
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lukas Heuberger | -- | 3155 | 2022-06-01 14:27:35 | | | |
| 2 | Peter Tang | Meta information modification | 3155 | 2022-06-02 03:14:27 | | |
Video Upload Options
Nano- and micrometer-sized compartments composed of synthetic polymers are designed to mimic spatial and temporal divisions found in nature. Self-assembly of polymers into compartments such as polymersomes, giant unilamellar vesicles (GUVs), layer-by-layer (LbL) capsules, capsosomes, or polyion complex vesicles (PICsomes) allows for the separation of defined environments from the exterior. These compartments can be further engineered through the incorporation of (bio)molecules within the lumen or into the membrane, while the membrane can be decorated with functional moieties to produce catalytic compartments with defined structures and functions. Nanometer-sized compartments are used for imaging, theranostic, and therapeutic applications as a more mechanically stable alternative to liposomes, and through the encapsulation of catalytic molecules, i.e., enzymes, catalytic compartments can localize and act in vivo. On the micrometer scale, such biohybrid systems are used to encapsulate model proteins and form multicompartmentalized structures through the combination of multiple compartments, reaching closer to the creation of artificial organelles and cells.
1. Introduction
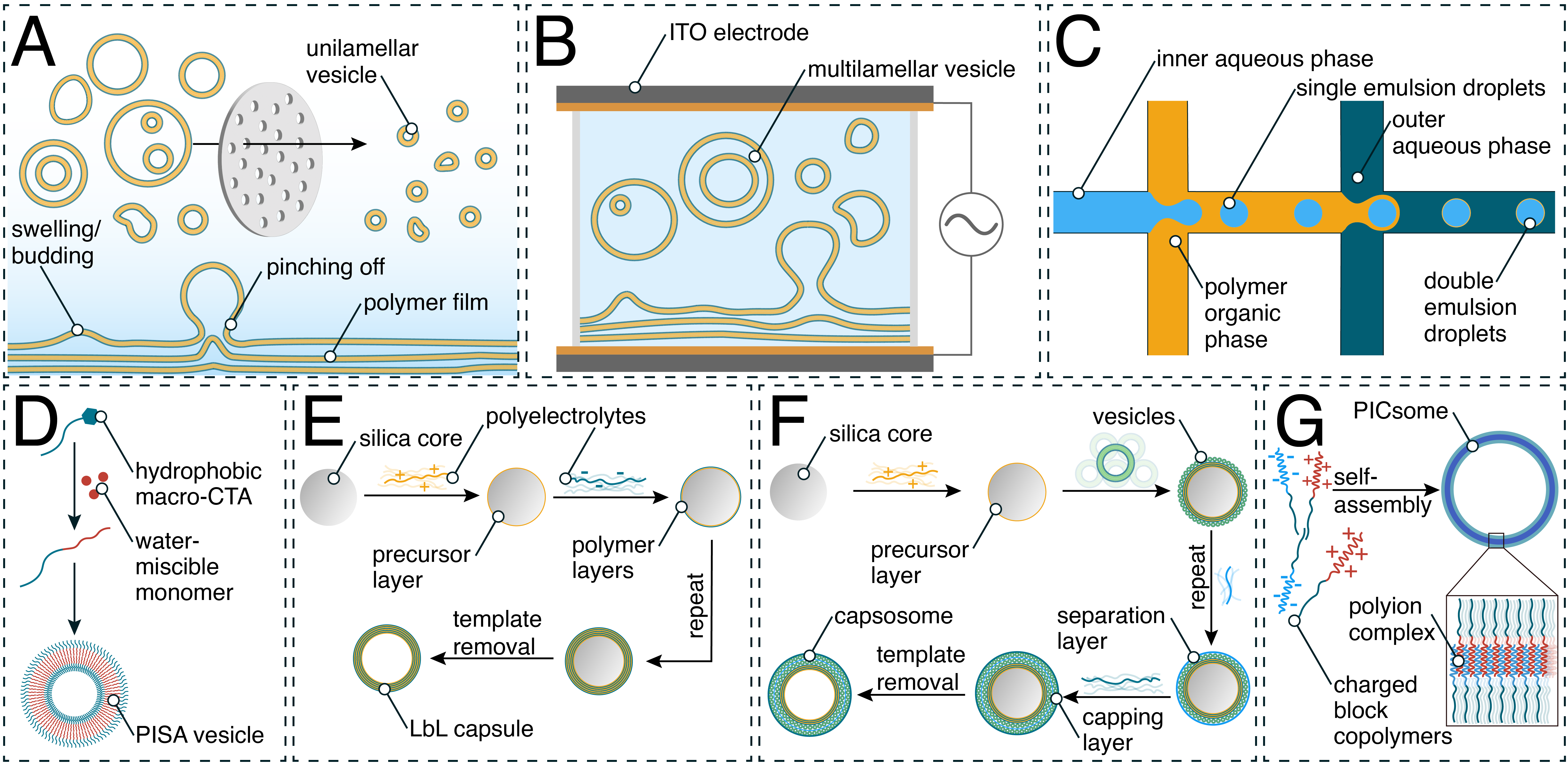
2. Generation of Synthetic Compartments
|
Polymer |
Method of Self-Assembly |
Characteristics |
|---|---|---|
|
Carbohydrate-b-PPG |
Direct hydration method [50] |
Forms capsosomes, inherently permeable to low-molecular-weight compounds |
|
Chitosan |
Biocompatible, natural polymer |
|
|
CTAB |
LbL [52] |
Surfactant, forms micelles in the absence of another polymer |
|
PA/DEX |
LbL [53] |
Biocompatible polysaccharide (anionic) |
|
P(OEGMA300-grad-HPMA) |
PISA [39] |
Biocompatible assembly, monomers and a macromolecular precursor need to be: (i) solvophilic and (ii) compatible with each other |
|
PA/PLA |
LbL [53] |
Biocompatible cationic polyelectrolyte |
|
PAA |
LbL [54] |
Anionic polyelectrolyte |
|
PAH |
Cationic polyelectrolyte |
|
|
PAMAM |
Mixing (PICsomes) [57] |
Dendrimer (branched structure) |
|
P(Asp-AP) |
Anionic polyelectrolyte, forms PICsomes, cannot form vesicles on its own |
|
|
PATK |
Mixing (PICsomes) [61] |
Cationic polyelectrolyte |
|
PBd-b-PEG |
Double emulsion microfluidics [33] |
Biocompatible |
|
PBd–b-PEO |
Emulsion centrifugation [30], Electroformation [62], Film rehydration [63] |
Pure or as hybrid (with POPC) polymersomes for membrane protein insertion, assembly of asymmetric polymer/lipid (POPC) hybrid membranes |
|
PBO-b-PG |
Microfluidic double emulsion, solvent switch [64] |
Biocompatible |
|
PCL-b-P[Glu-stat-(Glu-ADA)] |
Solvent switch [65] |
Biodegradable, bone-targeting |
|
PCL-b-PTrp-b-P(Lys-statPhe) |
Solvent switch [66] |
Biocompatible, biodegradable, antibacterial |
|
PDMS-b-heparin |
Film rehydration [67] |
Forms polymersomes in combination with PMOXA-b-PDMS-b-PMOXA, forms micelles by itself |
|
PDMS-g-PEO |
Electroformation, Film rehydration [68] |
Pure or as hybrid (with PC) polymersomes and GUVs for membrane protein insertion |
|
PEG-b-PCL |
Multidomain membrane formation with lipids (DPPC) |
|
|
PEG-P(CLgTMC) |
Direct hydration method [71] |
Biodegradable, intrinsic fluorescence |
|
PEG-b-P(CPTKMA-co-PEMA) |
Solvent exchange method [72] |
Biocompatible, conjugated with campthothecin |
|
PEG-GPLGVRG-PCL-PGPMA |
Film hydration method [73] |
Biocompatible, MMP-cleavable peptide and CPP-mimicking polymer |
|
PEG-b-PHPMA |
PISA [74] |
Highly hydrated membrane, size-selective transport of molecules |
|
PEG-b-PIC |
Solvent exchange [75] |
Biocompatible, iodine-rich for SPECT/CT and radioisotope therapy |
|
PEG-b-PLA |
Forms polymersomes with and without lipid mixing, biodegradable |
|
|
PEG-b-polypeptide |
Mixing (PICsomes) [76] |
pH-responsive, biocompatible |
|
PEG-b-PAsp |
Linear polymer, forms PICsomes, micelles or hydrogels, biocompatible |
|
|
PEG-b-PS |
Biocompatible, formation of stomatocytes, rigid assemblies |
|
|
PEI-b-PDLLA |
Microfluidic double emulsion [79] |
Biocompatible, cationic assemblies, can form polymer stomatocytes |
|
PEO-b-PBO |
Film rehydration [80] |
Forms asymmetric polymersomes |
|
PEO-b-PCL |
Emulsification-induced assembly [81] |
Low interfacial tension solvent or SDS is needed to control the assembly |
|
PEO-b-PCL-b-PMOXA |
Film rehydration [82] |
Rehydration at 62 °C due to the semi crystalline nature of the PCL block |
|
PEO-b-P(CMA-stat-DEA-stat-GEMA) |
Solvent exchange method [83] |
Biocompatible, CMA photocrosslinking stabilization |
|
PEO-b-PEHOx-b-PEtOz |
Solvent switch, film rehydration [22] |
Asymmetric membrane, can be used for directed protein insertion |
|
PEO-b-PPO-b-PEO (Pluronics L121) |
Double emulsion microfluidics [33] |
Assembly via DNA linkage |
|
PiB-b-PEG |
Freeze–thaw extrusion [84] |
Biocompatible, high chemical and thermal stability |
|
PLys |
Mixing (PICsomes) [85] |
Cationic polyelectrolyte |
|
PMA |
LbL [56] |
Labor-intensive LbL assembly |
|
PMOXA-b-PDMS |
microfluidic double emulsion [35] |
Formation of nano- and micro-sized vesicles in biocompatible, aqueous conditions, various channels and proteins can be inserted |
|
PMOXA-b-PDMS-b-PMOXA |
Formation of nano and micro-sized vesicles in biocompatible, aqueous conditions, various channels and proteins can be inserted |
|
|
PMPC-b-PDPA |
Formation of (asymmetric) polymersomes, can be electroporated |
|
|
POEGMA-b-P(ST-co-VBA) |
PISA [37] |
Biocompatible assembly, monomers and a macromolecular precursor need to be: (i) solvophilic and (ii) compatible with each other |
|
Poly(dopamine) |
LbL [90] |
Simplified LbL capsule formation |
|
PS-b-PEO |
Emulsification [91] |
High capacity of ammonia capture in bile salt-containing buffer |
|
PSMA-PBzMA |
PISA [38] |
Biocompatible assembly, monomers and a macromolecular precursor need to be: (i) solvophilic and (ii) compatible with each other |
|
PSS-b-PEO-b-PSS |
Mixing (PICsomes) [57] |
Forms PICsomes with loops within the membrane when combined with poly(amidoamine) dendrimers |
|
PVP |
LbL [92] |
Work-intensive LbL assembly |
3. Requirements for Compartments to Be Used in Biomedical Applications
4. Applications of Compartments in the Biomedical Field
|
Biomolecule |
Polymer |
Location in Assembly |
Application/Function |
|---|---|---|---|
|
Actin |
PMOXA-b-PDMS-b-PMOXA [67] |
Encapsulated (in GUVs) |
Polymerization to form a cytoskeleton |
|
ATP synthase |
Incorporated within membrane (GUVs) |
ATP generation |
|
|
Bacteriorhodopsin |
PDMS-g-PEO, PBd- b-PEO [117] |
Incorporated within membrane (GUVs) |
Pumping protons across membrane |
|
Catalase |
Encapsulated with the stomata of polymer stomatocytes and LbL capsules |
Conversion of hydrogen peroxide to oxygen and water for self-propelled movement |
|
|
Cholesterol–DNA |
PEO-b-PPO-b-PEO (Pluronics L121), PBd-b-PEG, PLA-b-PEG [33] |
Incorporated within membrane (GUVs) |
Clustering of polymersomes |
|
Cytochrome bo3 ubiquinol oxidase (Cyt bo3) |
PBd–PEO:POPC hybrid [118], PDMS-g-PEO and PDMS-g-PEO/PC hybrid [68][119] |
Incorporated within membrane (polymersomes, GUVs) |
Pumping protons across membrane |
|
DNA nanopore NP-3c |
PMPC-b-PDPA [89] |
Incorporated within membrane (GUVs) |
Pore formation for cross-membrane diffusion |
|
Dopa decarboxylase (DDC) |
PMOXA-b-PDMS [120] |
Encapsulated (in polymersomes) |
Production of dopamine |
|
Erythrosine B (and its ester derivatives) |
F127 Pluronic (mixed with DPPC lipids) [121] |
Incorporated within membrane (polymersomes) |
Photodynamic therapy |
|
Glucose oxidase (Gox) |
PMOXA-b-PDMS [35][87], PEG-b-P(CPTKMA-co-PEMA) [72], PATK and PEG-b-Pasp [61] |
Encapsulated (in GUVs, polymersomes, and PICsomes) |
Catalysis of glucose oxidation to hydrogen peroxide and D-glucono-δ-lactone |
|
Gramicidin |
Incorporated within membrane |
Membrane permeabilization towards ions |
|
|
Horseradish peroxidase (HRP) |
PMOXA-b-PDMS [35], PMOXA-b-PDMS-b-PMOXA [86][101], carbohydrate-b-PPG [50] |
Encapsulated (in GUVs, polymersomes, capsosomes) |
Catalysis of oxidation of organic substrates by hydrogen peroxide |
|
Icosane |
PAA and PAH (LbL) [54] |
Encapsulated (in capsules) |
Acting as a phase change material for thermal energy storage |
|
Inducible nitric oxide synthase (iNOS) |
PMOXA-b-PDMS-b-PMOXA [122] |
Encapsulated (in polymersomes) |
Oxidation of l-arginine to l-citrulline and nitric oxide (NO) |
|
Ionomycin |
PMOXA-b-PDMS-b-PMOXA [67] |
Incorporated within membrane |
Membrane permeabilization towards ions |
|
Laccase |
PMOXA-b-PDMS [123] |
Encapsulated (in polymersomes) |
Oxidation of phenolic and nonphenolic compounds |
|
Lactoperoxidase (LPO) |
PMOXA-b-PDMS [87] |
Encapsulated (in polymersomes) |
Oxidation of Amplex red using hydrogen peroxide |
|
L-asparaginase |
PMPC-b-PDPA and PEO-b-PBO [80], PEG-b-Pasp and P(Asp-AP) [60], PEG-b-PHPMA [74] |
Encapsulated (in polymersomes, PICsomes) |
Catalysis of L-asparagine to l-aspartic acid and ammonia |
|
Lipase |
PMOXA-b-PDMS-b-PMOXA [67] |
Encapsulated (in polymersomes) |
Catalysis of the hydrolysis of fats |
|
Luciferase |
PMOXA-b-PDMS [124] |
Encapsulated (in polymersomes) |
Bioluminescence |
|
Melittin |
Incorporated within membrane (polymersomes, GUVs) |
Pore formation for cross-membrane diffusion |
|
|
Methionine γ-lyase (MGL) |
Encapsulated (in PICsomes) |
Cancer therapy |
|
|
Outer membrane protein F from E. coli (OmpF) |
Incorporated within membrane (GUVs, polymersomes) |
Pore formation for cross-membrane diffusion |
|
|
Penicillin acylase |
PMOXA-b-PDMS-b-PMOXA [126] |
Encapsulated (in polymersomes) |
Production of antibiotic cephalexin |
|
Rnase H |
PEG-b-polypeptide (with single-stranded oligonucleotides) [76] |
Encapsulated (in PICsomes) |
Gene knockout therapy |
|
β-galactosidase |
PMOXA-b-PDMS [35] carbohydrate-b-PPG [50] |
Encapsulated (in GUVs, capsosomes) |
Catalysis of the hydrolysis of β-galactosides into monosaccharides |
|
β-glucuronidase |
PMOXA-b-PDMS [125] |
Encapsulated (in polymersomes) |
Cleavage of the glucuronide moiety from glucuronide-conjugates |
|
Soluble guanylyl cyclase (sGC) |
PMOXA-b-PDMS-b-PMOXA [122] |
Encapsulated (in polymersomes) |
Production of cyclic 3,5-guanosine monophosphate (cGMP) |
|
Trypsin |
PMPC-b-PDPA [89] |
Encapsulated (in polymersomes) |
Hydrolyzation of proteins |
|
Tyrosinase |
PMOXA-b-PDMS [127] |
Encapsulated (in polymersomes) |
Oxidation of L-DOPA |
|
Urate oxidase (UOX) |
PMOXA-b-PDMS-b-PMOXA [12] |
Encapsulated (in polymersomes) |
Production of hydrogen peroxide for a cascade reaction |
References
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411.
- Matoori, S.; Leroux, J.C. Twenty-five years of polymersomes: Lost in translation? Mater. Horiz. 2020, 7, 1297–1309.
- Feng, H.; Lu, X.; Wang, W.; Kang, N.-G.; Mays, J.W. Block copolymers: Synthesis, self-assembly, and applications. Polymers 2017, 9, 494.
- Kim, K.T.; Meeuwissen, S.A.; Nolte, R.J.; van Hest, J.C. Smart nanocontainers and nanoreactors. Nanoscale 2010, 2, 844–858.
- Go, Y.K.; Leal, C. Polymer–lipid hybrid materials. Chem. Rev. 2021, 121, 13996–14030.
- Thoma, J.; Belegrinou, S.; Rossbach, P.; Grzelakowski, M.; Kita-Tokarczyk, K.; Meier, W. Membrane protein distribution in composite polymer—Lipid thin films. Chem. Commun. 2012, 48, 8811–8813.
- Fauquignon, M.; Ibarboure, E.; Meins, J.-F. Membrane reinforcement in giant hybrid polymer lipid vesicles achieved by controlling the polymer architecture. Soft Matter 2021, 17, 83–89.
- Gilbert, R.J.; Serra, M.D.; Froelich, C.J.; Wallace, M.I.; Anderluh, G. Membrane pore formation at protein—Lipid interfaces. Trends Biochem. Sci. 2014, 39, 510–516.
- Hu, Z.; Ho, J.C.; Nallani, M. Synthetic (polymer) biology (membrane): Functionalization of polymer scaffolds for membrane proteins. Curr. Opin. Biotechnol. 2017, 46, 51–56.
- Hindley, J.W.; Law, R.V.; Ces, O. Membrane functionalization in artificial cell engineering. SN Appl. Sci. 2020, 2, 593.
- Goers, R.; Thoma, J.; Ritzmann, N.; Di Silvestro, A.; Alter, C.; Gunkel-Grabole, G.; Fotiadis, D.; Müller, D.J.; Meier, W. Optimized reconstitution of membrane proteins into synthetic membranes. Commun. Chem. 2018, 1, 35.
- Belluati, A.; Craciun, I.; Liu, J.; Palivan, C.G. Nanoscale enzymatic compartments in tandem support cascade reactions in vitro. Biomacromolecules 2018, 19, 4023–4033.
- Rideau, E.; Wurm, F.R.; Landfester, K. Self-assembly of giant unilamellar vesicles by film hydration methodologies. Adv. Biosyst. 2019, 3, 1800324.
- Cabukusta, B.; Neefjes, J. Mechanisms of lysosomal positioning and movement. Traffic 2018, 19, 761–769.
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267–277.
- Discher, D.E. Polymer vesicles. Science 2002, 297, 967–973.
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333.
- Daubian, D.; Gaitzsch, J.; Meier, W. Synthesis and complex self-assembly of amphiphilic block copolymers with a branched hydrophobic Poly(2-Oxazoline) into multicompartment micelles, pseudo-vesicles and Yolk/Shell nanoparticles. Polym. Chem. 2020, 11, 1237–1248.
- Wehr, R.; Gaitzsch, J.; Daubian, D.; Fodor, C.; Meier, W. Deepening the insight into poly(butylene oxide)-Block-poly(Glycidol) synthesis and self-assemblies: Micelles, worms and vesicles. RSC Adv. 2020, 10, 22701–22711.
- Li, B.-Y.; Li, Y.-C.; Lu, Z.-Y. The important role of cosolvent in the amphiphilic diblock copolymer self-assembly process. Polymer 2019, 171, 1–7.
- Balasubramanian, V.; Herranz-Blanco, B.; Almeida, P.V.; Hirvonen, J.; Santos, H.A. Multifaceted polymersome platforms: Spanning from self-assembly to drug delivery and protocells. Prog. Polym. Sci. 2016, 60, 51–85.
- Daubian, D.; Fillion, A.; Gaitzsch, J.; Meier, W. One-pot synthesis of an amphiphilic ABC triblock copolymer PEO- b -PEHOx- b -PEtOz and its self-assembly into nanoscopic asymmetric polymersomes. Macromolecules 2020, 53, 11040–11050.
- Parnell, A.J.; Tzokova, N.; Topham, P.D.; Adams, D.J.; Adams, S.; Fernyhough, C.M.; Ryan, A.J.; Jones, R.A. The efficiency of encapsulation within surface rehydrated polymersomes. Faraday Discuss. 2009, 143, 29–46.
- Belluati, A.; Craciun, I.; Meyer, C.E.; Rigo, S.; Palivan, C.G. Enzymatic reactions in polymeric compartments: Nanotechnology meets nature. Curr. Opin. Biotechnol. 2019, 60, 53–62.
- Pachioni-Vasconcelos, J.D.; Apolinário, A.C.; Lopes, A.M.; Pessoa, A., Jr.; Barbosa, L.R.; Rangel-Yagui, C.D. Compartmentalization of therapeutic proteins into semi-crystalline PEG-PCL polymersomes. Soft Mater. 2021, 19, 222–230.
- Lomora, M.; Garni, M.; Itel, F.; Tanner, P.; Spulber, M.; Palivan, C.G. Polymersomes with engineered ion selective permeability as stimuli-responsive nanocompartments with preserved architecture. Biomaterials 2015, 53, 406–414.
- Ibarboure, E.; Fauquignon, M.; Le Meins, J.-F. Obtention of giant unilamellar hybrid vesicles by electroformation and measurement of their mechanical properties by micropipette aspiration. J. Vis. Exp. 2020, 155, 60199.
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 2010, 11, 848–865.
- Garni, M.; Wehr, R.; Avsar, S.Y.; John, C.; Palivan, C.; Meier, W. Polymer membranes as templates for bio-applications ranging from artificial cells to active surfaces. Eur. Polym. J. 2019, 112, 346–364.
- Peyret, A.; Ibarboure, E.; Le Meins, J.-F.; Lecommandoux, S. Asymmetric hybrid polymer—Lipid giant vesicles as cell membrane mimics. Adv. Sci. 2018, 5, 1700453.
- dos Santos, E.C.; Angelini, A.; Hürlimann, D.; Meier, W.; Palivan, C.G. Giant polymer compartments for confined reactions. Chemistry 2020, 2, 470–489.
- Shum, H.C.; Kim, J.-W.; Weitz, D.A. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. J. Am. Chem. Soc. 2008, 130, 9543–9549.
- Luo, R.; Göpfrich, K.; Platzman, I.; Spatz, J.P. DNA-based assembly of multi-compartment polymersome networks. Adv. Funct. Mater. 2020, 30, 2003480.
- Deshpande, S.; Caspi, Y.; Meijering, A.E.; Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 1–9.
- dos Santos, E.C.; Belluati, A.; Necula, D.; Scherrer, D.; Meyer, C.E.; Wehr, R.P.; Lörtscher, E.; Palivan, C.G.; Meier, W. Combinatorial strategy for studying biochemical pathways in double emulsion templated cell-sized compartments. Adv. Mater. 2020, 32, 2004804.
- Weiss, M.; Frohnmayer, J.P.; Benk, L.T.; Haller, B.; Janiesch, J.W.; Heitkamp, T.; Börsch, M.; Lira, R.B.; Dimova, R.; Lipowsky, R.; et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 2018, 17, 89–95.
- Phan, H.; Taresco, V.; Penelle, J.; Couturaud, B. Polymerisation-induced self-assembly (PISA) as a straightforward formulation strategy for stimuli-responsive drug delivery systems and biomaterials: Recent advances. Biomater. Sci. 2021, 9, 38–50.
- Dorsman, I.R.; Derry, M.J.; Cunningham, V.J.; Brown, S.L.; Williams, C.N.; Armes, S.P. Tuning the vesicle-to-worm transition for thermoresponsive block copolymer vesicles prepared via polymerisation-induced self-assembly. Polym. Chem. 2021, 12, 1224–1235.
- Xu, S.; Corrigan, N.; Boyer, C. Forced gradient copolymerisation: A simplified approach for polymerisation-induced self-assembly. Polym. Chem. 2021, 12, 57–68.
- Canning, S.L.; Smith, G.N.; Armes, S.P. A critical appraisal of RAFT-mediated polymerization-induced self-assembly. Macromolecules 2016, 49, 1985–2001.
- Donath, E.; Sukhorukov, G.B.; Caruso, F.; Davis, S.A.; Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 1998, 37, 2201–2205.
- Eivazi, A.; Medronho, B.; Lindman, B.; Norgren, M. On the development of all-cellulose capsules by vesicle-templated layer-by-layer assembly. Polymers 2021, 13, 589.
- Elizarova, I.S.; Luckham, P.F. Layer-by-layer adsorption: Factors affecting the choice of substrates and polymers. Adv. Colloid Interface Sci. 2018, 262, 1–20.
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent developments in layer-by-layer technique for drug delivery applications. ACS Appl. Bio Mater. 2019, 2, 5512–5527.
- Campbell, J.; Vikulina, A.S. Layer-by-layer assemblies of biopolymers: Build-Up, mechanical stability and molecular dynamics. Polymers 2020, 12, 1949.
- Francesch, M.B. Capsosomes: The revolutionary enzyme carriers. Eur. Pharm. Rev. 2019, 24, 34–36.
- Städler, B.; Chandrawati, R.; Goldie, K.; Caruso, F. Capsosomes: Subcompartmentalizing polyelectrolyte capsules using liposomes. Langmuir 2009, 25, 6725–6732.
- Sun, J.; Li, Z. Polyion complexes via electrostatic interaction of oppositely charged block copolymers. Macromolecules 2020, 53, 8737–8740.
- Liu, Y.; Maruyama, T.; Kc, B.; Mori, T.; Katayama, Y.; Kishimura, A. Inducible dynamic behavior of polyion complex vesicles by disrupting charge balance. Chem. Lett. 2021, 50, 1034–1037.
- Nishimura, T.; Sasaki, Y.; Akiyoshi, K. Biotransporting self-assembled nanofactories using polymer vesicles with molecular permeability for enzyme prodrug cancer therapy. Adv. Mater. 2017, 29, 1702406.
- Odrobińska, J.; Gumieniczek-Chłopek, E.; Szuwarzyński, M.; Radziszewska, A.; Fiejdasz, S.; Strączek, T.; Kapusta, C.; Zapotoczny, S. Magnetically navigated core—Shell polymer capsules as nanoreactors loadable at the oil/water interface. ACS Appl. Mater. Interfaces 2019, 11, 10905–10913.
- Cai, H.; Wang, P.; Zhang, D. PH-responsive linkages-enabled layer-by-layer assembled antibacterial and antiadhesive multilayer films with polyelectrolyte nanocapsules as biocide delivery vehicles. J. Drug Deliv. Sci. Technol. 2019, 54, 101251.
- Svenskaya, Y.; Garello, F.; Lengert, E.; Kozlova, A.; Verkhovskii, R.; Bitonto, V.; Ruggiero, M.R.; German, S.; Gorin, D.; Terreno, E. Biodegradable polyelectrolyte/magnetite capsules for mr imaging and magnetic targeting of tumors. Nanotheranostics 2021, 5, 362–377.
- Seitz, S.; Ajiro, H. Self-assembling weak polyelectrolytes for the layer-by-layer encapsulation of paraffin-type phase change material icosane. Sol. Energy Mater. Sol. Cells 2019, 190, 57–64.
- Larrañaga, A.; Isa, I.L.; Patil, V.; Thamboo, S.; Lomora, M.; Fernández-Yague, M.A.; Sarasua, J.-R.; Palivan, C.G.; Pandit, A. Antioxidant functionalized polymer capsules to prevent oxidative stress. Acta Biomater. 2018, 67, 21–31.
- Sharma, V.; Vijay, J.; Ganesh, M.R.; Sundaramurthy, A. Multilayer capsules encapsulating nimbin and doxorubicin for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 582, 119350.
- Huang, J.; Li, C.; Gao, Y.; Cai, Y.; Guo, X.; Cohen Stuart, M.A.; Wang, J. Dendrimer-based polyion complex vesicles: Loops make loose. Macromol. Rapid Commun. 2022, 43, e2100594.
- Hori, M.; Cabral, H.; Toh, K.; Kishimura, A.; Kataoka, K. Robust polyion complex vesicles (PICsomes) under physiological conditions reinforced by multiple hydrogen bond formation derived by guanidinium groups. Biomacromolecules 2018, 19, 4113–4121.
- Koval, V.; Morozova, E.; Revtovich, S.; Lyfenko, A.; Chobanian, A.; Timofeeva, V.; Solovieva, A.; Anufrieva, N.; Kulikova, V.; Demidkina, T. Characteristics and stability assessment of therapeutic methionine γ-Lyase-loaded polyionic vesicles. ACS Omega 2022, 7, 959–967.
- Sueyoshi, D.; Anraku, Y.; Komatsu, T.; Urano, Y.; Kataoka, K. Enzyme-loaded polyion complex vesicles as in vivo nanoreactors working sustainably under the blood circulation: Characterization and functional evaluation. Biomacromolecules 2017, 18, 1189–1196.
- Li, J.; Anraku, Y.; Kataoka, K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew. Chem. Int. Ed. 2020, 59, 13526–13530.
- Seneviratne, R.; Catania, R.; Rappolt, M.; Jeuken, L.J.; Beales, P.A. Membrane mixing and dynamics in hybrid POPC/poly(1,2-butadiene-Block-ethylene oxide) (PBd-b-PEO) lipid/block co-polymer giant vesicles. Soft Matter 2022, 18, 1294–1301.
- Peyret, A.; Ibarboure, E.; Pippa, N.; Lecommandoux, S. Liposomes in polymersomes: Multicompartment System with temperature-triggered release. Langmuir 2017, 33, 7079–7085.
- Wehr, R.; dos Santos, E.C.; Muthwill, M.S.; Chimisso, V.; Gaitzsch, J.; Meier, W. Fully amorphous atactic and isotactic block copolymers and their self-assembly into nano- and microscopic vesicles. Polym. Chem. 2021, 12, 5377–5389.
- Zhou, X.; Cornel, E.J.; Fan, Z.; He, S.; Du, J. Bone-targeting polymer vesicles for effective therapy of osteoporosis. Nano Lett. 2021, 21, 7998–8007.
- Yang, Y.-Y.; Chen, L.-S.; Sun, M.; Wang, C.-Y.; Fan, Z.; Du, J.-Z. Biodegradable polypeptide-based vesicles with intrinsic blue fluorescence for antibacterial visualization. Chin. J. Polym. Sci. 2021, 39, 1412–1420.
- Belluati, A.; Thamboo, S.; Najer, A.; Maffeis, V.; von Planta, C.; Craciun, I.; Palivan, C.G.; Meier, W. Multicompartment polymer vesicles with artificial organelles for signal-triggered cascade reactions including cytoskeleton formation. Adv. Funct. Mater. 2020, 30, 2002949.
- Marušič, N.; Otrin, L.; Zhao, Z.; Lira, R.B.; Kyrilis, F.L.; Hamdi, F.; Kastritis, P.L.; Vidaković-Koch, T.; Ivanov, I.; Sundmacher, K.; et al. Constructing artificial respiratory chain in polymer compartments: Insights into the interplay between Bo3 oxidase and the membrane. Proc. Natl. Acad. Sci. USA 2020, 117, 15006–15017.
- Go, Y.K.; Kambar, N.; Leal, C. Hybrid unilamellar vesicles of phospholipids and block copolymers with crystalline domains. Polymers 2020, 12, 1232.
- Khan, S.; McCabe, J.; Hill, K.; Beales, P.A. Biodegradable hybrid block copolymer—Lipid vesicles as potential drug delivery systems. J. Colloid Interface Sci. 2020, 562, 418–428.
- Cao, S.; Xia, Y.; Shao, J.; Guo, B.; Dong, Y.; Pijpers, I.A.; Zhong, Z.; Meng, F.; Abdelmohsen, L.K.; Williams, D.S.; et al. Biodegradable polymersomes with structure inherent fluorescence and targeting capacity for enhanced photo-dynamic therapy. Angew. Chem. Int. Ed. 2021, 60, 17629–17637.
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic polymersome nanoreactors with tumor-specific activable cascade reactions for cooperative cancer therapy. ACS Nano 2019, 13, 2357–2369.
- Li, J.; Ge, Z.; Toh, K.; Liu, X.; Dirisala, A.; Ke, W.; Wen, P.; Zhou, H.; Wang, Z.; Xiao, S.; et al. Enzymatically transformable polymersome-based nanotherapeutics to eliminate minimal relapsable cancer. Adv. Mater. 2021, 33, 2105254.
- Blackman, L.D.; Varlas, S.; Arno, M.C.; Houston, Z.H.; Fletcher, N.L.; Thurecht, K.J.; Hasan, M.; Gibson, M.I.; O’Reilly, R.K. Confinement of therapeutic enzymes in selectively permeable polymer vesicles by polymerization-induced self-assembly (pisa) reduces antibody binding and proteolytic susceptibility. ACS Cent. Sci. 2018, 4, 718–723.
- Cao, J.; Wei, Y.; Zhang, Y.; Wang, G.; Ji, X.; Zhong, Z. Iodine-rich polymersomes enable versatile SPECT/CT imaging and potent radioisotope therapy for tumor in vivo. ACS Appl. Mater. Interfaces 2019, 11, 18953–18959.
- Kim, B.S.; Naito, M.; Chaya, H.; Hori, M.; Hayashi, K.; Min, H.S.; Yi, Y.; Kim, H.J.; Nagata, T.; Anraku, Y.; et al. Noncovalent stabilization of vesicular polyion complexes with chemically modified/single-stranded oligonucleotides and peg-b-guanidinylated polypeptides for intracavity encapsulation of effector enzymes aimed at cooperative gene knockdown. Biomacromolecules 2020, 21, 4365–4376.
- Choi, H.; Lee, G.-H.; Kim, K.S.; Hahn, S.K. Light-guided nanomotor systems for autonomous photothermal cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 2338–2346.
- Sun, J.; Mathesh, M.; Li, W.; Wilson, D.A. Enzyme-powered nanomotors with controlled size for biomedical applications. ACS Nano 2019, 13, 10191–10200.
- Pijpers, I.A.; Cao, S.; Llopis-Lorente, A.; Zhu, J.; Song, S.; Joosten, R.R.; Meng, F.; Friedrich, H.; Williams, D.S.; Sánchez, S.; et al. Hybrid biodegradable nanomotors through compartmentalized synthesis. Nano Lett. 2020, 20, 4472–4480.
- Bueno, C.Z.; Apolinário, A.C.; Duro-Castano, A.; Poma, A.; Pessoa, A.; Rangel-Yagui, C.O.; Battaglia, G. L-asparaginase encapsulation into asymmetric permeable polymersomes. ACS Macro Lett. 2020, 9, 1471–1477.
- Jin, S.-M.; Jeon, J.; Park, M.-K.; Kim, G.H.; Lee, E. Multicompartment vesicles formation by emulsification-induced assembly of poly(ethylene oxide)-block-poly(ε-Caprolactone) and their dual-loading capability. Macromol. Rapid Commun. 2018, 39, 1700545.
- Konishcheva, E.V.; Daubian, D.; Rigo, S.; Meier, W.P. Probing membrane asymmetry of abc polymersomes. Chem. Commun. 2019, 55, 1148–1151.
- Xiao, Y.; Sun, H.; Du, J. Sugar-breathing glycopolymersomes for regulating glucose level. J. Am. Chem. Soc. 2017, 139, 7640–7647.
- Askes, S.H.; Pomp, W.; Hopkins, S.L.; Kros, A.; Wu, S.; Schmidt, T.; Bonnet, S. Imaging upconverting polymersomes in cancer cells: Biocompatible antioxidants brighten triplet-triplet annihilation upconversion. Small Weinh. Bergstr. Ger. 2016, 12, 5579–5590.
- Kulikova, V.V.; Morozova, E.A.; Anufrieva, N.V.; Koval, V.S.; Lyfenko, A.D.; Lesnova, E.I.; Kushch, A.A.; Revtovich, S.V.; Demidkina, T.V. Kinetic and pharmacokinetic characteristics of therapeutic methinonine γ-Lyase encapsulated in polyion complex vesicles. Biochimie 2022, 194, 13–18.
- Garni, M.; Einfalt, T.; Goers, R.; Palivan, C.G.; Meier, W. Live follow-up of enzymatic reactions inside the cavities of synthetic giant unilamellar vesicles equipped with membrane proteins mimicking cell architecture. ACS Synth. Biol. 2018, 7, 2116–2125.
- Maffeis, V.; Belluati, A.; Craciun, I.; Wu, D.; Novak, S.; Schoenenberger, C.-A.; Palivan, C.G. Clustering of catalytic nanocompartments for enhancing an extracellular non-native cascade reaction. Chem. Sci. 2021, 12, 12274–12285.
- Thamboo, S.; Najer, A.; Belluati, A.; von Planta, C.; Wu, D.; Craciun, I.; Meier, W.; Palivan, C.G. Mimicking cellular signaling pathways within synthetic multicompartment vesicles with triggered enzyme activity and induced ion channel recruitment. Adv. Funct. Mater. 2019, 29, 1904267.
- Messager, L.; Burns, J.R.; Kim, J.; Cecchin, D.; Hindley, J.; Pyne, A.L.; Gaitzsch, J.; Battaglia, G.; Howorka, S. Biomimetic hybrid nanocontainers with selective permeability. Angew. Chem. Int. Ed. 2016, 55, 11106–11109.
- Hosta-Rigau, L.; York-Duran, M.J.; Zhang, Y.; Goldie, K.N.; Städler, B. Confined Multiple enzymatic (cascade) reactions within poly(dopamine)-based capsosomes. ACS Appl. Mater. Interfaces 2014, 6, 12771–12779.
- Matoori, S.; Bao, Y.; Schmidt, A.; Fischer, E.J.; Ochoa-Sanchez, R.; Tremblay, M.; Oliveira, M.M.; Rose, C.F.; Leroux, J.-C. An investigation of PS-b-PEO polymersomes for the oral treatment and diagnosis of hyperammonemia. Small 2019, 15, 1902347.
- Kulygin, O.; Price, A.D.; Chong, S.-F.; Städler, B.; Zelikin, A.N.; Caruso, F. Subcompartmentalized polymer hydrogel capsules with selectively degradable carriers and subunits. Small 2010, 6, 1558–1564.
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable hydrophobic injectable polymers for drug delivery and regenerative medicine. Adv. Funct. Mater. 2021, 31, 2010284.
- Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Biomolecules turn self-assembling amphiphilic block co-polymer platforms into biomimetic interfaces. Front. Chem. 2019, 6, 645.
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent advancements in polymer/liposome assembly for drug delivery: From surface modifications to hybrid vesicles. Polymers 2021, 13, 1027.
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610.
- Itel, F.; Najer, A.; Palivan, C.G.; Meier, W. Dynamics of membrane proteins within synthetic polymer membranes with large hydrophobic mismatch. Nano Lett. 2015, 15, 3871–3878.
- Lomora, M.; Itel, F.; Dinu, I.A.; Palivan, C.G. Selective ion-permeable membranes by insertion of biopores into polymersomes. Phys. Chem. Chem. Phys. 2015, 17, 15538–15546.
- Garni, M.; Thamboo, S.; Schoenenberger, C.-A.; Palivan, C.G. Biopores/membrane proteins in synthetic polymer membranes. Biochim. Biophys. Acta BBA Biomembr. 2017, 1859, 619–638.
- Draghici, C.; Kowal, J.; Darjan, A.; Meier, W.; Palivan, C.G. “Active surfaces” formed by immobilization of enzymes on solid-supported polymer membranes. Langmuir 2014, 30, 11660–11669.
- Einfalt, T.; Witzigmann, D.; Edlinger, C.; Sieber, S.; Goers, R.; Najer, A.; Spulber, M.; Onaca-Fischer, O.; Huwyler, J.; Palivan, C.G. Biomimetic artificial organelles with in vitro and in vivo activity triggered by reduction in microenvironment. Nat. Commun. 2018, 9, 1127.
- Edlinger, C.; Einfalt, T.; Spulber, M.; Car, A.; Meier, W.; Palivan, C.G. Biomimetic strategy to reversibly trigger functionality of catalytic nanocompartments by the insertion of PH-responsive biovalves. Nano Lett. 2017, 17, 5790–5798.
- Sun, X.; Liu, C.; Liu, D.; Li, P.; Zhang, N. Novel biomimetic vectors with endosomal-escape agent enhancing gene transfection efficiency. Int. J. Pharm. 2012, 425, 62–72.
- Rehman, Z.U.; Hoekstra, D.; Zuhorn, I.S. Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: Real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano 2013, 7, 3767–3777.
- Wong, A.S.; Mann, S.K.; Czuba, E.; Sahut, A.; Liu, H.; Suekama, T.C.; Bickerton, T.; Johnston, A.P.; Such, G.K. Self-assembling dual component nanoparticles with endosomal escape capability. Soft Matter 2015, 11, 2993–3002.
- Gaur, D.; Dubey, N.C.; Tripathi, B.P. Biocatalytic self-assembled synthetic vesicles and coacervates: From single compartment to artificial cells. Adv. Colloid Interface Sci. 2022, 299, 102566.
- Belluati, A.; Mikhalevich, V.; Yorulmaz Avsar, S.; Daubian, D.; Craciun, I.; Chami, M.; Meier, W.P.; Palivan, C.G. How do the properties of amphiphilic polymer membranes influence the functional insertion of peptide pores? Biomacromolecules 2020, 21, 701–715.
- Xing, X.; Ma, W.; Zhao, X.; Wang, J.; Yao, L.; Jiang, X.; Wu, Z. Interaction between surface charge-modified gold nanoparticles and phospholipid membranes. Langmuir 2018, 34, 12583–12589.
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 5577.
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.-H.; Lee, B.L.; Jung, Y.; Yoo, J.-W. Bacteria-targeted clindamycin loaded polymeric nanoparticles: Effect of surface charge on nanoparticle adhesion to MRSA, antibacterial activity, and wound healing. Pharmaceutics 2019, 11, 236.
- Jeon, S.; Clavadetscher, J.; Lee, D.-K.; Chankeshwara, S.; Bradley, M.; Cho, W.-S. Surface charge-dependent cellular uptake of polystyrene nanoparticles. Nanomaterials 2018, 8, 1028.
- Du, X.-J.; Wang, J.-L.; Iqbal, S.; Li, H.-J.; Cao, Z.-T.; Wang, Y.-C.; Du, J.-Z.; Wang, J. The effect of surface charge on oral absorption of polymeric nanoparticles. Biomater. Sci. 2018, 6, 642–650.
- Kohli, A.K.; Alpar, H.O. Potential use of nanoparticles for transcutaneous vaccine delivery: Effect of particle size and charge. Int. J. Pharm. 2004, 275, 13–17.
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287.
- Deprey, K.; Becker, L.; Kritzer, J.; Plückthun, A. Trapped! A critical evaluation of methods for measuring total cellular uptake versus cytosolic localization. Bioconjug. Chem. 2019, 30, 1006–1027.
- Sharma, A.R.; Kundu, S.K.; Nam, J.-S.; Sharma, G.; Priya Doss, C.G.; Lee, S.-S.; Chakraborty, C. Next generation delivery system for proteins and genes of therapeutic purpose: Why and how? BioMed Res. Int. 2014, 2014, e327950.
- Kleineberg, C.; Wölfer, C.; Abbasnia, A.; Pischel, D.; Bednarz, C.; Ivanov, I.; Heitkamp, T.; Börsch, M.; Sundmacher, K.; Vidaković-Koch, T. Light-driven ATP regeneration in diblock/grafted hybrid vesicles. ChemBioChem 2020, 21, 2149–2160.
- Khan, S.; Li, M.; Muench, S.P.; Jeuken, L.J.C.; Beales, P.A. Durable proteo-hybrid vesicles for the extended functional lifetime of membrane proteins in bionanotechnology. Chem. Commun. 2016, 52, 11020–11023.
- Otrin, L.; Witkowska, A.; Marušič, N.; Zhao, Z.; Lira, R.B.; Kyrilis, F.L.; Hamdi, F.; Ivanov, I.; Lipowsky, R.; Kastritis, P.L.; et al. En route to dynamic life processes by SNARE-mediated fusion of polymer and hybrid membranes. Nat. Commun. 2021, 12, 4972.
- Artificial Melanogenesis by Confining Melanin/Polydopamine Production inside Polymersomes-Meyer-2021-Macromolecular Bioscience-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/mabi.202100249 (accessed on 27 April 2022).
- de Freitas, C.F.; Calori, I.R.; da Silva, A.C.P.; de Castro, L.V.; Sato, F.; Pellosi, D.S.; Tessaro, A.L.; Caetano, W.; Hioka, N. PEG-coated vesicles from pluronic/lipid mixtures for the carrying of photoactive erythrosine derivatives. Colloids Surf. B Biointerfaces 2019, 175, 530–544.
- Belluati, A.; Craciun, I.; Palivan, C.G. Bioactive catalytic nanocompartments integrated into cell physiology and their amplification of a native signaling cascade. ACS Nano 2020, 14, 12101–12112.
- Liu, J.; Craciun, I.; Belluati, A.; Wu, D.; Sieber, S.; Einfalt, T.; Witzigmann, D.; Chami, M.; Huwyler, J.; Palivan, C.G. DNA-directed arrangement of soft synthetic compartments and their behavior in vitro and in vivo. Nanoscale 2020, 12, 9786–9799.
- Catalytic Polymersomes to Produce Strong and Long-Lasting Bioluminescence—Nanoscale (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2021/NR/D0NR07178A#!divAbstract (accessed on 27 April 2022).
- Korpidou, M.; Maffeis, V.; Dinu, I.A.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Inverting glucuronidation of hymecromone in situ by catalytic nanocompartments. J. Mater. Chem. B 2022.
- Langowska, K.; Palivan, C.G.; Meier, W. Polymer nanoreactors shown to produce and release antibiotics locally. Chem. Commun. 2012, 49, 128–130.
- Meyer, C.E.; Schoenenberger, C.-A.; Wehr, R.P.; Wu, D.; Palivan, C.G. Artificial melanogenesis by confining melanin/polydopamine production inside polymersomes. Macromol. Biosci. 2021, 21, 2100249.




