Oncol. 2010, 28, 4831–4841.
- Van der Perk, M.M.; Van der Kooi, A.L.L.; Van de Wetering, M.D.; IJgosse, I.M.; Van Dulmen-den Broeder, E.; Broer, S.L.; Klijn, A.J.; Versluys, A.B.; Arends, B.; Oude Ophuis, R.J.A. Oncofertility care for newly diagnosed girls with cancer in a national pediatric oncology setting, the first full year experience from the Princess Máxima Center, the PEARL study. PLoS ONE 2021, 16, e0246344.
- Donnez, J.; Dolmans, M.M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013, 9, 735–749.
- Oktay, K. Ovarian tissue cryopreservation and transplantation: Preliminary findings and implications for cancer patients. Hum. Reprod. Update 2001, 7, 526–534.
- Seshadri, T.; Gook, D.; Lade, S.; Spencer, A.; Grigg, A.; Tiedemann, K.; McKedrick, J.; Mitchell, J.; Stern, C.; Seymour, J.F. Lack of evidence of disease contamination in ovarian tissue harvested for cryopreservation from patients with Hodgkin lymphoma and analysis of factors predictive of oocyte yield. Br. J. Cancer 2006, 94, 1007–1010.
- Dolmans, M.M.; Donnez, J.; Cacciottola, L. Fertility preservation: The challenge of freezing and transplanting ovarian tissue. Trends Mol. Med. 2021, 27, 777–791.
- Donnez, J.; Dolmans, M.M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J. Assist. Reprod. Genet. 2015, 32, 1167–1170.
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410.
- Janse, F.; Donnez, J.; Anckaert, E.; De Jong, F.H.; Fauser, B.C.; Dolmans, M.M. Limited value of ovarian function markers following orthotopic transplantation of ovarian tissue after gonadotoxic treatment. J. Clin. Endocrinol. Metab. 2011, 96, 1136–1144.
- Dolmans, M.M.; De Ouderaen, S.H.; Demylle, D.; Pirard, C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J. Assist. Reprod. Genet. 2015, 32, 1233–1237.
- Oktay, K.; Turan, V.; Bedoschi, G.; Pacheco, F.S.; Moy, F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J. Clin. Oncol. 2015, 33, 2424.
- Cobo, A.; García-Velasco, J.A.; Coello, A.; Domingo, J.; Pellicer, A.; Remohí, J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil. Steril. 2016, 105, 755–764.
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: A prospective cohort study. Fertil. Steril. 2018, 109, 478–485.
- Specchia, C.; Baggiani, A.; Immediata, V.; Ronchetti, C.; Cesana, A.; Smeraldi, A.; Scaravelli, G.; Levi-Setti, P.E. Oocyte cryopreservation in oncological patients: Eighteen years experience of a tertiary care referral center. Front. Endocrinol. 2019, 10, 600.
- Donnez, J.; Dolmans, M.M.; Diaz, C.; Pellicer, A. Ovarian cortex transplantation: Time to move on from experimental studies to open clinical application. Fertil. Steril. 2015, 104, 1097–1098.
- Van der Ven, H.; Liebenthron, J.; Beckmann, M.; Toth, B.; Korell, M.; Krüssel, J.; Frambach, T.; Kupka, M.; Hohl, M.K.; Winkler-Crepaz, K.; et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: Tissue activity, pregnancy and delivery rates. Hum. Reprod. 2016, 31, 2031–2041.
- Meirow, D.; Ra’anani, H.; Shapira, M.; Brenghausen, M.; Chaim, S.D.; Aviel-Ronen, S. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil. Steril. 2016, 106, 467–474.
- Jensen, A.K.; Macklon, K.T.; Fedder, J.; Ernst, E.; Humaidan, P.; Andersen, C.Y. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: Focus on birth and perinatal outcome in 40 of these children. J. Assist. Reprod. Genet. 2017, 34, 325–336.
- Shapira, M.; Dolmans, M.M.; Silber, S.; Meirow, D. Evaluation of ovarian tissue transplantation: Results from three clinical centers. Fertil. Steril. 2020, 114, 388–397.
- Terenziani, M.; Piva, L.; Meazza, C.; Gandola, L.; Cefalo, G.; Merola, M. Oophoropexy: A relevant role in preservation of ovarian function after pelvic irradiation. Fertil. Steril. 2009, 91, 935.e15–935.e16.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
4 times
(View History)
Update Date:
10 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




