
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Ehsan | + 1779 word(s) | 1779 | 2020-09-28 10:03:18 | | | |
| 2 | Vicky Zhou | -555 word(s) | 1224 | 2020-10-09 11:45:20 | | | | |
| 3 | Vicky Zhou | -72 word(s) | 1152 | 2020-10-27 07:03:21 | | |
Video Upload Options
Haemonchosis/barber’s pole disease is a socio-economically important disease caused by one of the most pathogenic nematode parasite of ruminants called Haemonchus contortus. The control practices through anthelmintics, and resistance to these drugs demands for an effective, safe and durable vaccine against H. contortus. During host-parasite relationship, parasites excrete and secrete large number of molecules into the host, which perform adversative immune-regulatory functions upon bindings to host immune cells. Various potential vaccine antigens have been tested by different ways and strategies applied in animal models, and significant progress has been made in the development of vaccines against H. contortus.
1. Introduction
The gastrointestinal nematode parasite Haemonchus contortus (H. contortus) is a resident of tropical and subtropical regions worldwide that imposes significant production losses, economic losses, and animal health issues in the small ruminant industry, particularly sheep and goats. Considerable efforts have been made to understand how immunity is elicited against H. contortus infection. Various potential vaccine antigens have been tested by different methods and strategies applied in animal models, and significant progress has been made in the development of vaccines against H. contortus.
2. Mechanisms of Helminths-Associated Immunity
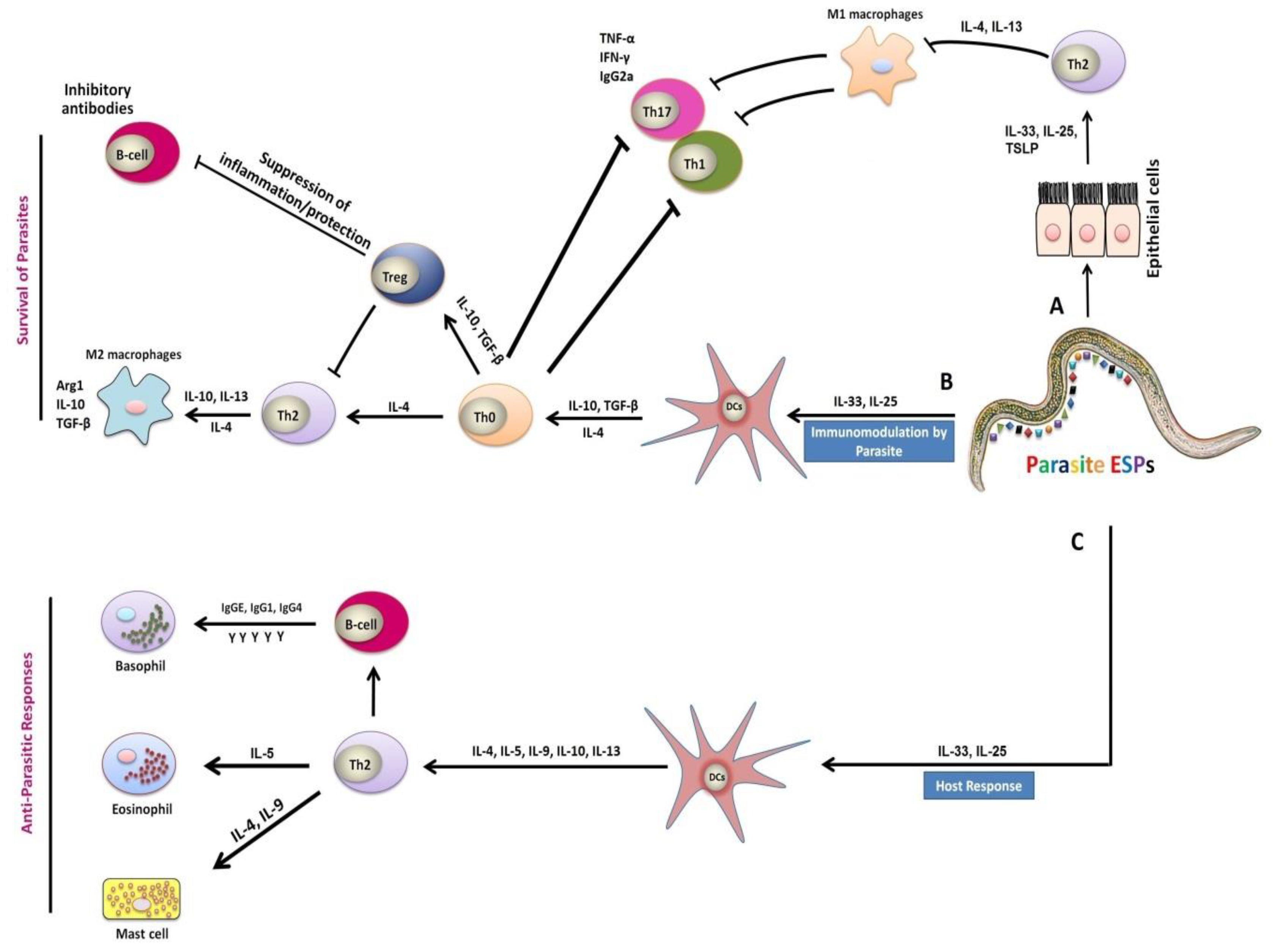
The immune responses to helminth parasites within their host are quite complex and characterized into two different stages: the immune responses to infective larvae and the immune responses against adult parasites. The T-helper cell type 2 (Th2) responses are considered the most effective resistance against helminths associated with high levels of serum and mucosal immunoglobulin-A (IgA), E (IgE), G (IgG), and eosinophilia at the site of infection [1][2][3]. The resistance against H. contortus infection is mainly associated with the breed and age of the host, as well as previous exposure to parasitic infection; especially, lambs are more susceptible to parasitism during the first 6 months of their lives, as they cannot develop immunity against H. contortus. This could differentially happen in older sheep, which usually can handle a moderate infection due to acquired immunity; however, this immunological phenomenon still not well understood. Several events are required for the clearance of nematode from the immunized host, including the activation of nonspecific defense mechanisms and the recognition of somatic and excretory/secretory (ES) antigens by dendritic cells (DCs), which act as antigen-presenting cells (APCs) for T cells [4]. The effector constituents of immunity are ‘‘allergic” inflammatory responses involving mucosal mast cells and eosinophils that secrete inflammatory mediators, which suppress egg production, prevent infective larvae and adult worm establishment, paralyze worm motility, and possibly mystify incoming larvae [5][6].
Generally, in case of helminths infection, Th2 immune responses are the part of the humoral responses associated with the specific expression of cytokines at the site of infection and cytokines acting as critical mediators for any immunological response, which evolved in CD4+ T cells stimulation and differentiation [4][7]. Helminths infection elicited strong Th2 cell responses associated with significant productions of interleukin (IL)-4, IL-5, IL-9, IL-10, IL-13, IL-25, and IL-31 [8][9][10] (Figure 2). Furthermore, these responses were also associated with high levels of IgE, IgG1, IgG4, and stable eosinophil and mast cell responses [11] (Figure 2). The cytokines IL-4 and IL-13 were considered as key regulators in humoral immunity, inducing B-cell class switching to IgE and regulating major histocompatibility complex (MHC) class II production . These cytokines were involved in increased smooth-muscle-cell contractility, stimulation of epithelial cell permeability, and enhanced mucous production by goblet cells . Mast cells, eosinophils, specific antibodies, and inhibitory molecules were generally involved in immunity to H. contortus parasite [3] (Figure 2). Some previous studies have shown that Th2 immune responses associated with helminths infection were characterized by the induction of IL-4, IL-13, IgG, IgG1, and IgE antibodies in rats , and the generation of IL-3, IL-4, IL-5, and IL-13 in mice . Some studies reported that helminths have established various strategies to escape or modulate the host immune responses with advantages on both sides; for example, a modification in the Th2 response toward immunosuppression takes place as a result of the upregulation of regulatory T cells (Tregs), which suppressed protective Th2 as well as inflammatory Th1 responses in Ancylostoma caninum infection . Furthermore, helminth-mediated Th2 responses could also prevent the harmful inflammatory Th1 responses by inducing suppressive Tregs, which contributed to the formation of IL-10 and transforming growth factor-β (TGF-β) cytokines. An earlier investigation has shown that Trichinella spiralis excretory/secretory products (ESPs) suppressed in vitro DCs maturation and induced the inflation of functional Tregs induced by both S- and R-form lipopolysaccharide (LPS) .
3. Concluding Remarks
Since the global emergence of anthelmintics resistance, substantial progress has been made, and still, work is on the way to identify and evaluate protective efficiencies of various vaccine candidate antigens. However, none have shown robust and passable protective efficacy yet. Prospective impediments and elucidations during the developmental phase of an effective haemonchosis vaccine have been discussed. There is still the need to fully understand the immunopathology of H. contortus during parasite–host interactions for the accurate identification of better immune correlates and vaccination candidates, and most importantly, the precise choice of delivery vehicle or adjuvant for vaccination. Therefore, a deep insight into the immunopathology of H. contortus in interaction with domestic hosts (sheep, goats) is still needed to establish a road map in the development of vaccines, which will have a pivotal role in the prevention and control of H. contortus infection. Finally, the incorporation of the most advanced innovational strategies such as genomics, transcriptomics, proteomics, and metabolomics will boost up the progress of a more effective, safe, and durable vaccine against H. contortus in the near future.
References
- Lacroux, C.; Nguyen, T.H.C.; Andréoletti, O.; Prévot, F.; Grisez, C.; Bergeaud, J.-P.; Gruner, L.; Brunel, J.-C.; Francois, D.; Dorchies, P.; et al. Haemonchus contortus (Nematoda: Trichostrongylidae) infection in lambs elicits an unequivocal Th2 immune response. Vet. Res. 2006, 37, 607–622.
- Shakya, K.; Miller, J.; Lomax, L.; Burnett, D. Evaluation of immune response to artificial infections of Haemonchus contortus in Gulf Coast Native compared with Suffolk lambs. Vet. Parasitol. 2011, 181, 239–247.
- Balic, A.; Cunningham, C.P.; Meeusen, E.N.T. Eosinophil interactions with Haemonchus contortus larvae in the ovine gastrointestinal tract. Parasite Immunol. 2006, 28, 107–115.
- Meeusen, E.N.T.; Balic, A.; Bowles, V.M. Cells, cytokines and other molecules associated with rejection of gastrointestinal nematode parasites. Vet. Immunol. Immunopathol. 2005, 108, 121–125.
- Jones, W.; Emery, D.; McClure, S.; Wagland, B. Changes in inflammatory mediators and larval inhibitory activity in intestinal contents and mucus during primary and challenge infections of sheep with Trichostrongylus colubriformis. Int. J. Parasitol. 1994, 24, 519–525.
- Emery, D. Vaccination against worm parasites of animals. Vet. Parasitol. 1996, 64, 31–45.
- Anthony, R.M.; Urban, J.F.; Alem, F.; Hamed, H.A.; Rozo, C.T.; Boucher, J.-L.; Van Rooijen, N.; Gause, W.C. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 2006, 12, 955–960.
- Jackson, J.A.; Friberg, I.M.; Little, S.; Bradley, J.E. Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: Coevolutionary legacies? Immunology 2009, 126, 18–27.
- Maizels, R.M.; Yazdanbakhsh, M. Immune Regulation by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2003, 3, 733–744.
- Wang, L.J.; Cao, Y.; Shi, H.N. Helminth infections and intestinal inflammation. World J. Gastroenterol. 2008, 14, 5125–5132.
- Ditgen, D.; Anandarajah, E.M.; Meissner, K.A.; Brattig, N.; Wrenger, C.; Liebau, E. Harnessing the helminth secretome for therapeutic immunomodulators. Biomed Res. Int. 2014, 1–14.




