Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guofei Qin | -- | 1266 | 2022-05-31 11:19:52 | | | |
| 2 | Conner Chen | + 2 word(s) | 1268 | 2022-06-01 03:25:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Qin, G.; , .; Zhu, F.; Zhang, X.; Liang, H.; Yao, J.; Zhang, G. X-ray Single-Crystal Diffraction. Encyclopedia. Available online: https://encyclopedia.pub/entry/23593 (accessed on 10 March 2026).
Qin G, , Zhu F, Zhang X, Liang H, Yao J, et al. X-ray Single-Crystal Diffraction. Encyclopedia. Available at: https://encyclopedia.pub/entry/23593. Accessed March 10, 2026.
Qin, Guofei, , Feng Zhu, Xingwang Zhang, Hong-Bao Liang, Jing-Chun Yao, Guimin Zhang. "X-ray Single-Crystal Diffraction" Encyclopedia, https://encyclopedia.pub/entry/23593 (accessed March 10, 2026).
Qin, G., , ., Zhu, F., Zhang, X., Liang, H., Yao, J., & Zhang, G. (2022, May 31). X-ray Single-Crystal Diffraction. In Encyclopedia. https://encyclopedia.pub/entry/23593
Qin, Guofei, et al. "X-ray Single-Crystal Diffraction." Encyclopedia. Web. 31 May, 2022.
Copy Citation
X-ray single-crystal diffraction (XRSCD) is regarded as the most reliable strategy for absolute configuration determination. Differences in the X-ray anomalous scattering effect of each atom are used to determine the absolute configuration of molecules and can provide the precise spatial position of all of the atoms in a compound in the solid state, including how the atoms are connected, the molecular conformation, and accurate bond length and bond angle data. The strength of the anomalous scattering effect is proportional to the electron cloud density of the atom, which is manifested as the stronger anomalous scattering effect of the atom with a larger atomic number.
flexible marine natural products
configuration determinations
X-ray single-crystal diffraction
1. Introduction
Oceans are a treasure trove for the discovery of novel small molecules and drugs. To date, more than 35,000 marine natural products (MNPs) have been discovered, and this number is growing at a rate of about 1500 per year (more than 400 papers) [1]. According to the statistics, 71% of the skeletons of new compounds can only be found in marine resources [2]. Meanwhile, it is worth mentioning that there are 13 MNP-sourced drugs that are entering the EU and US drug markets, 4 of which have been approved in the past 3 years [3]. In addition, four drugs of marine origin or containing marine ingredients are in phase III clinical trials; eight are in phase II clinical trials; and twenty are in phase I clinical trials [3][4]. Among them, a considerable number include molecules with flexible backbones, such as eribulin, bryostatin, taltobulin, plocabulin, and monomethyl auristatin E (Figure 1) [5]. Flexible MNPs are attracting considerable critical attention in the development of modern marine drugs.
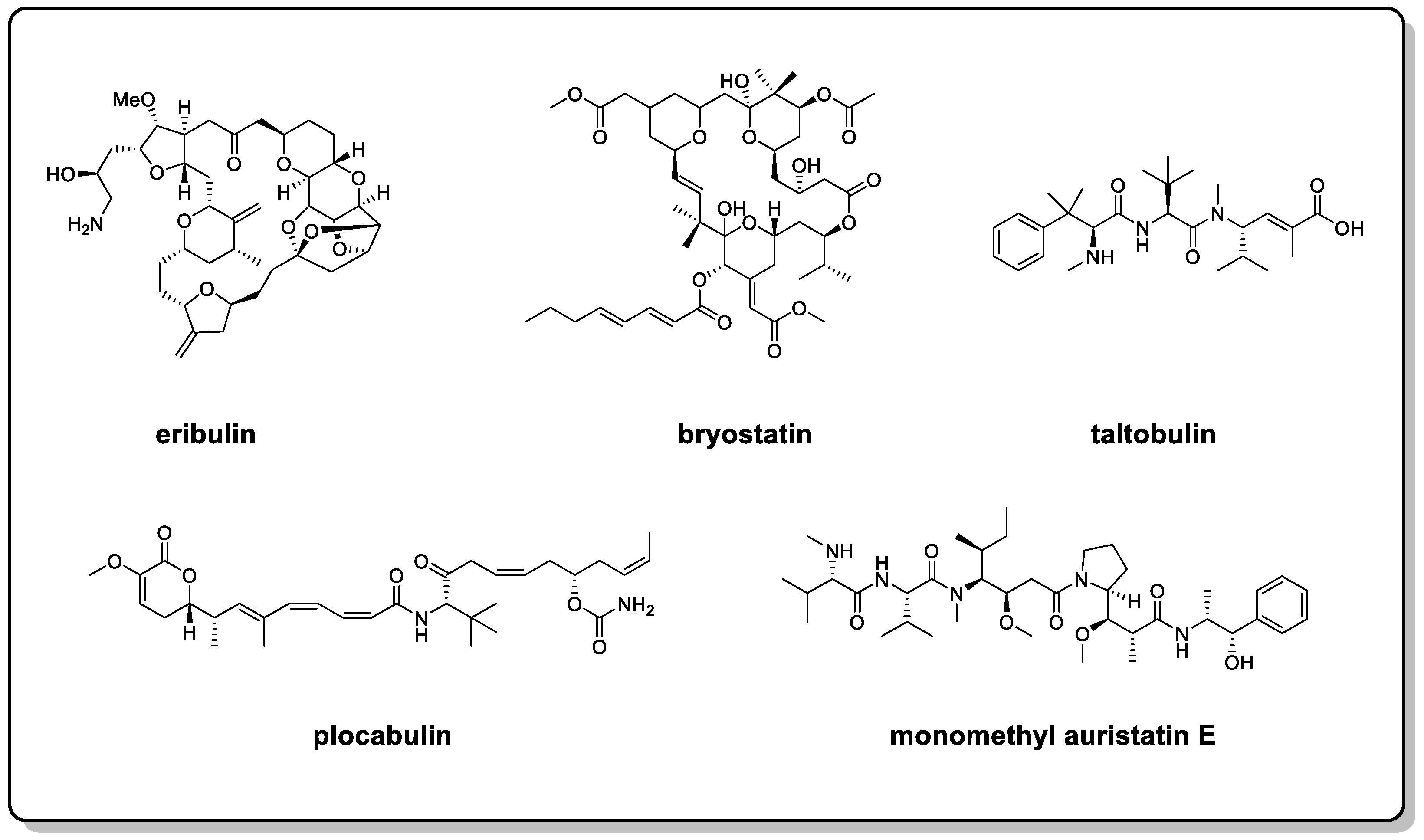
Figure 1. Structures of eribulin, bryostatin, taltobulin, plocabulin, and monomethyl auristatin E.
2. X-ray Single-Crystal Diffraction (XRSCD)
X-ray single-crystal diffraction (XRSCD) is regarded as the most reliable strategy for absolute configuration determination. Differences in the X-ray anomalous scattering effect of each atom are used to determine the absolute configuration of molecules and can provide the precise spatial position of all of the atoms in a compound in the solid state, including how the atoms are connected, the molecular conformation, and accurate bond length and bond angle data [6]. The strength of the anomalous scattering effect is proportional to the electron cloud density of the atom, which is manifested as the stronger anomalous scattering effect of the atom with a larger atomic number [7][8]. The anomalous scattering effect is also positively related to the wavelength of the irradiated radiation. Cu-kα or Mo-kα radiation is usually selected as the light source in single-crystal diffraction experiments. Due to the longer wavelength of Cu-kα rays, its scattering ability is 6–10 times that of Mo-kα. If the molecule to be tested contains heavy atoms, both Cu-kα and Mo-kα radiation can be used to determine its absolute configuration [8]. However, most of the molecules in natural products are mainly composed of C, H, O, and N and do not contain heavy atoms, so their absolute configurations can only be assigned when Cu-kα is used as a radiation source. Furthermore, for crystalline compounds that are devoid of strong scatterers, the combined X-ray/ECD (solid-state CD/TDDFT approach) technique is another effective tactic that can be implemented to solve absolute configuration issues [9]. In crystal structure analysis, the Flack parameter x (0 < x < 1) and its standard uncertainty u are introduced to determine the absolute configuration of the crystal. If u is small (u < 0.04) and the Flack parameter x is close to zero (generally x < 0.1), it indicates that the configuration is correct; otherwise, the correct configuration is its enantiomer [7]. Nevertheless, the bottleneck of this technique is the requirement of preparing a single crystal that is of sufficient size, quality, and stability. Unfortunately, for flexible molecules, it is difficult to generate a suitable crystal because of their special properties (structure, solubility, and molecular weight). Accordingly, several approaches based on X-ray single-crystal diffraction have emerged to address this limitation.
Bastimolide A (1), a macrolide isolated from marine cyanobacterium Okeania hirsute in 2015, possesses a flexible skeleton [10]. Since bastimolide A shows viscous properties in various solvents, it is impossible to crystallize. Thus, derivatization strategies were smoothly pursued by Shao and colleagues to generate crystalline compounds from an organic solvent [10]. Four derivatives (1a–1d) were prepared, three of which were acetonide derivatives, and one of which was a nona-p-nitrobenzoate derivative (Figure 2). The latter (1d) generated suitable crystals, and X-ray single-crystal diffraction was carried out to determine its absolute configuration: 9S, 11R, 15S, 19R, 21R, 23R, 27S, 31R, 35R, 39S (Figure 2). This approach of derivatizing the original molecule to improve its crystallization properties is well established, and Burns and co-workers successfully assigned the relative and absolute structures of alkenes by means of common Mo X-ray analysis using crystalline osmate derivatives [11].
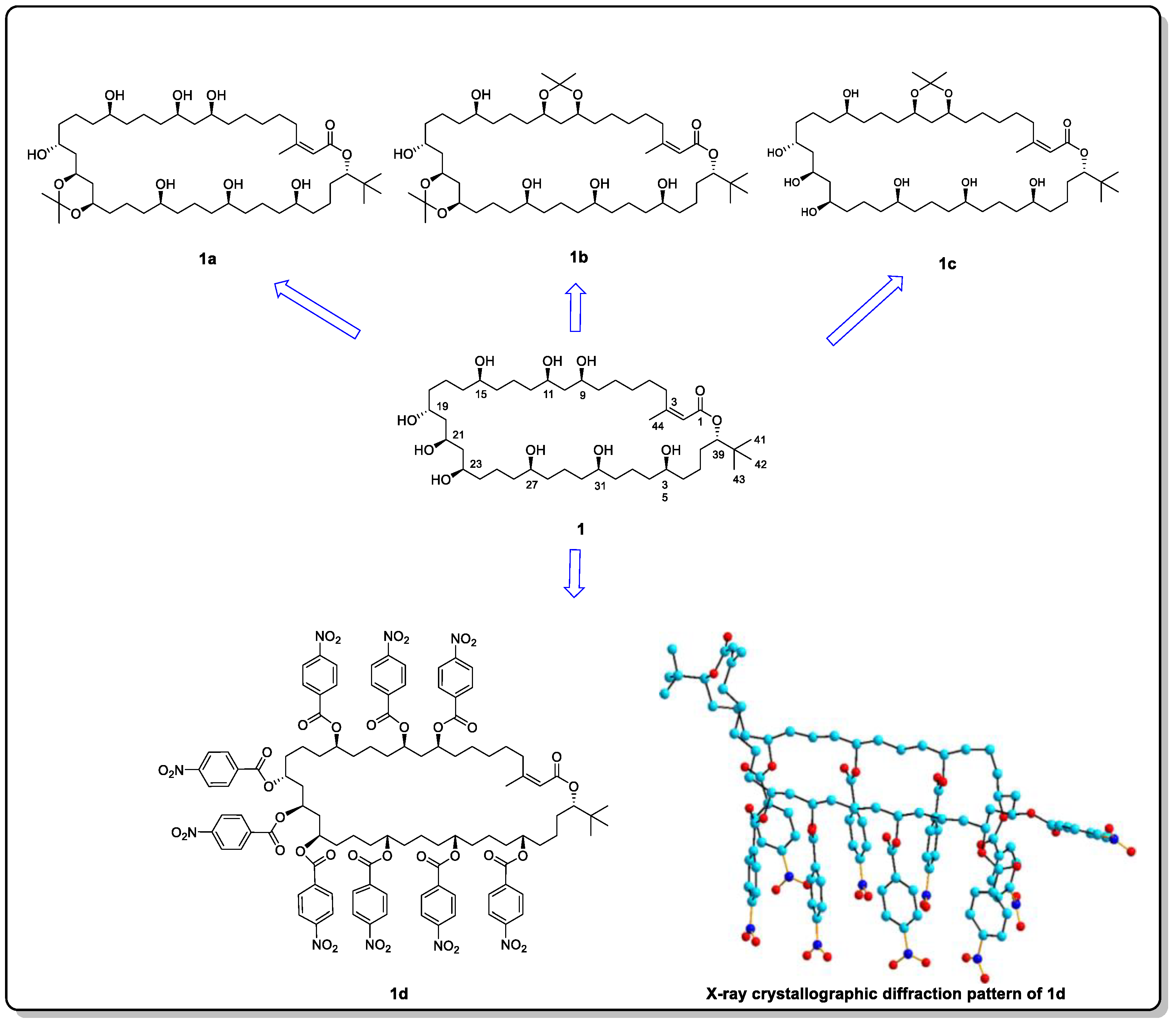
Figure 2. Acetonide derivatives (1a–1c) and nona-p-nitrobenzoate derivatives (1d) of 1, and the X-ray crystallographic diffraction pattern of 1d.
Further, crystallization chaperones can also be used to induce the co-crystallization of target molecules. Crystallization chaperones are molecules that bind covalently or non-covalently to the analyte, helping it to assemble into a crystalline lattice that is then generally applied for protein analysis [12][13]. The Richert group discovered that tetraaryladamantanes (TAAs) are easily co-crystallized with small molecules in the form of inclusive complexes [14]. A total of 3 TAAs were selected as crystallization chaperones for 52 small molecules to evaluate their ability to induce crystallization (Figure 3). A total of 88% of the small molecules were crystallized, and 77% of them obtained high-resolution crystal structures [14]. The results showed that this crystallization chaperone had a great crystallization ability and good versatility. Although marine natural products were not contained in the test sets, several representative flexible compounds, such as 2b–2f and 2s–2w, have demonstrated the potential and suitability of this method to resolve the absolute configuration of flexible skeletons [14].
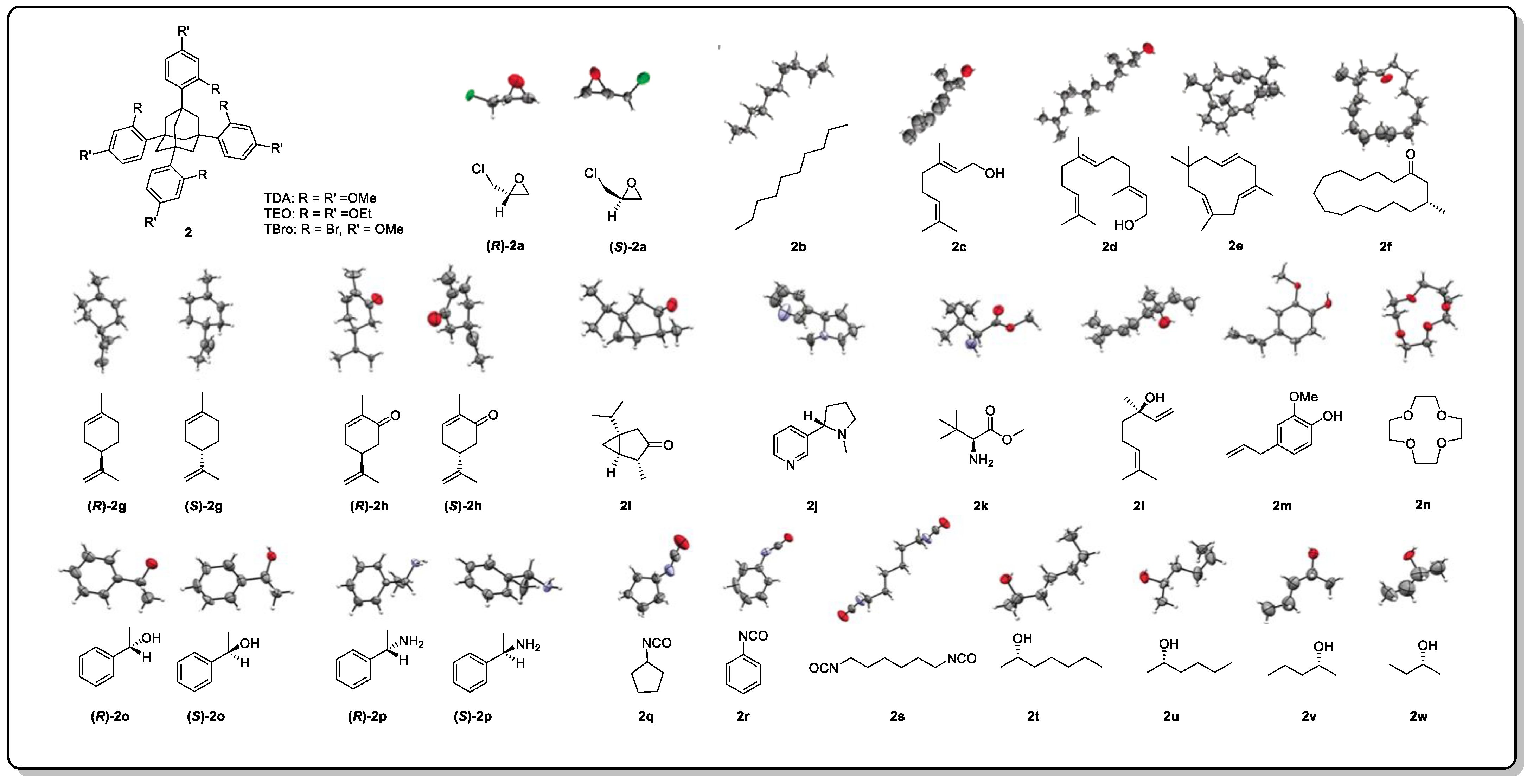
Figure 3. Structures of tetraaryl adamantanes and the X-ray diffraction patterns of some of the test compounds.
In addition, crystalline sponges (CS), single-crystalline porous coordination networks that do not involve sample crystallization, have been proposed. They contain large and regular cavities that can absorb small molecules in the same way that sponges can and arrange them in an orderly manner so that their structures can be determined with X-ray diffraction [15][16][17].
Elatenyne (3) is a marine natural product that possesses a special pseudo mirror-symmetric skeleton and whose absolute configuration cannot be determined by regular methods because of its pseudo-meso core structure [18]. Crystalline sponges greatly contributed to Fujita et al.’s absolute-structure determination of 3 [19]. Additionally, to confirm the accuracy of this method in determining absolute configurations, a crystalline sponge analysis of a click adduct (3a) was performed, achieving positive results. It is worth mentioning that the total amount required for this experiment was only 100 µg, and 95 µg could be easily recovered after the experiment, showing the obvious advantages of using this method.
Using this method, the absolute configuration of miyakosyne A (4), a cytotoxic and flexible acetylene isolated from a marine sponge Petrosia sp., was revealed (Figure 4) [15][20]. However, when comparing the synthetic stereoisomers and subsequent chemical degradation of 4, it was found that the absolute configuration at C14 should be R instead of S [15][21][22] (Figure 5). Although there was an error in the judgment of the absolute configuration of 4, the accuracy of the other compounds that were tested was appreciable. Therefore, by continuously improving CS, it can be believed that this method will be groundbreaking in determining the absolute configurations of complex natural products.
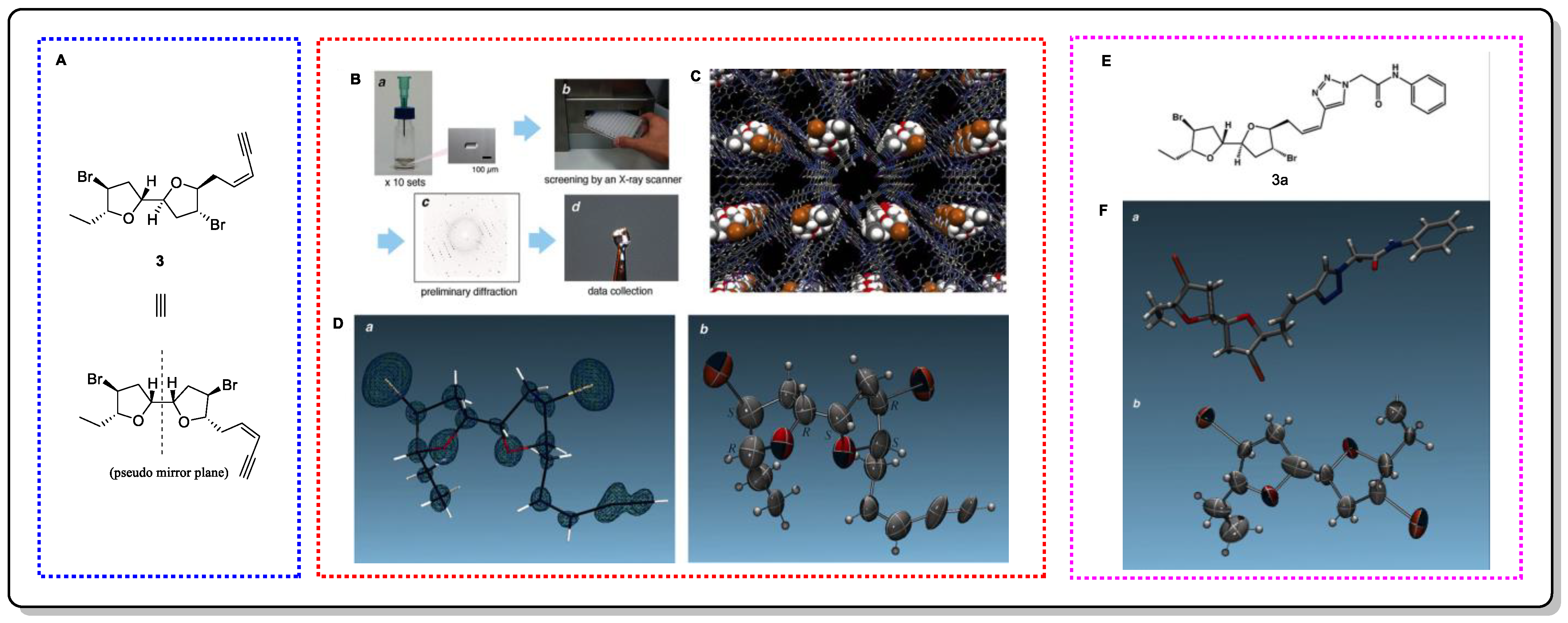
Figure 4. The structure of 3 and its X-ray analysis schematic. (A) Structure of compound 3. (B) Sample preparation. (C) Crystal structure of the guest-absorbed crystalline sponge. (D) Molecular structure of elatenyne found in 3. (E) Structure of 3a. (F) Stick representation of 3a and ORTEP drawing of the elatenyne moiety.
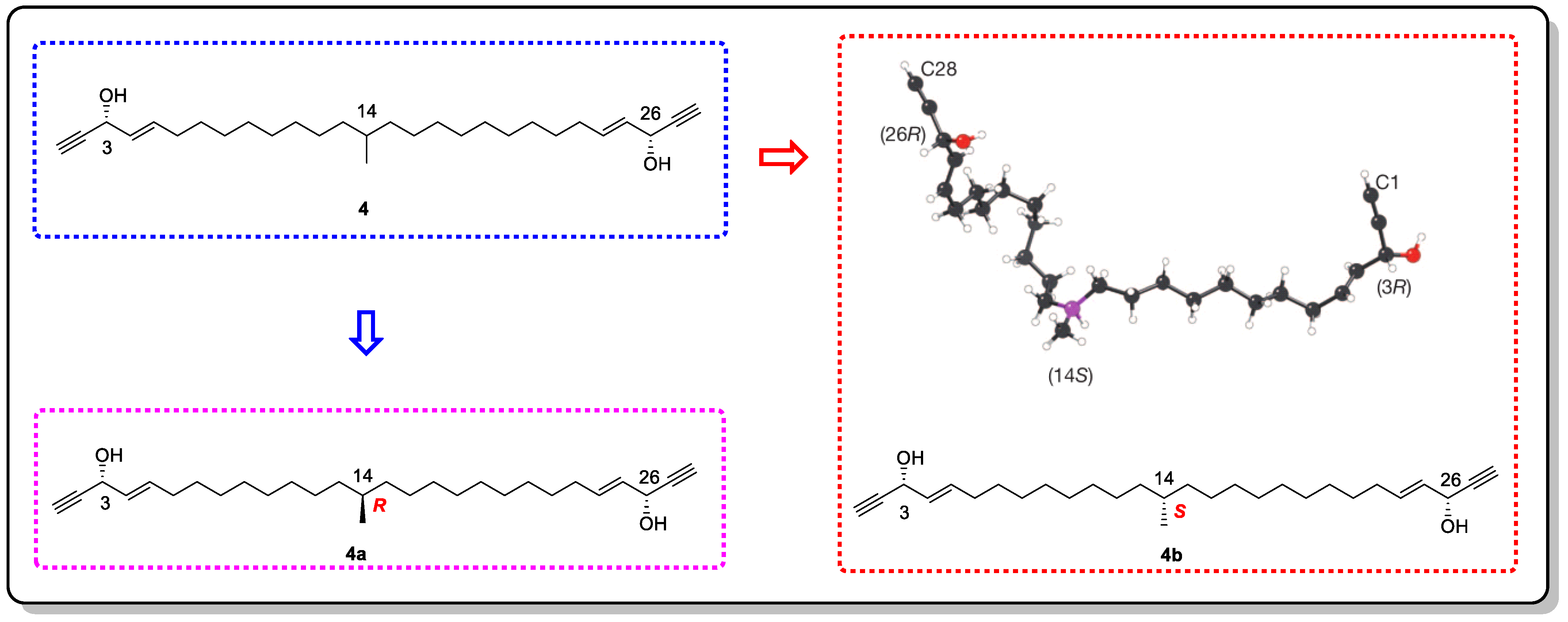
Figure 5. The structure of 4 and its correct configuration (4a) determined by synthesizing the model compound and the wrong configuration (4b) derived from the crystallization sponge method.
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2021, 38, 362–413.
- Lu, W.Y.; Li, H.J.; Li, Q.Y.; Wu, Y.C. Application of marine natural products in drug research. Bioorganic Med. Chem. 2021, 35, 116058.
- Cappello, E.; Nieri, P. From Life in the Sea to the Clinic: The marine drugs approved and under clinical trial. Life 2021, 11, 1390.
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about clinically approved and preclinically investigated marine natural products. Curr. Res. Biotechnol. 2020, 2, 88–102.
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258.
- Valentín-Pérez, A.; Rosa, P.; Hillard, E.A.; Giorgi, M. Chirality determination in crystals. Chirality 2022, 34, 163–181.
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690.
- Thompson, A.L.; Watkin, D.J. X-ray crystallography and chirality: Understanding the limitations. Tertahedron Asymmetry 2009, 20, 712–717.
- Pescitelli, G.; Kurtán, T.; Flörke, U.; Krohn, K. Absolute structural elucidation of natural products—A focus on quantum-mechanical calculations of solid-state CD spectra. Chirality 2009, 21, E181–E201.
- Shao, C.L.; Linington, R.G.; Balunas, M.J.; Centeno, A.; Boudreau, P.; Zhang, C.; Engene, N.; Spadafora, C.; Mutka, T.S.; Kyle, D.E. Bastimolide A, a potent antimalarial polyhydroxy macrolide from the marine cyanobacterium Okeania hirsuta. J. Org. Chem. 2015, 80, 7849–7855.
- Burns, A.S.; Dooley, C.; Carlson, P.R.; Ziller, J.W.; Rychnovsky, S.D. Relative and absolute structure assignments of alkenes using crystalline osmate derivatives for X-ray analysis. Org. Lett. 2019, 21, 10125–10129.
- Johnson, J.L.; Entzminger, K.C.; Hyun, J.; Kalyoncu, S.; Heaner, D.P.; Morales, I.A.; Sheppard, A.; Gumbart, J.C.; Maynard, J.A.; Lieberman, R.L. Structural and biophysical characterization of an epitope-specific engineered Fab fragment and complexation with membrane proteins: Implications for co-crystallization. Acta Crystallogr. Sect. D 2015, 71, 896–906.
- Bhatt, P.M.; Desiraju, G.R. Co-crystal formation and the determination of absolute configuration. CrystEngComm 2008, 10, 1747–1749.
- Krupp, F.; Frey, W.; Richert, C. Absolute configuration of small molecules by co-crystallization. Angew. Chem. Int. Ed. 2020, 59, 15875–15879.
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Hitora, Y.; Takada, K.; Matsunaga, S.; Rissanen, K.; Fujita, M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 2013, 495, 461–466, Erratum in Nature 2013, 501, 262.
- Du, Q.; Peng, J.; Wu, P.; He, H. Review: Metal-organic framework based crystalline sponge method for structure analysis. Trend. Anal. Chem. 2018, 102, 290–310.
- Zigon, N.; Duplan, V.; Wada, N.; Fujita, M. Crystalline sponge method: X-ray structure analysis of small molecules by post-orientation within porous crystals—Principle and proof-of-concept studies. Angew. Chem. Int. Ed. 2021, 60, 25204–25222.
- Hall, J.G.; Reiss, J.A. Elatenyne—A pyranopyranyl vinyl acetylene from the red alga. Aust. J. Chem. 1986, 39, 1401–1409.
- Urban, S.; Brkljača, R.; Hoshino, M.; Lee, S.; Fujita, M. Determination of the absolute configuration of the pseudo-symmetric natural product elatenyne by the crystalline sponge method. Angew. Chem. Int. Ed. 2016, 128, 2728–2732.
- Hitora, Y.; Takada, K.; Okada, S.; Matsunaga, S. Miyakosynes A–F, cytotoxic methyl branched acetylenes from a marine sponge Petrosia sp. Tetrahedron 2011, 67, 4530–4534.
- Hitora, Y.; Takada, K.; Matsunaga, S. On the assignment of the absolute configuration at the isolated methyl branch in miyakosyne A, cytotoxic linear acetylene, from the deep-sea marine sponge Petrosia sp. Tetrahedron 2013, 69, 11070–11073.
- Mori, K.; Akasaka, K.; Matsunaga, S. Chemoenzymatic synthesis and HPLC analysis of the stereoisomers of miyakosyne A , a cytotoxic metabolite of a marine sponge Petrosia sp., to determine the absolute configuration of its major component as 3R,14R,26R. Tetrahedron 2014, 70, 392–401.
More
Information
Subjects:
Medical Laboratory Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
01 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





