Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christophe Le Terrier | -- | 3109 | 2022-05-30 11:31:22 | | | |
| 2 | Catherine Yang | + 10 word(s) | 3119 | 2022-05-31 03:02:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Le Terrier, C.; Ackfeld, T.; , .; Youcef, G. Blood Transfusion Reactions. Encyclopedia. Available online: https://encyclopedia.pub/entry/23554 (accessed on 07 March 2026).
Le Terrier C, Ackfeld T, , Youcef G. Blood Transfusion Reactions. Encyclopedia. Available at: https://encyclopedia.pub/entry/23554. Accessed March 07, 2026.
Le Terrier, Christophe, Theresa Ackfeld, , Guechi Youcef. "Blood Transfusion Reactions" Encyclopedia, https://encyclopedia.pub/entry/23554 (accessed March 07, 2026).
Le Terrier, C., Ackfeld, T., , ., & Youcef, G. (2022, May 30). Blood Transfusion Reactions. In Encyclopedia. https://encyclopedia.pub/entry/23554
Le Terrier, Christophe, et al. "Blood Transfusion Reactions." Encyclopedia. Web. 30 May, 2022.
Copy Citation
Blood transfusions have been the cornerstone of life support since the introduction of the ABO classification in the 20th century. The physiologic goal is to restore adequate tissue oxygenation when the demand exceeds the offer. Although it can be a life-saving therapy, blood transfusions can lead to serious adverse effects, and it is essential that physicians remain up to date with the current literature and are aware of the pathophysiology, initial management and risks of each type of transfusion reaction.
erythrocyte transfusion
blood cell transfusion
anemia treatment
adverse transfusion reactions
1. Epidemiology
Mortality, defined as deaths per total number of transfusions, differs between the different countries, but so does the reporting rate of transfusion reactions. Germany especially seems to underestimate the rate of minor adverse events, since hemovigilance reporting is only required for serious reactions and the PEI only sporadically receives information on non-serious events, so that these were not included in their evaluation [1].
The numbers presented here for different countries should, however, be evaluated carefully. Not only do the reporting systems and the designations of imputability differ from one country to another but also the definition of adverse events. In Germany, for example, a febrile non-hemolytic transfusion reaction (FNHTR) is defined as a fever ≥39 °C with an increase of ≥2 °C compared with the value before transfusion, versus ≥38 °C with an increase of ≥1 °C following the definition of the National Healthcare Safety Network (NHSN) [1][2]. Other authors also noted great variability in transfusion-associated adverse event rates in different countries, mostly due to passive surveillance, different definitions of each transfusion reaction and the use of different blood products, underlining the difficulty of interpretation of these hemovigilance data [3].
2. Definition of Blood Transfusion Reactions and Initial Management
Transfusion reactions are defined as adverse events following a blood transfusion, with the severity ranging from minor to life-threatening. In a clinical setting, any new symptom or change in vital signs occurring within 24 h of a blood transfusion should be considered a transfusion reaction until proven otherwise [4]. The diagnosis is often difficult to establish, due to a wide range of symptoms that are mostly overlapping. Each adverse event following a blood transfusion should be considered severe until further work-up is performed.
The symptomatic patient should be evaluated immediately. Initial management for all types of transfusion reactions includes stopping the transfusion, keeping the intravenous line open, providing supportive and symptomatic therapy and checking the patient identification and the blood product labeling [5][6]. Figure 1 gives an overview of the definition and specific management of each transfusion reaction.
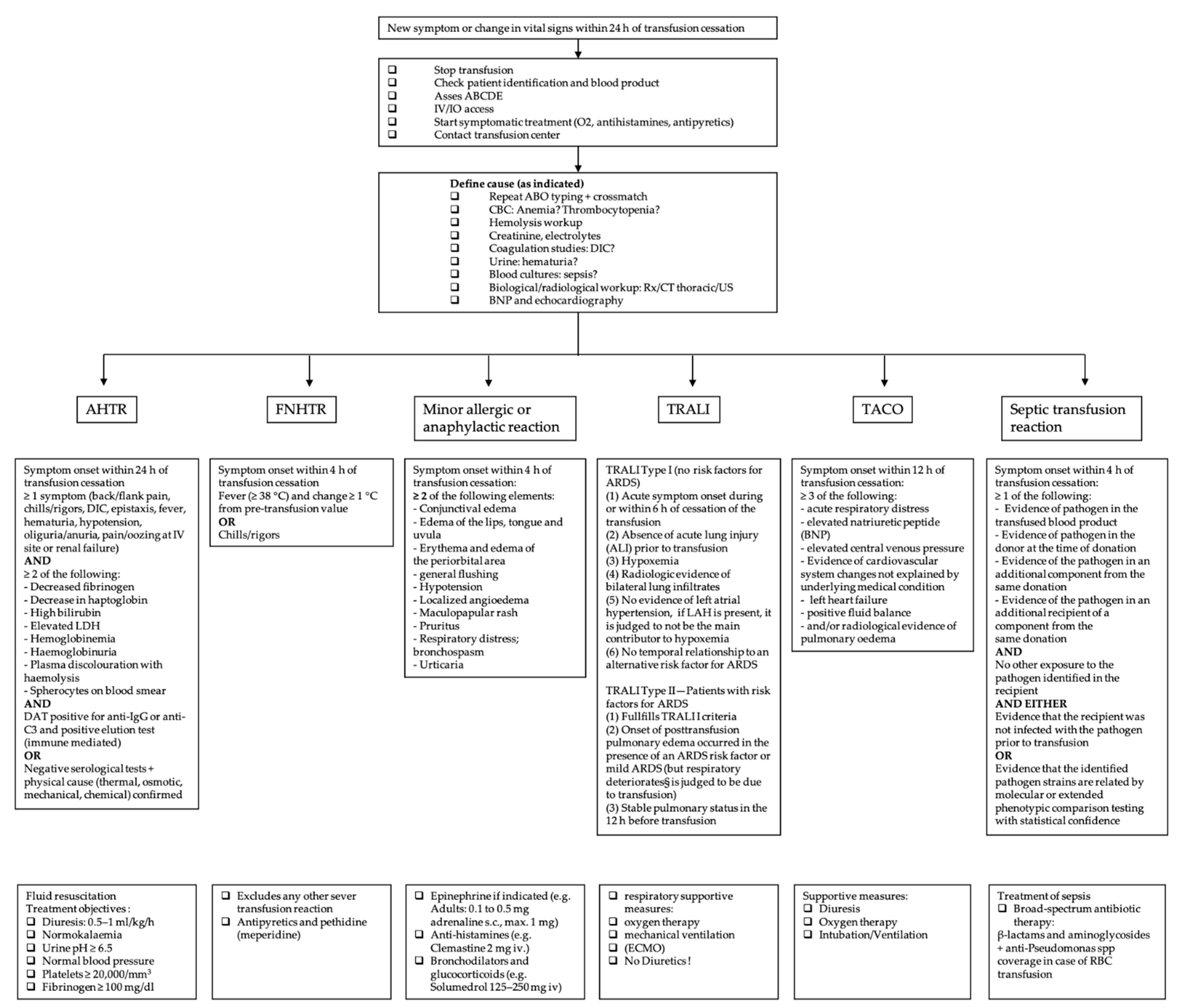
Figure 1. Overview of the most common acute transfusion reactions with treatment propositions. CBC = complete blood count; DIC = disseminated intravascular coagulation; BNP = brain natriuretic peptide; AHTR = acute hemolytic transfusion reaction; FNHTR = febrile non-hemolytic transfusion reaction; TRALI = transfusion-related acute lung injury; TACO = transfusion-associated circulatory overload; LDH = lactate dehydrogenase; ARDS = acute respiratory distress syndrome; LAH = left atrial hypertension; RBC = red blood cell.
3. Acute Hemolytic Transfusion Reaction
Immune-mediated acute hemolytic transfusion reactions (AHTR) are most often related to ABO incompatibility but can also be caused by non-ABO antigens (e.g., irregular antibodies, anti-K, 1 anti-Fya, 1, anti-Jkb, mixed antibodies including anti-Jka, anti-Jkb and anti-Jk3 and anti-E and anti-K). The extent of hemolysis, and therefore the severity of the reaction, depends on multiple factors such as the involved immunoglobulin class, subclass and antibodies. Pathophysiological AHTR involves intravascular or extravascular hemolysis, with or without complement activation. As the expression of ABO antigens on RBC is higher than other antigens, more antigen–antibody complexes are formed, and therefore more sites for complement activation are present. This may explain the severity of ABO incompatibility. Other reasons are the lower titers of irregular antibodies and the dilution in the recipient’s plasma. The volume of the ABO-incompatible blood product may also play a role. A higher mortality is associated with transfused volumes over 200 mL, yet fatal blood transfusions have also been reported with small volumes (25 mL), especially in pediatric patients. Laboratory parameters, however, do not predict the severity [7][8][9].
Despite no clear data being reported, immunological incompatibility seems to be the most frequent cause of hemolytic transfusion reactions, generally caused by misidentification of the patient or the blood sample at the time of collection or transfusion [8]. Therefore, careful pre-transfusion testing is indispensable, in order to match RBC donors and recipients and to prepare immunologically compatible blood products.
Of special importance in the emergency department is the emergency transfusion of non-compatible blood products, which is practiced in trauma centers worldwide. Nevertheless, there is a small but potentially serious risk of acute hemolytic transfusion reactions (<1/1000) that the clinician should be aware of [10].
On the other hand, non-immune-mediated reactions are the result of red blood cell destruction due to mechanical, thermal, chemical or osmotic damage [8].
Symptoms of AHTR usually occur within 24 h of the transfusion [2]. Although the classic triad involves fever, kidney pain and hemoglobinuria, the symptoms can be very varied and non-specific [8]. Patients may present with pruritus, jaundice, hypotension, tachycardia, tachypnoea, pain (at the side of the veinous access or in the renal compartment), nausea, disseminated intravascular coagulation, acute renal failure, shock and even death [2]. Biologically, two of the following elements must be found: a decrease in fibrinogen or haptoglobin, an increase in bilirubin or LDH, hemoglobinuria and hemoglobinemia leading to plasma discoloration or spherocytes on the blood smear. In addition to this work-up, an immune analysis with repeat crossmatching, grouping and an elution test should be performed [2]. Since fever and chills may be the only early signs, it is important to stop the transfusion immediately and begin the diagnostic investigation.
AHTR is a medical emergency. There is no specific treatment, and management consists mainly of fluid resuscitation with a target diuresis of 0.5–1 mL/kg/h. Treatment objectives are normokalaemia, urine pH ≥ 6.5, normal blood pressure, platelets ≥ 20,000/mm3 and fibrinogen ≥ 100 mg/dL [8]. Some authors propose steroids, plasma exchange and continuous hemodiafiltration, and others propose immunoglobulins or complement inhibitors. The pathophysiological mechanism behind this is the elimination of cytokine release in the early phase of AHTR via steroids or plasma exchange (after incompatible blood transfusion plasma exchange therapy removes anti-A or anti-B antibodies, which inhibit the antigen–antibody reaction, and free hemoglobin is also removed, which inhibits disseminated intravascular coagulation and acute kidney injury) or cytokine action, for example, via JAK-STAT inhibitor Ruxolotinib or via Eculizumab, a monoclonal antibody against complement 5 that inhibits the formation of membrane attack complex [11][12][13].
4. Febrile Non-Hemolytic Transfusion Reaction (FNHTR)
FNHTR, with an incidence of 1.44 per 1000 transfusions in 2020 in Switzerland, is a frequent complication [14]. It is characterized by a fever (+>1 °C or >38°) without hemolysis, which may be accompanied by chills, tachypnoea, anxiety, headache, transient hypertension and discomfort within 4 h of transfusion [2][6][15]. Two etiologies are described. In immune-mediated FNHTR, the symptoms are attributable to the release of endogenous pyrogens from white blood cells (WBCs) (either from the patient or the recipient), following a reaction between the recipient’s antibodies and the donor’s antigens [16]. Non-immune-mediated FNHTR is described by the release and accumulation of pro-inflammatory cytokines by WBCs in the blood product during storage. Critical factors are thought to be WBC content and age [15].
5. Anaphylactic Transfusion Reaction (ATR) and Minor Allergic Transfusion Reaction
ATRs and minor transfusion reactions are type 1 hypersensitivity reactions, accounting for 9% of all possible transfusion reactions in Switzerland in 2020 [14]. The severity of these reactions varies from simple skin and mucous membrane damage to upper and lower airway and cardiovascular system involvement. The diagnosis is clinical. Symptoms appear within 4 h of the transfusion and are related to the release of histamine from mast cells and basophils [17]. They do not differ from those of other allergic reactions, and the therapeutic management is superimposable on other anaphylactic reactions [2]. Tryptase blood level can help to confirm the diagnosis but does not rule it out if it is negative (half-life of 2 h). A basophil activation test (BAT) performed with residual transfused blood and the patient’s own blood often confirms the allergic reaction [17]. A simple mucocutaneous reaction does not contraindicate future transfusions.
6. Lung Transfusion Complications
Transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) are serious, life-threatening pulmonary transfusion reactions. Despite the parallels between TACO and TRALI, it is important to distinguish these two diagnoses as their treatment and prevention differs considerably.
7. Transfusion-Related Acute Lung Injury (TRALI)
TRALI was cited in 0.15% of hemovigilance reports in 2020 in Switzerland. This incidence is, however, probably underestimated [14]. Although the reported incidence of TRALI is low, mortality is high; the Food and Drug Administration (FDA) reported a transfusion-associated fatality rate of 27% due to TRALI in the fiscal year 2019, highlighting the importance of recognizing this complication [18].
One reason for this underestimation is a lack of understanding among clinicians, especially due to the difficulty of distinguishing TRALI from other entities. The main differential diagnosis is acute respiratory distress syndrome (ARDS). Several classifications have been developed in order to distinguish TRALI from ARDS and to provide accurate data on adverse transfusion reactions [19][20].
TRALI is an acute non-cardiogenic pulmonary oedema associated with hypoxemia. It was initially classified by the 2004 definition of TRALI and possible TRALI (pTRALI) at the Canadian Consensus Conference CCC [19]. In this definition, patients who present with symptoms of TRALI but who also have ARDS risk factors are classified as pTRALI, to underline the fact that ARDS cannot be excluded. In 2019, these definitions, as well as those for TRALI including criteria for diagnosis, clinical findings, timing of onset and relationship to ARDS risk factors were reconsidered, and a more clinical approach was advocated [20]. This new definition dropped the term pTRALI and defines TRALI type I as new, acute respiratory distress within 6 h of blood transfusion in the absence of temporally associated risk factors for ARDS. The definition is based on five mandatory criteria: (1) absence of acute lung injury prior to transfusion; (2) occurrence of acute lung injury during or within 6 h of cessation of transfusion; (3) hypoxemia; (4) radiographic evidence of bilateral lung infiltrates; and (5) no evidence of left atrial hypertension (LAH) or if LAH is present, it is judged to be not the main contributor to the hypoxemia [2][20]. TRALI type II is defined by three criteria: (1) it must fulfill the same clinical criteria as TRALI type I; (2) the onset of post-transfusion pulmonary edema occurred in the presence of an ARDS risk factor or mild ARDS; and (3) there was a stable pulmonary status in the 12 h before transfusion [20].
TRALI remains a clinical diagnosis, and the clinician’s judgement plays an important role. However, patients with risk factors who develop ARDS within 6 h of transfusion and who already had pulmonary deterioration 12 h before transfusion should be considered as displaying ARDS and not TRALI [21].
8. Transfusion-Associated Circulatory Overload (TACO)
According to the latest version of the National Healthcare Safety Network (NHSN), TACO is defined as the new onset or exacerbation of respiratory symptoms within 12 h of transfusion. This changed from the 2016 definition, which specified symptom onset within 6 h. Three or more of the following must be present: acute respiratory distress, elevated natriuretic peptide (BNP), elevated central venous pressure, left heart failure, positive fluid balance and/or radiological evidence of pulmonary oedema [2]. This definition is also supported by the International Society of Blood Transfusion [22]. TACO is related to circulatory overload, but several studies suggest additional pathophysiological factors [21][23]. The pathophysiological mechanism of TACO is still incompletely understood, and as in TRALI, a “two-hit” theory is proposed [23]. The 2017 study by Parmar et al. highlights a new fever in one third of patients, suggesting other components such as pro-inflammatory reactions that deserve further investigation [24]. A further hint on pathogenesis may be the finding of a 2010 study showing a 50% decrease in the occurrence of TACO with the introduction of universal leukoreduced products [25]. Further studies are needed to shine light on the role of inflammation in TACO. Prevention of TACO and identification of high-risk patients is essential. According to a 2013 retrospective study, the risk factors for developing TACO are a history of heart failure (41%), renal failure (44%) and age over 70 years (65%), and special attention should be played to these patient groups [21]. Swiss data from 2020 also suggest that TACO was mostly observed in the high-risk group (>70 years), with 54 cases of TACO out of a total of 88 cases. This may be due to the unadjusted transfusion rate in the presence of risk factors [14]. Identifying patients at risk and the use of slower transfusion rates in selected patients, as well as prophylactic volume reduction with diuretics, may be beneficial [26][27]. At present, there is no causal treatment, and the management of TACO is similar to that of acute heart failure, consisting of diuresis, oxygen and ventilation or intubation if needed [23].
9. Massive Transfusion-Associated Complications
No universal definition for massive transfusion (MT) is found in the current literature, but the persistent transfusion requirement of >4 packed red cells (approximately 1000 mL) within 1 h or the transfusion of 10 units of packed red blood cells within a 24 h period is a commonly accepted definition in clinical settings [28].
Complication prevention starts with the correct administration of blood products when MT is indicated. Several scores have been developed in order to predict clinical situations that warrant MT, such as the German Trauma-Associated Severe Hemorrhage (TASH) score, with a correct classification rate of over 90%, the Prince of Wales Hospital/Rainer score (PWH score) with a correct classification rate of 97%, the American Vandromme score with a positive predictive value of 75% and many more [29][30][31]. The TASH score, however, is the most well-validated score. The choice of score needs to be individualized, considering the available skill set as well as hospital resources [32].
Furthermore, massive transfusion protocols (MTPs), which are used in most trauma centers, help to standardize the resuscitation approach in traumatic hemorrhagic shock. Figure 2 displays the following information in a flow chart. MTPs provide guidance to the blood bank on the use of blood products and are associated with reduced blood utilization and improved outcomes [33][34][35]. Indications to start the MTP are as follows: blood lactate level ≥ 5 mmol/L; arterial base excess (BE) < −6 mmol/L; blood hemoglobin (Hb) concentration ≤ 9 g/dL; systolic blood pressure (SBP) ≤ 90 mmHg [36]. Although MT improves patient outcome, it is associated with various complications. In addition to the general adverse transfusion reactions, patients receiving MT are especially prone to developing coagulation abnormalities, hypothermia and acidosis. Hypoperfusion and lactate release by RBC during storage, as well as by sodium citrate (an anticoagulant used in stored blood products), further enhance these complications. Hyperkalemia, on the one hand, can occur due to high potassium levels in stored blood products, and cases of hyperkalemic cardiac arrest have been described, especially associated with critically ill patients and fast transfusion rates exceeding 100–150 mL/min [29][37]. Hypokalemia, on the other hand, can develop due to metabolic alkalosis following citrate administration, as well as the use of potassium-poor solutions including crystalloid solutions, platelets and fresh frozen plasma (FFP) [29]. Calcium and magnesium can bind to citrate, and this is used for anticoagulation of the blood products, and ionized calcium levels (total serum calcium concentrations should not be used because of hemodilution, which occurs during resuscitation) and magnesium levels should be monitored and kept in the normal range [29]. In addition, it is important to be aware of increasing bacterial infections in MT patients due to transfusion-related immunomodulation [38].
Post-traumatic hemorrhage, however, is the major cause of death in patients who sustained severe trauma and is generally attributable to two mechanisms: bleeding caused by the direct injury of blood vessels and bleeding due to trauma-induced coagulopathy (TIC) [39]. Approximately one third of patients who receive MT present TIC. TIC is caused by three variables: acute traumatic coagulopathy (ATC), coagulopathy induced by resuscitation maneuvers and detrimental factors such as acidosis, hypothermia, shock, male sex, comorbidities, genetic background, inflammation and premedication, e.g., oral anticoagulants [39]. The pathophysiology of ATC is multifactorial due to protein C activation, endothelial glycocalyx disruption, consumption of fibrinogen and platelet dysfunction, but improper medical management can worsen the outcome [40]. TIC was initially thought to be caused solely by the dilution of clotting factors due to massive transfusion and fluid resuscitation. This was thought to enhance the development of acidosis and hypothermia, also known as the “lethal triad” [6][39]. Newer research, however, has shown that TIC appears early in trauma, before medical intervention, acidemia or hypothermia occurs. ATC and coagulopathy induced by resuscitation can coexist, but pathogenies must be distinguished [39].
When MT protocols are indicated, the RBC target is 7–9 g/dL. Supportive measures such as hypothermia prevention, permissive hypotension, early clotting support, hypovolemic resuscitation and isotonic balanced crystalloids with vasopressors in cases of life-threatening hypotension and shock are the cornerstones of therapy [39]. In order to uncover TIC, early monitoring of coagulation is imperative. When an increase in aPTT, PT and INR is observed (PTT or aPTT > 1.5× normal value), FFP or coagulation factor concentrates (PPCs) are indicated, although PPCs have been proven to be better than FFP for rapidly reversing vitamin K antagonists [39]. Fibrinogen supplementation should be started when under 1.5 g/L (Clauss method), with a suggested initial dose of 3–4 g or 50 mg/kg of concentrated fibrinogen. Platelet concentrates are indicated with a target value of >50 × 109/L or >100 × 109/L in cases of persisting hemorrhage or traumatic brain injury [39].
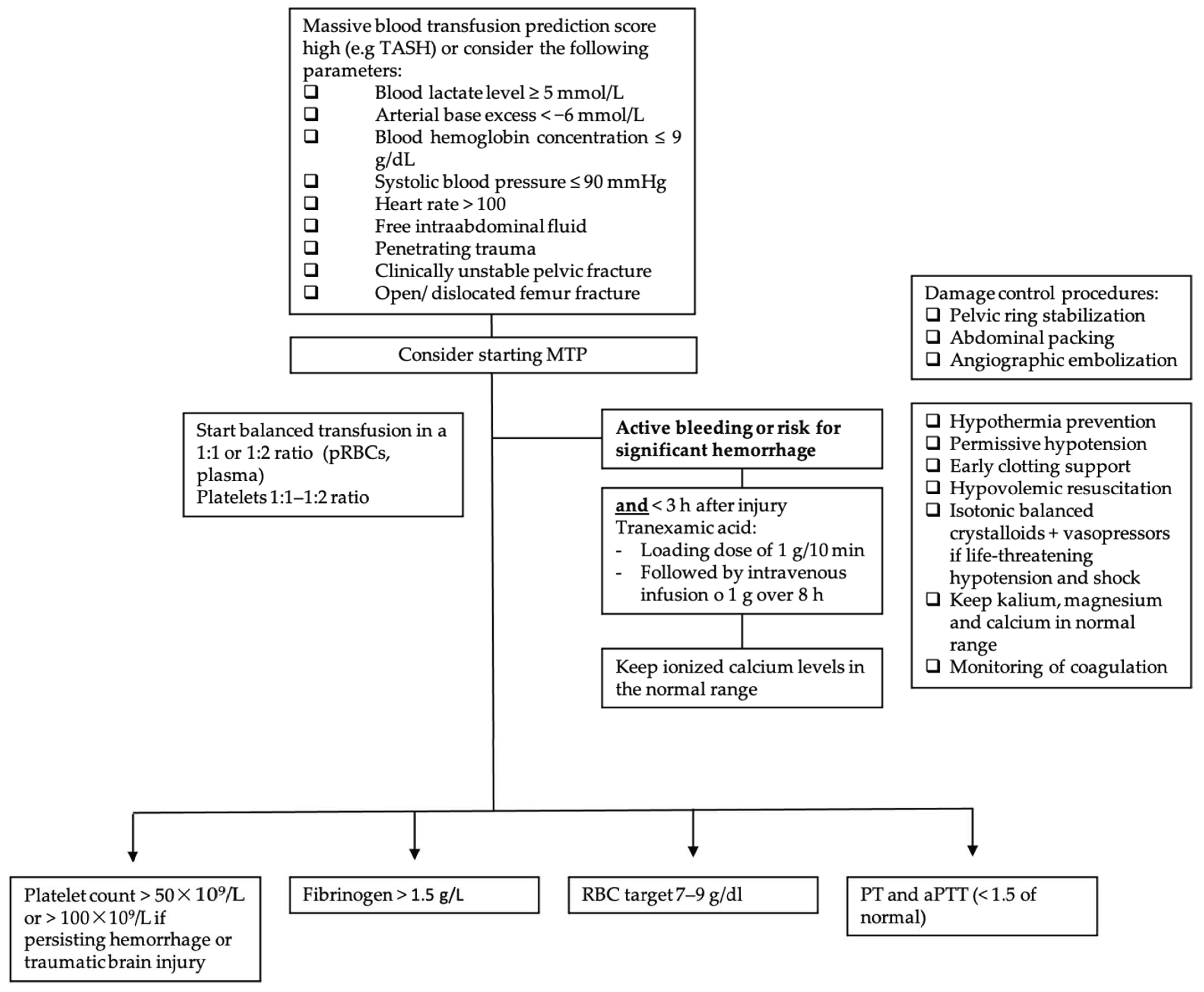
Figure 2. Massive transfusion protocol algorithm. TASH score = Trauma Associated Severe Hemorrhage; RBC = red blood cell; pRBC = packed red blood cell; PCC = prothrombin complex concentrate (PCC); PT = prothrombin time; aPTT = activated partial thromboplastin time; MTP = massive transfusion protocol.
10. Septic Transfusion Reaction
Septic transfusion reactions occur within 4 h of transfusion with fever, hypotension, chills and other signs of bacterial infection (qSOFA criteria) [6].
When post-transfusion bacterial infection is suspected, bacterial samples should be taken from the patient and from every transfused blood product (culture and Gram stain). Definitive diagnosis requires isolation of the same microorganism in the blood sample and in the patient. Bacterial contamination is still presumed in the case of negative cultures in a septic patient with confirmed blood product contamination [41]. However, microorganisms from a positive component culture cannot be interpreted in isolation. Patients with sepsis symptoms at any time should always be investigated with blood cultures [42]. Furthermore, it is important to highlight the vulnerability of residual component cultures to secondary contamination, and results must be carefully evaluated in the clinical context [42]. The most common contaminants found in platelet units are Staphylococcus aureus or Gram-negative organisms caused by skin microbiota after needle insertion, but contaminants may also arise from an asymptomatic donor [43][44][45]. In the case of red blood cells, Gram-negative organisms (Pseudomonas spp., Yersinia spp. and Serratia spp.) in particular are found [46]. It should be noted that mortality increases in cases of contamination with Gram-negative organisms [46]. The treatment of the septic transfusion reaction is superposable on the treatment of sepsis and should cover the most common organisms detected associated with the septic transfusion reaction, with, in particular, a broad-spectrum antibiotic therapy [6]. There are no consensus guidelines, but antimicrobial treatment should be individualized to the local resistance patterns. A parenteral combination of vancomycin and a broad-spectrum beta-lactam or aminoglycoside may cover most likely pathogens.
References
- Funk, M.B.; Heiden, M.; Muller, S. Hämovigilanz-Bericht des Paul-Ehrlich-Instituts 2020: Auswertung der Meldungen von Reaktionen und Zwischenfällen nach § 63i AMG. 2021. Available online: www.pei.de/haemovigilanzbericht (accessed on 26 March 2022).
- National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol. Available online: https://www.cdc.gov/nhsn/pdfs/biovigilance/bv-hv-protocol-current.pdf (accessed on 11 February 2022).
- Rogers, M.A.M.; Rohde, J.M.; Blumberg, N. Haemovigilance of reactions associated with red blood cell transfusion: Comparison across 17 Countries. Vox Sang. 2016, 110, 266–277.
- Elliott, M.; Coventry, A. Critical care: The eight vital signs of patient monitoring. Br. J. Nurs. 2012, 21, 621–625.
- Hoffbrand, A.V.; Higgs, D.R.; Keeling, D.M.; Mehta, A.B. Postgraduate Haematology, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 214–246.
- Delaney, M.; Wendel, S.; Bercovitz, R.S.; Cid, J.; Cohn, C.; Dunbar, N.M.; Apelseth, T.O.; Popovsky, M.; Stanworth, S.J.; Tinmouth, A.; et al. Transfusion reactions: Prevention, diagnosis, and treatment. Lancet 2016, 388, 2825–2836.
- Strobel, E. Hemolytic Transfusion Reactions. Transfus. Med. Hemother. 2008, 35, 346–353.
- Panch, S.R.; Montemayor-Garcia, C.; Klein, H.G. Hemolytic Transfusion Reactions. N. Engl. J. Med. 2019, 381, 150–162.
- Arthur, C.M.; Chonat, S.; Fasano, R.; Yee, M.; Josephson, C.D.; Roback, J.D.; Stowell, S.R. Examining the Role of Complement in Predicting, Preventing, and Treating Hemolytic Transfusion Reactions. Transfus. Med. Rev. 2019, 33, 217–224.
- Fiorellino, J.; Elahie, A.L.; Warkentin, T.E. Acute haemolysis, DIC and renal failure after transfusion of uncross-matched blood during trauma resuscitation: Illustrative case and literature review. Transfus. Med. 2018, 28, 319–325.
- Namikawa, A.; Shibuya, Y.; Ouchi, H.; Takahashi, H.; Furuto, Y. A case of ABO-incompatible blood transfusion treated by plasma exchange therapy and continuous hemodiafiltration. CEN Case Rep. 2018, 7, 114–120.
- Deveci, B.; Saba, R.; Altunay, H.; Toptas, T.; Kublashvilli, G.; Karadogan, I. Severe Acute Hemolytic Transfusion Reaction Treated with Ruxolitinib and Plasma Exchange. Transfus. Med. Hemother. 2021, 48, 250–253.
- Weinstock, C.; Möhle, R.; Dorn, C.; Weisel, K.; Höchsmann, B.; Schrezenmeier, H.; Kanz, L. Successful use of eculizumab for treatment of an acute hemolytic reaction after ABO-incompatible red blood cell transfusion. Transfusion 2015, 55, 605–610.
- Swissmedic. Analyse des Annonces D’hémovigilance. 2020. Available online: https://www.swissmedic.ch/swissmedic/fr/home/humanarzneimittel/marktueberwachung/haemovigilance/haemovigilance-publications-events/haemovigilance-report-2020.html (accessed on 31 January 2022).
- Goel, R.; Tobian, A.A.R.; Shaz, B.H. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood 2019, 133, 1831–1839.
- Addas-Carvalho, M.; Salles, T.S.I.; Saad, S.T.O. The association of cytokine gene polymorphisms with febrile non-hemolytic transfusion reaction in multitransfused patients. Transfus. Med. 2006, 16, 184–191.
- Hirayama, F. Current understanding of allergic transfusion reactions: Incidence, pathogenesis, laboratory tests, prevention and treatment. Br. J. Haematol. 2013, 160, 434–444.
- Food and Drug Administration. Fatalities Reported to FDA Following Blood Collection and Transfusion Annual Summary for FY2019. Available online: https://www.fda.gov/media/147628/download (accessed on 28 March 2022).
- Kleinman, S.; Caulfield, T.; Chan, P.; Davenport, R.; McFarland, J.; McPhedran, S.; Meade, M.; Morrison, D.; Pinsent, T.; Robillard, P.; et al. Toward an understanding of transfusion-related acute lung injury: Statement of a consensus panel. Transfusion 2004, 44, 1774–1789.
- Vlaar, A.P.J.; Toy, P.; Fung, M.; Looney, M.R.; Juffermans, N.P.; Bux, J.; Bolton-Maggs, P.; Peters, A.L.; Silliman, C.C.; Kor, D.J.; et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion 2019, 59, 2465–2476.
- Van den Akker, T.A.; Grimes, Z.M.; Friedman, M.T. Transfusion-Associated Circulatory Overload and Transfusion-Related Acute Lung Injury. Am. J. Clin. Pathol. 2021, 156, 529–539.
- Transfusion-Associated Circulatory Overload (TACO) Definition. 2018. Available online: https://www.aabb.org/docs/default-source/default-document-library/resources/taco-2018-definition.pdf?sfvrsn=e1bcfce4_0 (accessed on 28 March 2022).
- Semple, J.W.; Rebetz, J.; Kapur, R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood 2019, 133, 1840–1853.
- Parmar, N.; Pendergrast, J.; Lieberman, L.; Lin, Y.; Callum, J.; Cserti-Gazdewich, C. The association of fever with transfusion-associated circulatory overload. Vox Sang. 2017, 112, 70–78.
- Blumberg, N.; Heal, J.M.; Gettings, K.F.; Phipps, R.P.; Masel, D.; Refaai, M.A.; Kirkley, S.A.; Fialkow, L.B. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion 2010, 50, 2738–2744.
- Roubinian, N.; Murphy, E.L. Adjusting the Focus on Transfusion-associated Circulatory Overload. Anesthesiology 2017, 126, 363–365.
- Clifford, L.; Jia, Q.; Subramanian, A.; Yadav, H.; Schroeder, D.R.; Kor, D.J. Risk Factors and Clinical Outcomes Associated with Perioperative Transfusion-associated Circulatory Overload. Anesthesiology 2017, 126, 409–418.
- Patil, V.; Shetmahajan, M. Massive transfusion and massive transfusion protocol. Indian J. Anaesth. 2014, 58, 590–595.
- Guerado, E.; Medina, A.; Mata, M.I.; Galvan, J.M.; Bertrand, M.L. Protocols for massive blood transfusion: When and why, and potential complications. Eur. J. Trauma Emerg. Surg. 2016, 42, 283–295.
- Yücel, N.; Lefering, R.; Maegele, M.; Vorweg, M.; Tjardes, T.; Ruchholtz, S.; Neugebauer, E.A.; Wappler, F.; Bouillon, B.; Rixen, D.; et al. Trauma Associated Severe Hemorrhage (TASH)-Score: Probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J. Trauma 2006, 60, 1228–1237.
- Rainer, T.H.; Ho, A.M.-H.; Yeung, J.H.H.; Cheung, N.K.; Wong, R.S.M.; Tang, N.; Ng, S.K.; Wong, G.K.; Lai, P.B.; Graham, C.A. Early risk stratification of patients with major trauma requiring massive blood transfusion. Resuscitation 2011, 82, 724–729.
- Shih, A.W.; Al Khan, S.; Wang, A.Y.-H.; Dawe, P.; Young, P.Y.; Greene, A.; Hudoba, M.; Vu, E. Systematic reviews of scores and predictors to trigger activation of massive transfusion protocols. J. Trauma Acute Care Surg. 2019, 87, 717–729.
- Camazine, M.N.; Hemmila, M.R.; Leonard, J.C.; Jacobs, R.A.; Horst, J.A.; Kozar, R.A.; Bochicchio, G.V.; Nathens, A.B.; Cryer, H.M.; Spinella, P.C. Massive transfusion policies at trauma centers participating in the American College of Surgeons Trauma Quality Improvement Program. J. Trauma Acute Care Surg. 2015, 78 (Suppl. S1), S48–S53.
- Bawazeer, M.; Ahmed, N.; Izadi, H.; McFarlan, A.; Nathens, A.; Pavenski, K. Compliance with a massive transfusion protocol (MTP) impacts patient outcome. Injury 2015, 46, 21–28.
- Cotton, B.A.; Au, B.K.; Nunez, T.C.; Gunter, O.L.; Robertson, A.M.; Young, P.P. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J. Trauma 2009, 66, 41–49.
- Johnson, J.W.; Gracias, V.H.; Schwab, C.W.; Reilly, P.M.; Kauder, D.R.; Shapiro, M.B.; Dabrowski, G.P.; Rotondo, M.F. Evolution in damage control for exsanguinating penetrating abdominal injury. J. Trauma 2001, 51, 261–271.
- Smith, H.M.; Farrow, S.J.; Ackerman, J.D.; Stubbs, J.R.; Sprung, J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: A case series. Anesth. Analg. 2008, 106, 1062–1069.
- Dunne, J.R.; Malone, D.; Tracy, J.K.; Gannon, C.; Napolitano, L.M. Perioperative anemia: An independent risk factor for infection, mortality, and resource utilization in surgery. J. Surg. Res. 2002, 102, 237–244.
- Savioli, G.; Ceresa, I.F.; Caneva, L.; Gerosa, S.; Ricevuti, G. Trauma-Induced Coagulopathy: Overview of an Emerging Medical Problem from Pathophysiology to Outcomes. Medicines 2021, 8, 16.
- Simmons, J.W.; Powell, M.F. Acute traumatic coagulopathy: Pathophysiology and resuscitation. Br. J. Anaesth. 2016, 117 (Suppl. S3), iii31–iii43.
- Eder, A.F.; Goldman, M. How do I investigate septic transfusion reactions and blood donors with culture-positive platelet donations? Transfusion 2011, 51, 1662–1668.
- Martin, I.W.; Cohn, C.S.; Delaney, M.; Fontaine, M.J.; Shih, A.W.; Dunbar, N.M.; SCARED Study Investigators on behalf of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Limitations of current practices in detection of bacterially contaminated blood products associated with suspected septic transfusion reactions. Transfusion 2021, 61, 2414–2420.
- Ramirez-Arcos, S. Bacterial contamination. In Tranfusion Reactions, 4th ed.; Popovsky, M.D., Mark, A., Eds.; AABB (Association for the Advancement of Blood & Biotherapies): Bethesda, MD, USA, 2012.
- Haass, K.A.; Sapiano, M.R.P.; Savinkina, A.; Kuehnert, M.J.; Basavaraju, S.V. Transfusion-transmitted Infections reported to the National Healthcare Safety Network Hemovigilance Module. Transfus. Med. Rev. 2019, 33, 84–91.
- Heroes, A.-S.; Ndalingosu, N.; Kalema, J.; Luyindula, A.; Kashitu, D.; Akele, C.; Kabinda, J.; Lagrou, K.; Vandekerckhove, P.; Jacobs, J.; et al. Bacterial contamination of blood products for transfusion in the Democratic Republic of the Congo: Temperature monitoring, qualitative and semi-quantitative culture. Blood Transfus. 2020, 18, 348–358.
- Kuehnert, M.J.; Roth, V.R.; Haley, N.R.; Gregory, K.R.; Elder, K.V.; Schreiber, G.B.; Arduino, M.J.; Holt, S.C.; Carson, L.A.; Banerjee, S.N.; et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion 2001, 41, 1493–1499.
More
Information
Subjects:
Emergency Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
31 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





