Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md. Habibur Rahman | -- | 1895 | 2022-06-06 13:42:23 | | | |
| 2 | Dean Liu | Meta information modification | 1895 | 2022-06-07 03:10:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rahman, M.H.; Cavalu, S.; Zehravi, M.; , .; Akter, R.; Malik, S.; Ashraf, G.M.; Tagde, P.; Ramproshad, S.; Mohan, A.G. Neurological Diseases and Their Biomarkers. Encyclopedia. Available online: https://encyclopedia.pub/entry/23747 (accessed on 07 February 2026).
Rahman MH, Cavalu S, Zehravi M, , Akter R, Malik S, et al. Neurological Diseases and Their Biomarkers. Encyclopedia. Available at: https://encyclopedia.pub/entry/23747. Accessed February 07, 2026.
Rahman, Md. Habibur, Simona Cavalu, Mehrukh Zehravi, , Rokeya Akter, Sumira Malik, Ghulam Md Ashraf, Priti Tagde, Sarker Ramproshad, Aurel George Mohan. "Neurological Diseases and Their Biomarkers" Encyclopedia, https://encyclopedia.pub/entry/23747 (accessed February 07, 2026).
Rahman, M.H., Cavalu, S., Zehravi, M., , ., Akter, R., Malik, S., Ashraf, G.M., Tagde, P., Ramproshad, S., & Mohan, A.G. (2022, June 06). Neurological Diseases and Their Biomarkers. In Encyclopedia. https://encyclopedia.pub/entry/23747
Rahman, Md. Habibur, et al. "Neurological Diseases and Their Biomarkers." Encyclopedia. Web. 06 June, 2022.
Copy Citation
Neurodegenerative diseases (NDDs) are disorders that affect both the central and peripheral nervous systems. To name a few causes, NDDs can be caused by ischemia, oxidative and endoplasmic reticulum (ER) cell stress, inflammation, abnormal protein deposition in neural tissue, autoimmune-mediated neuron loss, and viral or prion infections.
neurological disorder
neuroinflammation
proteinopathies
1. Neurodegeneration, Inflammation, and Tumorigenesis in the Central Nervous System
Although the pathogenic function and basic molecular processes underpinning neurodegeneration are complicated, involving genetic, environmental, and endogenous variables linked with aging, their pathogenic function and basic molecular mechanisms are unknown [1][2]. Now, NDDs are categorized based on their known genetic pathways and the primary chemicals found in their protein deposits. These disorders are called ‘protein misfolding’ diseases or proteinopathies because significant structural abnormalities cause them in proteins [3][4]. Numerous fundamental mechanisms underlying neurodegeneration may be initiated at various stages of the neurodegenerative cascade by inflammatory cells and mediators.
Apoptosis: Apoptosis is a form of planned cell death controlled by caspases [5][6][7][8]. It is characterized by the production of membrane-encased apoptotic bodies that are rapidly phagocytozed by macrophages or neighboring cells. Although evidence of apoptotic pathways has been found in animal models of a variety of neurodegenerative illnesses, there is less evidence in human tissues. In Huntington’s disease (HD) models, activation of caspase-1, -3, -8, and -9 as well as cytochrome c release were found in human striatal brain tissue. Similarly, in amyotrophic lateral sclerosis (ALS) and HIV-associated neurodegeneration, caspase activation and neuronal death have been observed [9].
Necroptosis: Necroptosis is a type of programmed cell death defined by the loss of plasma membrane integrity and occurs in the absence of caspase activation. The receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and mixed-lineage kinase domain-like are the two key effector proteins in necroptosis (MLKL). TNF-α, FasL-, and TRAIL are released by astrocytes and can cause necroptosis by activating RIPK1 and MLKL, as shown in ALS mice models [10]. Axonal disease caused by RIPK1 was detected in pathological specimens from ALS patients [11]. In MS pathology samples, necroptotic pathways were also detected [12].
Autophagy, also known as type II programmed cell death, is defined by the buildup of autophagic vacuoles during cell death, along with potentially harmful components such as proteins or damaged organelles. Excessive autophagy can result in cell death and self-destruction. Autophagosomes were found in AD, HD, and PD patients’ damaged neurons. Numerous other triggers, such as food deprivation, mitochondrial toxins, hypoxia, and oxidative stress, can cause autophagy [13][14].
Axonal damage or transection can result in retrograde degeneration of the proximal neuronal cell body, which is associated with a range of degenerative alterations within the cell body, including apoptosis and neuronal perikaryon chromatolysis. Because of the relationship between neuronal apoptosis and axonal damage, inflammation-induced axotomy may result in retrograde (secondary) apoptosis of neuronal cell bodies [15].
Astrogliopathy: Astrogliopathy is a broad word that refers to astrocyte dysfunction. The abnormal buildup of inappropriately phosphorylated tau protein in astrocytes seen in AD, frontal temporal lobe dementia (FTLD), and corticobasal degeneration is referred to as aging-related tau astrogliopathy (ARTAG) [16][17]. Optic neuritis and myelitis characterize neuromyelitis optica (NMO), which can mimic MS. Antibodies against aquaporin-4 (AQP4), which binds to astrocyte water channels, are linked to NMO. NMO is characterized pathologically by a significant loss of immunoreactivity for the astrocytic proteins AQP4 and glial fibrillary acidic protein (GFAP), perivascular deposition of immunoglobulins, and complement activation, even in lesions containing some myelin [18].
Inflammation begins when the body’s immune cells start inflammatory cascades to avoid tissue damage caused by injury or invading pathogens. If the inflammatory response is successful, it eliminates pathogens, initiates wound healing and angiogenesis, and eventually decreases. When the neuroinflammatory response is acute, it is required and even helpful for the neuronal environment, as it aids in pathogen elimination and brain restoration. When serious threats to the neural environment, such as protein aggregates (Lewy bodies, neurofibrillary tangles), build in the brain and sustain inflammation for an extended length of time, continuous gliosis and apoptosis can occur as a result of uncontrolled inflammatory cytokine production. Chronic inflammation is associated to nearly all neurological diseases, including AD, PD, and ALS, as a result of persistent activation [19][20]. In contrast to this protective homeostatic mechanism, inflammation has been implicated in a wide variety of diseases. In recent years, its impact on neurological disorders has been hypothesized as a crucial role in disease progression. Microglia in the CNS form phagocytic morphologies and secrete pro-inflammatory cytokines to interact with astrocytes and neurons. This can result in neurodegeneration, synaptic phagocytosis, reduced neuronal function, microglial activation, inflammatory cytokine release, and even more microglial activation until the neural environment is no longer threatened. Astrocytes are also activated during the inflammatory process, a process known as astrogliosis. Aging is a significant risk factor for neurodegeneration [21][22][23]. In general, older adults have dysregulated cytokine expression (i.e., increased synthesis of pro-inflammatory cytokines and decreased availability of anti-inflammatory cytokines), resulting in a chronic low-grade inflammatory state. Inflammaging is a term that refers to this type of auto-inflammatory disorder that occurs throughout aging [24].
Aside from blood and lymphatic vessels, data suggests that neurogenesis (the growth of new neurons) and axonogenesis (tumor-induced neural sprouting toward the tumor microenvironment) are important in carcinogenesis and cancer progression. Neurogenesis and axonogenesis have been seen in pre-neoplastic lesions, implying that they play a role in the onset of cancer as an early occurrence in the pre-malignant phase [25].
2. New Potential Biomarkers
In contrast to medical symptoms, which are simply indications of a patient’s health as expressed and perceived by the patient, biomarkers, or “biological markers“. In 1998, the Biomarkers Definitions Working Group of the National Institutes of Health defined biomarkers as “evidence of any biological, pathogenic, or pharmacogenomic response to any therapy modification” [26]. Biological markers are any substances, structures, or processes that may be measured inside or outside the body and can influence any changes in the body or the chance of disease prevalence of neurological diseases [27].
2.1. Alzheimer’s Disease
AD is a slowly progressing neurological disease for which there is now no effective cure. Deposition of 42-amino-acid-long amyloid (Aβ) protein in extracellular plaques in the brain is the earliest identifiable disease, which occurs decades before clinical symptoms appear [28]. According to biomarker studies, a buildup is connected to synaptic dysfunction and increased tau phosphorylation and secretion, a microtubule-binding axonal protein abundantly produced in cortical neurons [29]. This dysregulated tau metabolism puts neurons at an elevated risk of degeneration, as intraneuronal neurofibrillary tangles formed of hyperphosphorylated and shortened tau proteins form. Neurodegeneration finally manifests as the AD clinical syndrome, characterized by progressive cognitive deficits [30][31][32]. The pathology of AD is shown in Figure 1.
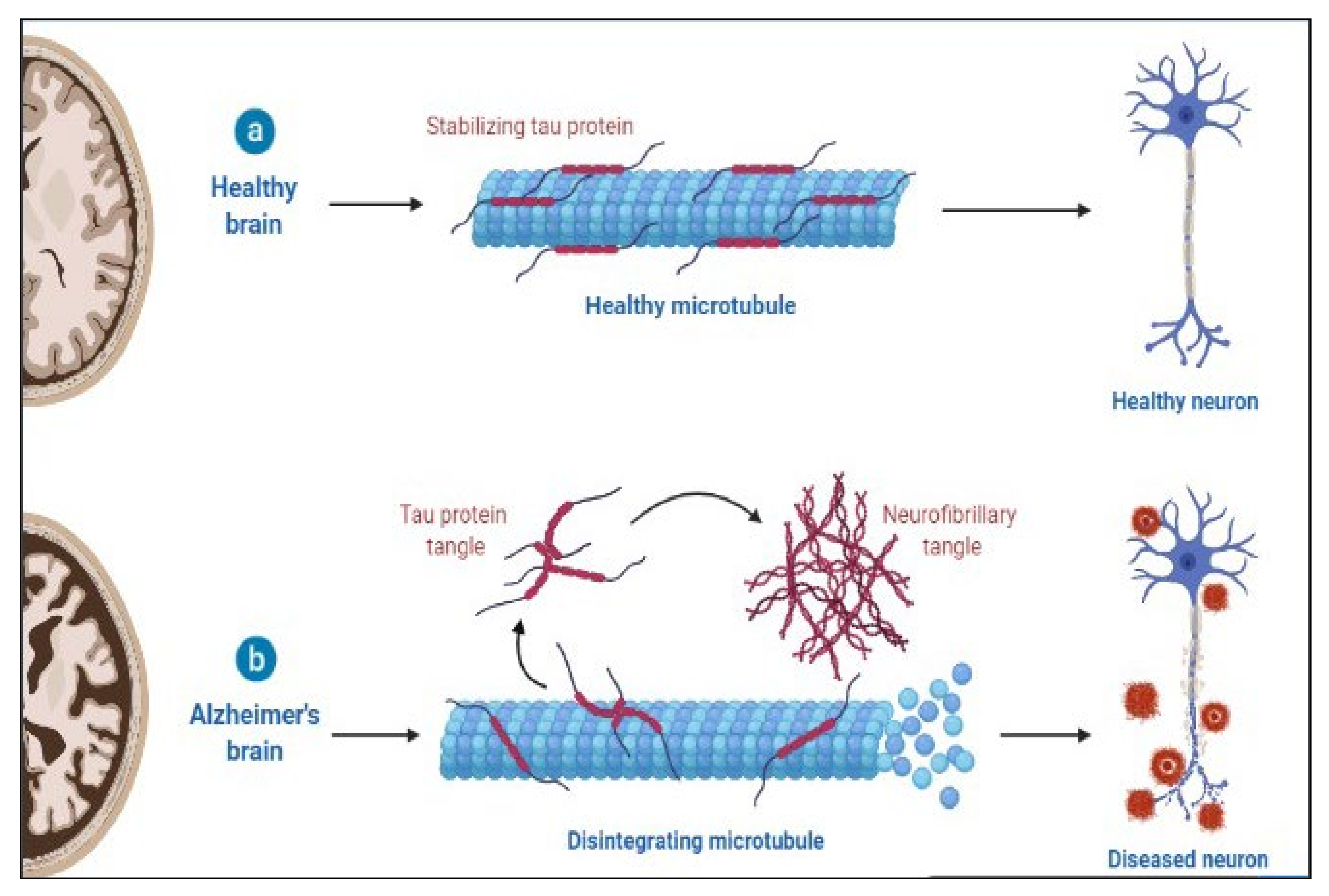
Figure 1. Pathology of AD.
2.2. Aβ Pathology Biomarkers
Extracellular deposition of Aβ, formed by BACE1 and γ-secretase cleavage of amyloid precursor protein (APP), forming plaques is a significant pathological characteristic of AD. It has been hypothesized to represent the primary pathogenic event in the illness [33]. Aβ42 is an APP breakdown product generally transported from the brain interstitial fluid to the CSF and blood via the glymphatic system [34]. Amyloid positron emission tomography (PET) has been validated in comparison with neuropathology, has undergone substantial standardization in terms of quantifying pathology and defining abnormality cut-points, and has adequate usage criteria [35][36][37].
2.3. Tau Pathology Biomarkers
A fundamental pathogenic hallmark of AD is the aggregation of hyperphosphorylated tau in the neuronal soma, generating neurofibrillary tangles. However, tau inclusions in neurons or glial cells are also observed in other neurodegenerative dementias [38]. Together with the CSF Aβ42/Aβ40 ratio, the cornerstone markers totaled tau (T-tau) and phosphorylated tau (P-tau) have been proposed as biomarkers for biologically defining AD and are considered diagnostic in the research criteria for AD [39][40]. Both T-tau and P-tau concentrations in the cerebrospinal fluid (CSF) reflect AD pathogenesis in all neurodegenerative dementias [41]. The most likely explanation is that the higher tau levels in the CSF result from enhanced tau phosphorylation and release by neurons in response to Aβ exposure [42][43].
2.4. Multiple Sclerosis
MS is a chronic autoimmune disease that causes demyelination of the central nervous system and neurodegeneration [44]. Through interplay with immune cells, energy metabolic problems and endocrine abnormalities have been demonstrated to begin MS [45]. Furthermore, viral infection and environmental pollutants have been demonstrated to impair immunological tolerance and trigger the release of proinflammatory factors such as IL-6 and NF-kB in hereditarily vulnerable people [46]. The disease is heterogeneous in radiological and histological alterations, clinical presentation and development, and response to therapy [47][48]. P-gp expression is also increased, which promotes CD4+ and CD8+ T cell migration and amplifies neuroinflammation [49]. T-cells and B-cells mediate inflammatory responses by secreting cytokines that activate inflammatory cells such as microglia behind the BBB [50]. The pathology of MS is shown in Figure 2.
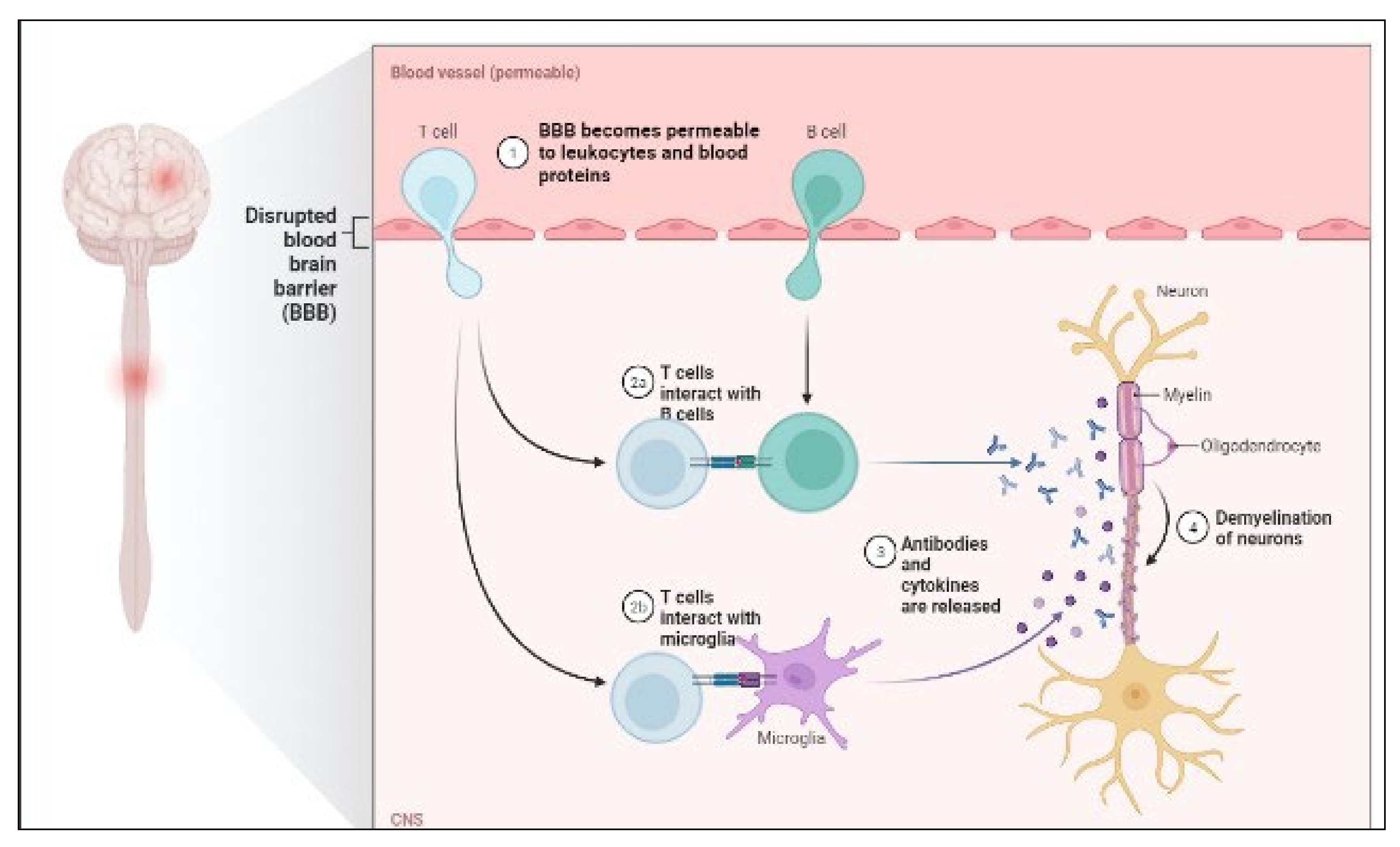
Figure 2. Pathology of MS.
When a patient’s blood serum and CSF fluid are analyzed at the same time, oligoclonal bands are discovered. It has long been known that oligoclonal bands (OCB) can be found in the CSF of MS patients (by isoelectric focusing). Plasma cells in the CNS use immunoglobulin G (IgG) and M to create them (IgM) [51].
After a period in which OCB were not employed for diagnosis according to the McDonald criteria, they have been reintroduced into the diagnostic algorithm in the 2017 update. This shift toward substituting a positive CSF result for dissemination in time rather than in space is pragmatic. However, it underlines clinical neurologists’ responsibility to obtain cutting-edge CSF tests. MS is the most likely diagnosis for patients with typical clinical presentations, typical lesions, and alternative diagnoses that have been ruled out. By demonstrating the presence of OCB, researchers may provide proof for the disease’s immunological and inflammatory nature without waiting for the spread to occur. Thus, OCB is a well-established biomarker with clinical relevance for MS diagnosis [52][53][54][55].
2.4.1. IgG Index
The immunoglobulin (Ig) G index is defined as the ratio of IgG’s CSF/serum quotient to albumin’s CSF/serum quotient. The albumin quotient, defined as albumin in CSF divided by albumin in serum, is used to assess blood-CSF barrier failure in MS [56]. The IgG index is used to quantify intrathecal immunoglobulin synthesis. An IgG index result greater than 0.7 implies an elevated intrathecal B cell response and, consequently, MS’s existence. Around 70% of people with MS have a high IgG index. As a result, this biomarker’s sensitivity is lower than that of the OCB [57][58].
2.4.2. Antinuclear antibodies
Antinuclear antibodies (ANA) are tissue-independent autoantibodies directed against components of the cell nucleus that are quantified in the serum [59]. A continuously high titer indicates collagenous SLE [60].
2.4.3. Anti-MOG antibodies
MOG is a myelin protein that is mostly located on the surface of myelin sheaths and oligodendrocyte membranes, and it could be a target for the autoimmune response in demyelinating disorders [61][62]. Therefore, anti-MOG antibodies, contrary to common perception, are only beneficial for differential diagnosis, not for MS diagnosis or prognosis. Using cutting-edge detection technologies, anti-MOG antibodies were found in a subset of pediatric patients with acute disseminated encephalomyelitis (ADEM), patients with clinical symptoms of NMOSD, and patients with bilateral optic neuritis in particular (cell-based approaches) [63].
2.4.4. Anti-aquaporin-4 antibodies
Aquaporin-4 (AQP-4) is a water channel protein that is expressed by astrocytes in the CNS and is necessary for brain water homeostasis [64][65]. Antibodies to this protein are found in around 75% of patients with neuromyelitis optica spectrum disorder (NMOSD), but not in MS patients. Anti-aquaporin-4 antibodies are thus appropriate for high-specificity biomarkers. It is the first molecular biomarker to be clinically proven for differentiating between distinct CNS inflammatory demyelinating disorders. Antibodies against aquaporin-4 are frequently seen in the serum of patients suspected of having NMOSD [66].
References
- Jellinger, K.A. Recent advances in our understanding of neurodegeneration. J. Neural Transm. 2009, 116, 1111–1162.
- Skovronsky, D.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Neurodegenerative Diseases: New Concepts of Pathogenesis and Their Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 151–170.
- Golde, T.E.; Miller, V.M. Proteinopathy-induced neuronal senescence: A hypothesis for brain failure in Alzheimer’s and other neurodegenerative diseases. Alzheimer’s Res. Ther. 2009, 1, 5.
- Vladimir, N.U. Intrinsic disorder in proteins associated with neurodegenerative diseases. Front. Biosci. 2009, 14, 5188–5238.
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, H.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; et al. Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed. Pharmacother. 2021, 141, 111928.
- Karthika, C.; Hari, B.; Rahman, M.H.; Akter, R.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Akhtar, M.F.; Abdel-Daim, M.M. Multiple strategies with the synergistic approach for addressing colorectal cancer. Biomed. Pharmacother. 2021, 140, 111704.
- Adeola, H.A.; Bano, A.; Vats, R.; Vashishtha, A.; Verma, D.; Kaushik, D.; Mittal, V.; Rahman, H.; Najda, A.; Albadrani, G.M.; et al. Bioactive compounds and their libraries: An insight into prospective phytotherapeutics approach for oral mucocutaneous cancers. Biomed. Pharmacother. 2021, 141, 111809.
- Cavalu, S.; Simon, V. Proteins adsorption to orthopaedic biomaterials: Vibrational spectroscopy evidence. J. Optoelectron. Adv. Mater. 2007, 9, 3297–3302.
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49.
- Re, D.B.; Le Verche, V.; Yu, C.; Amoroso, M.W.; Politi, K.A.; Phani, S.; Ikiz, B.; Hoffmann, L.; Koolen, M.; Nagata, T.; et al. Necroptosis Drives Motor Neuron Death in Models of Both Sporadic and Familial ALS. Neuron 2014, 81, 1001–1008.
- Ito, Y.; Ofengeim, D.; Najafov, A.; Das, S.; Saberi, S.; Li, Y.; Hitomi, J.; Zhu, H.; Chen, H.; Mayo, L.; et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science 2016, 353, 603–608.
- Ofengeim, D.; Ito, Y.; Najafov, A.; Zhang, Y.; Shan, B.; DeWitt, J.P.; Ye, J.; Zhang, X.; Chang, A.; Vakifahmetoglu-Norberg, H.; et al. Activation of Necroptosis in Multiple Sclerosis. Cell Rep. 2015, 10, 1836–1849.
- Bauer, P.O.; Goswami, A.; Wong, H.K.; Okuno, M.; Kurosawa, M.; Yamada, M.; Miyazaki, H.; Matsumoto, G.; Kino, Y.; Nagai, Y.; et al. Harnessing chaperone-mediated autophagy for the selective degradation of mutant huntingtin protein. Nat. Biotechnol. 2010, 28, 256–263.
- Vives-Bauza, C.; de Vries, R.L.A.; Tocilescu, M.; Przedborski, S. PINK1/Parkin direct mitochondria to autophagy. Autophagy 2010, 6, 315–316.
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587.
- Kovacs, G.G.; Robinson, J.L.; Xie, S.X.; Lee, E.B.; Grossman, M.; Wolk, D.A.; Irwin, D.; Weintraub, D.; Kim, C.F.; Schuck, T.; et al. Evaluating the Patterns of Aging-Related Tau Astrogliopathy Unravels Novel Insights Into Brain Aging and Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2017, 76, 270–288.
- Ling, H.; Kovacs, G.G.; Vonsattel, J.P.G.; Davey, K.; Mok, K.Y.; Hardy, J.; Morris, H.R.; Warner, T.T.; Holton, J.L.; Revesz, T. Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain 2016, 139, 3237–3252.
- Lucchinetti, C.F.; Guo, Y.; Popescu, B.F.G.; Fujihara, K.; Itoyama, Y.; Misu, T. The Pathology of an Autoimmune Astrocytopathy: Lessons Learned from Neuromyelitis Optica. Brain Pathol. 2013, 24, 83–97.
- Jung, Y.J.; Tweedie, D.; Scerba, M.T.; Greig, N.H. Neuroinflammation as a Factor of Neurodegenerative Disease: Thalidomide Analogs as Treatments. Front. Cell Dev. Biol. 2019, 7, 313.
- Bajgai, J.; Lee, K.-J.; Rahman, H.; Fadriquela, A.; Kim, C.-S. Role of Molecular Hydrogen in Skin Diseases and its Impact in Beauty. Curr. Pharm. Des. 2021, 27, 737–746.
- Boiko, D.I.; Skrypnikov, A.M.; Shkodina, A.D.; Hasan, M.M.; Ashraf, G.M.; Rahman, H. Circadian rhythm disorder and anxiety as mental health complications in post-COVID-19. Environ. Sci. Pollut. Res. 2022, 29, 28062–28069.
- Miere, F.; Fritea, L.; Cavalu, S.; Vicaș, S.I. Formulation, Characterization, and Advantages of Using Liposomes in Multiple Therapies. Pharmacophore 2020, 11, 1–12.
- Bhattacharya, T.; Chopra, H.; Rahman, M.M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 2022, 15, 804.
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590.
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.-P.; Firlej, V.; Allory, Y.; Roméo, P.-H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678.
- United Nations Environment Programme; International Labour Organization; World Health Organization; International Program on Chemical Safety. Biomarkers in Risk Assessment: Validity and Validation; World Health Organization: Geneva, Switzerland, 2014; pp. 1–21.
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466.
- De Ture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32.
- Palmqvist, S.; Insel, P.S.; Stomrud, E.; Janelidze, S.; Zetterberg, H.; Brix, B.; Eichenlaub, U.; Dage, J.; Chai, X.; Blennow, K.; et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol. Med. 2019, 11, e11170.
- Tagde, P.; Tagde, S.; Bhattacharya, T.; Tagde, P.; Chopra, H.; Akter, R.; Kaushik, D.; Rahman, H. Blockchain and artificial intelligence technology in e-Health. Environ. Sci. Pollut. Res. 2021, 28, 52810–52831.
- Jack, C.R.; Holtzman, D.M. Biomarker Modeling of Alzheimer’s Disease. Neuron 2013, 80, 1347–1358.
- Arya, A.; Chahal, R.; Rao, R.; Rahman, H.; Kaushik, D.; Akhtar, M.; Saleem, A.; Khalifa, S.; El-Seedi, H.; Kamel, M.; et al. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy. Biomolecules 2021, 11, 350.
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608.
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024.
- Cohen, A.D.; Landau, S.M.; Snitz, B.E.; Klunk, W.E.; Blennow, K.; Zetterberg, H. Fluid and PET biomarkers for amyloid pathology in Alzheimer’s disease. Mol. Cell. Neurosci. 2019, 97, 3–17.
- Klunk, W.E.; Koeppe, R.A.; Price, J.C.; Benzinger, T.L.; Devous, M.D.; Jagust, W.J.; Johnson, K.A.; Mathis, C.A.; Minhas, D.; Pontecorvo, M.J.; et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimer’s Dement. 2014, 11, 1–15.e4.
- Johnson, K.A.; Minoshima, S.; Bohnen, N.I.; Donohoe, K.J.; Foster, N.L.; Herscovitch, P.; Karlawish, J.H.; Rowe, C.C.; Hedrick, S.; Pappas, V.; et al. Update on appropriate use criteria for amyloid PET imaging: Dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimer’s Dement. 2013, 9, e106–e109.
- Irwin, D.J. Tauopathies as clinicopathological entities. Park. Relat. Disord. 2016, 22, S29–S33.
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer Dement. 2018, 14, 535–562.
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629.
- Skillbäck, T.; Farahmand, B.Y.; Rosén, C.; Mattsson, N.; Nägga, K.; Kilander, L.; Religa, D.; Wimo, A.; Winblad, B.; Schott, J.M.; et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 2015, 138, 2716–2731.
- Maia, L.F.; Kaeser, S.A.; Reichwald, J.; Hruscha, M.; Martus, P.; Staufenbiel, M.; Jucker, M. Changes in Amyloid-β and Tau in the Cerebrospinal Fluid of Transgenic Mice Overexpressing Amyloid Precursor Protein. Sci. Transl. Med. 2013, 5, 194re2.
- Sato, C.; Barthélemy, N.R.; Mawuenyega, K.G.; Patterson, B.W.; Gordon, B.; Jockel-Balsarotti, J.; Sullivan, M.; Crisp, M.J.; Kasten, T.; Kirmess, K.M.; et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron 2018, 98, 861–864.
- Dobson, R.; Giovannoni, G. Multiple sclerosis–A review. Eur. J. Neurol. 2019, 26, 27–40.
- De Rosa, V.; Galgani, M.; Porcellini, A.; Colamatteo, A.; Santopaolo, M.; Zuchegna, C.; Romano, A.; De Simone, S.; Procaccini, C.; La Rocca, C.; et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015, 16, 1174–1184.
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, C.J.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874.
- Ziemssen, T.; Kern, R.; Thomas, K. Multiple sclerosis: Clinical profiling and data collection as prerequisite for personalized medicine approach. BMC Neurol. 2016, 16, 124.
- Kuhlmann, T.; Ludwin, S.; Prat, A.; Antel, J.; Brück, W.; Lassmann, H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017, 133, 13–24.
- Kooij, G.; Kroon, J.; Paul, D.; Reijerkerk, A.; Geerts, D.; van der Pol, S.M.A.; Hof, B.V.H.; Drexhage, J.A.; van Vliet, S.J.; Hekking, L.H.P.; et al. P-glycoprotein regulates trafficking of CD8+ T cells to the brain parenchyma. Acta Neuropathol. 2014, 127, 699–711.
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H.G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11.
- Pryce, G.; Baker, D. Oligoclonal bands in multiple sclerosis; Functional significance and therapeutic implications. Does the specificity matter? Mult. Scler. Relat. Disord. 2018, 25, 131–137.
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173.
- Petzold, A. Applying the 2017 McDonald diagnostic criteria for multiple sclerosis. Lancet Neurol. 2018, 17, 496–497.
- Arrambide, G.; Tintore, M.; Montalban, X. Oligoclonal bands do not represent dissemination in time in the 2017 revisions to the McDonald criteria. Mult. Scler. J. 2019, 25, 1690–1691.
- Trojano, M.; Tintore, M.; Montalban, X.; Hillert, J.; Kalincik, T.; Iaffaldano, P.; Spelman, T.; Sormani, M.P.; Butzkueven, H. Treatment decisions in multiple sclerosis-insights from real-world observational studies. Nat. Rev. Neurol. 2017, 13, 105–118.
- Levine, S.M. Albumin and multiple sclerosis. BMC Neurol. 2016, 16, 47.
- Maggi, P.; Absinta, M.; Grammatico, M.; Vuolo, L.; Emmi, G.; Carlucci, G.; Spagni, G.; Barilaro, A.; Repice, A.M.; Emmi, L.; et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann. Neurol. 2018, 83, 283–294.
- Bonnan, M. Intrathecal IgG Synthesis: A Resistant and Valuable Target for Future Multiple Sclerosis Treatments. Mult. Scler. Int. 2015, 2015, 296184.
- Grygiel-Górniak, B.; Rogacka, N.; Puszczewicz, M. Antinuclear antibodies in healthy people and non-rheumatic diseases–diagnostic and clinical implications. Reumatologia 2018, 56, 243–248.
- Becker, J.; Geffken, M.; Diehl, R.R.; Berlit, P.; Krämer, M. Choosing wisely? Multiple Sclerosis and Laboratory Screening for Autoimmune Differential Diagnoses. Neurol. Int. Open 2017, 01, E256–E263.
- Narayan, R.; Simpson, A.; Fritsche, K.; Salama, S.; Pardo, S.; Mealy, M.; Paul, F.; Levy, M. MOG antibody disease: A review of MOG antibody seropositive neuromyelitis optica spectrum disorder. Mult. Scler. Relat. Disord. 2018, 25, 66–72.
- Jarius, S.; Paul, F.; Aktas, O.; Asgari, N.; Dale, R.C.; De Seze, J.; Franciotta, D.; Fujihara, K.; Jacob, A.; Kim, H.J.; et al. MOG encephalomyelitis: International recommendations on diagnosis and antibody testing. J. Neuroinflamm. 2018, 15, 134.
- Peschl, P.; Bradl, M.; Höftberger, R.; Berger, T.; Reindl, M. Myelin Oligodendrocyte Glycoprotein: Deciphering a Target in Inflammatory Demyelinating Diseases. Front. Immunol. 2017, 8, 529.
- Mitsdoerffer, M.; Kuchroo, V.; Korn, T. Immunology of neuromyelitis optica: A T cell-B cell collaboration. Ann. New York Acad. Sci. 2013, 1283, 57–66.
- Verkman, A.S. Aquaporins in Clinical Medicine. Annu. Rev. Med. 2012, 63, 303–316.
- McCreary, M.; Mealy, M.A.; Wingerchuk, D.M.; Levy, M.; DeSena, A.; Greenberg, B.M. Updated diagnostic criteria for neuromyelitis optica spectrum disorder: Similar outcomes of previously separate cohorts. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318815925.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
982
Revisions:
2 times
(View History)
Update Date:
07 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




