Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liangliang Song | -- | 1324 | 2022-05-30 03:11:26 | | | |
| 2 | Amina Yu | Meta information modification | 1324 | 2022-05-30 05:52:27 | | | | |
| 3 | Amina Yu | Meta information modification | 1324 | 2022-05-30 05:54:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Song, L.; Cai, L. Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles. Encyclopedia. Available online: https://encyclopedia.pub/entry/23528 (accessed on 07 February 2026).
Song L, Cai L. Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles. Encyclopedia. Available at: https://encyclopedia.pub/entry/23528. Accessed February 07, 2026.
Song, Liangliang, Lingchao Cai. "Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles" Encyclopedia, https://encyclopedia.pub/entry/23528 (accessed February 07, 2026).
Song, L., & Cai, L. (2022, May 30). Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles. In Encyclopedia. https://encyclopedia.pub/entry/23528
Song, Liangliang and Lingchao Cai. "Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles." Encyclopedia. Web. 30 May, 2022.
Copy Citation
Microwave-assisted post-Ugi reactions have been employed for the synthesis of diverse polycycles. From commercially available starting materials, transition metal-catalyzed or transition metal-free transformations are performed in an efficient, rapid, and step-economical manner. With the help of microwave irradiation, linear Ugi-adducts are converted into more complex drug-like polycycles with high chemo-, regio-, and stereoselectivity, exhibiting the robustness and potential of this strategy and unfolding a large window for organic synthesis.
microwave
post-Ugi
polycycles
copper
palladium
1. Copper Catalysis
In 2013, Liu and co-workers developed a copper-catalyzed intramolecular arylation of Ugi-adducts [1]. Under microwave heating, diverse tetracyclic benzo[e][1,4]diazepines were synthesized within 40 min in high yields and with excellent chemoselectivities (Scheme 1). Then they changed the amine and acid components, giving a series of new Ugi-adducts [2]. By using the same conditions in 30 min, the Ugi-adducts were performed to deliver various 5,6-dihydroindolo[1,2-a]quinoxalines with excellent yields and chemoselectivities (Scheme 2).
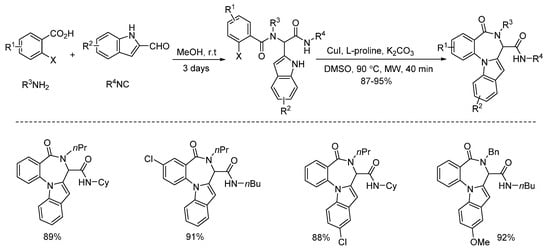
Scheme 1. Copper-catalyzed arylation for the synthesis of tetracyclic benzo[e][1,4]diazepines.
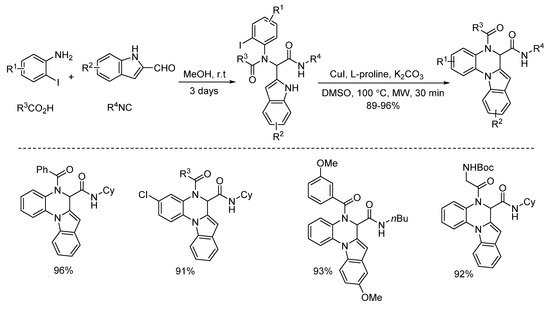
Scheme 2. Synthesis of 5,6-dihydroindolo[1,2-a]quinoxalines via copper catalysis.
Van der Eycken’s group reported a post-Ugi copper-catalyzed intramolecular Ullmann coupling in 2014 (Scheme 3). Microwave assistance promoted the diversity-oriented formation of 4H-benzo[f]imidazo[1,4]diazepin-6-ones with high yields, in 30 min [3]. Ugi-adducts derived from imidazole-4-carbaldehyde and imidazole-2-carbaldehyde reacted smoothly to give the corresponding products. Notably, substrates derived from C-2 or C-5 substituted imidazole-4-carbaldehyde failed to deliver the corresponding products due to the decomposition of the starting materials.
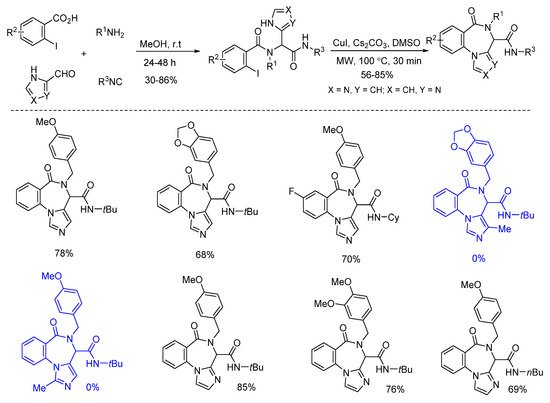
Scheme 3. Synthesis of 4H-benzo[f]imidazo[1,4]diazepin-6-ones through Ullmann coupling.
In 2016, a microwave-assisted intramolecular Ullmann etherification was established by Dai and co-workers for the efficient construction of dibenz[b,f][1,4]oxazepine scaffold. When optimizing the reaction conditions, it was found that conventional heating gave 75% yield after 48 h, while microwave irradiation delivered 64% yield in 30 min, dramatically speeding up the reaction (Scheme 4). Under the optimal reaction conditions of microwave irradiation, they explored the substrate limitation (Scheme 5). By using 2-bromobenzoic acids or 2-bromobenzaldehydes, the diverse 6/7/6-fused tricyclic heterocycles were synthesized from Ugi-adducts via copper-catalyzed Ullmann coupling in 30 min [4]. In contrast with their previous one through copper-catalyzed Goldberg amidation of Ugi-adducts [5], this reaction exhibited excellent chemoselectivity to undergo Ullmann etherification under microwave assistance.
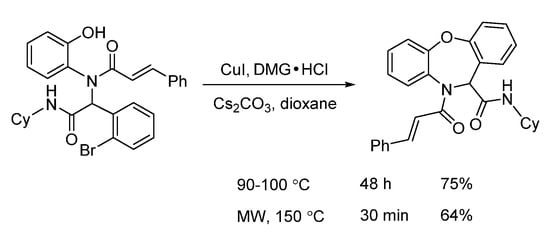
Scheme 4. Intramolecular Ullmann etherification under conventional and microwave heating.
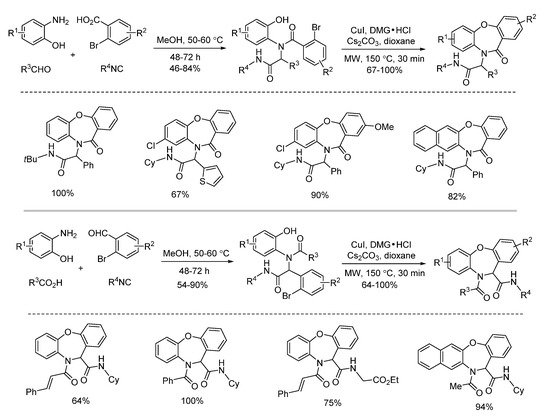
Scheme 5. Construction of dibenz[b,f][1,4]oxazepines via Ullmann etherification.
2. Palladium Catalysis
Gracias and co-workers described a microwave-assisted intramolecular Heck cyclization of Ugi-adducts in 2004 [6]. Through palladium catalysis, highly functionalized N-heterocyclic scaffolds were synthesized with excellent yields in 2 h (Scheme 6).
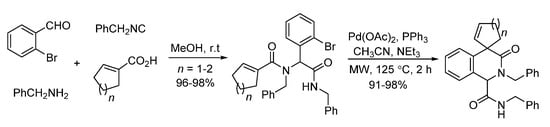
Scheme 6. Microwave-assisted intramolecular Heck cyclization of Ugi-adducts.
In 2007, Judd’s group reported a sequenced RCM/Heck reaction of diverse Ugi-adducts [7]. Under the assistance of microwave heating, various bridged bicyclic lactams were prepared with high yields and diastereoselectivities in 40 min (Scheme 8). Compared to the homogeneous palladium catalyst Pd(Ph3P)2Cl2, the immobilized palladium catalyst FibreCat 1032 showed similar performance for all cases. This method could also give [4.3.2] the bicycloundecane scaffold in high yield and diastereoselectivity, leading to a mixture of the alkene regioisomers (Scheme 7b). The more constrained indole RCM product afforded the bridged indole scaffold with excellent selectivity (Scheme 7c). Interestingly, by using microwave heating, the indole RCM product delivered the saturated bridged bicyclic lactam with high diastereoselectivity using FibreCat 1032 and sodium formate (Scheme 7d).
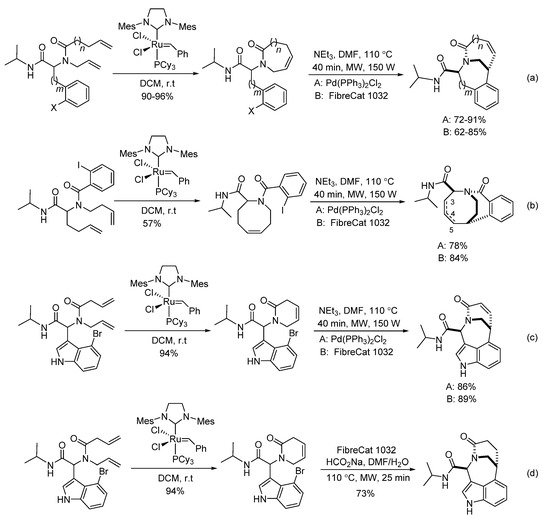
Scheme 7. Sequenced RCM/Heck reactions.
Under microwave irradiation, palladium and copper catalysts showed distinctly different catalytic properties [1][2]. Highly selective C3-arylation of Ugi-adducts was achieved by Liu’s group in 2013 [1]. Under microwave-assisted palladium catalysis, various benzo[5,6]azepino[3,4-b]indoles were constructed with high yields in 1 h (Scheme 8). Subsequently, the same group employed this strategy using the Ugi-adducts derived from 2-halogenated anilines instead of 2-halogenated benzoic acids [2]. With the assistance of microwave irradiation, diverse indole-fused 6,7-dihydroindolo[2,3-c]quinolines were obtained with high yields in 2 h (Scheme 9).
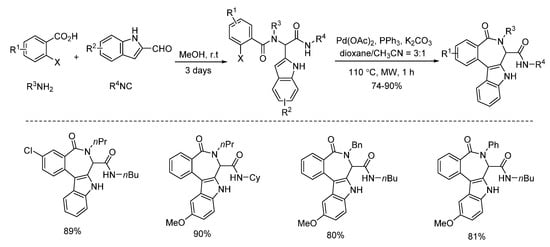
Scheme 8. Synthesis of benzo[5,6]azepino[3,4-b]indoles through palladium catalysis.
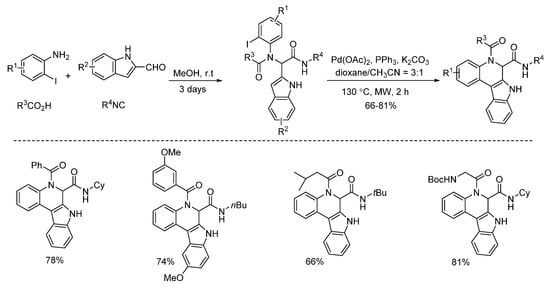
Scheme 9. Preparation of indole-fused 6,7-dihydroindolo[2,3-c]quinolines.
3. Other Transition Metal Catalysis
In 2018, Sieburth and Al-Tel discovered a zinc-catalyzed microwave-assisted post-Ugi cascade transformation (Scheme 10). Based on a build/couple/pair strategy, the diastereoselective synthesis of various chromenopyrroles was performed in a one-pot fashion in 50 min [8]. By using amino acids as chiral auxiliary, this method provided access to enantiopure chromenopyrroles. The polycyclic product was subjected to similar conditions, resulting in pyrrole formation in 62% yield through C-O bond cleavage and isomerization (Scheme 11a). By using 3,4,5-trimethoxy- and 4,5-dimethoxyphenylacetic acids, pentacyclic products were produced via additional intramolecular Friedel–Crafts acylation (Scheme 11b). As proposed, the Ugi-adduct first coordinates with Zn2+, giving intermediate A (Scheme 12). This is followed by nucleophilic addition of the tertiary amide and deprotonation, generating the azomethine ylide C. Sequential intramolecular [3 + 2] cyclization produces the strained intermediate D, which undergoes the expulsion of CO2 to give the desired chromenopyrrole product.
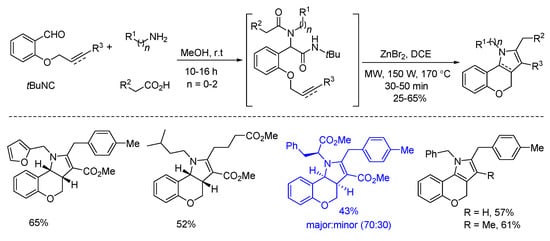
Scheme 10. Zinc-catalyzed post-Ugi transformation for the construction of chromenopyrroles.
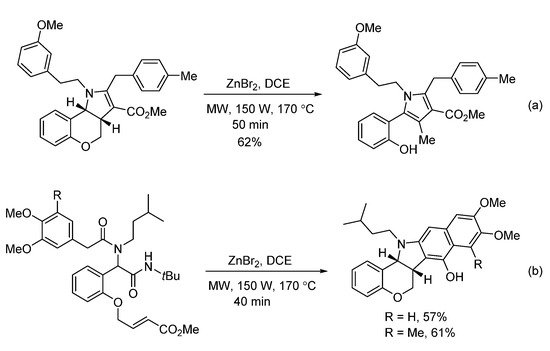
Scheme 11. Special examples for zinc-catalyzed transformation.
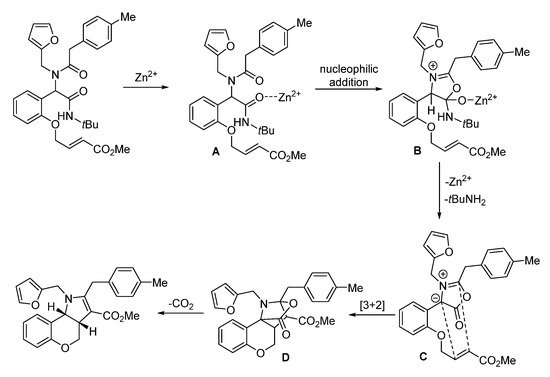
Scheme 12. Proposed mechanism for zinc-catalyzed post-Ugi transformation.
Van der Eycken and co-workers developed a microwave-assisted rhodium(III)-catalyzed intramolecular annulation of Ugi-adducts in 2019. It was observed that microwave heating (100 W) afforded the annulation product in 78% yield, while conventional heating only gave 36% yield in the same reaction time (Scheme 13). Through chemoselective C(sp2)-H activation (Scheme 14), without installing a directing group, modification of peptidomimetics and oligopeptides was accomplished in a rapid and step-economical fashion, delivering various indolizinone and quinolizinone scaffolds in 1 h (Scheme 16). Furthermore, this method was compatible with water and specifically added N-protected amino acids [9].
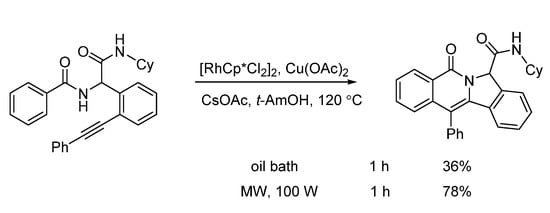
Scheme 13. Rhodium-catalyzed annulation under conventional and microwave heating.
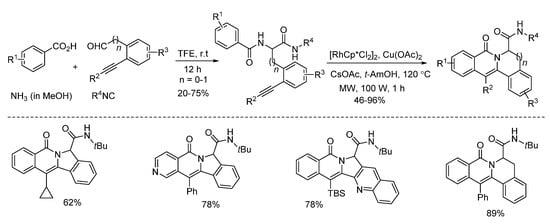
Scheme 14. Microwave-assisted rhodium(III)-catalyzed intramolecular annulation.
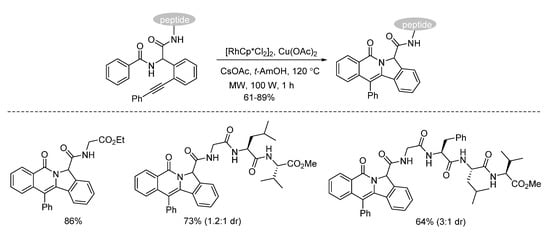
Scheme 15. Chemoselective modification of oligopeptides.
In 2019, Van der Eycken’s group reported a gold-triggered dearomative spirocarbocyclization/Diels–Alder reaction of Ugi-adducts. When optimizing the reaction conditions, it was observed that microwave irradiation could not only accelerate the reaction and enhance the yield, but also significantly improve the diastereomeric ratio (Scheme 16). By using microwave heating (Scheme 17), the diversity-oriented synthesis of complex bridged polycyclic N-heterocycles was achieved with excellent chemo- and diastereoselectivity, in 10 min [10]. According to the mechanism, the triple bond of the Ugi-adducts is activated by the in situ generated cationic gold(I) species, giving intermediate A (Scheme 18). This is followed by dearomatization, generating intermediate B in a 5-endo-dig fashion. The final products are formed by sequential [4 + 2] intramolecular cycloaddition.
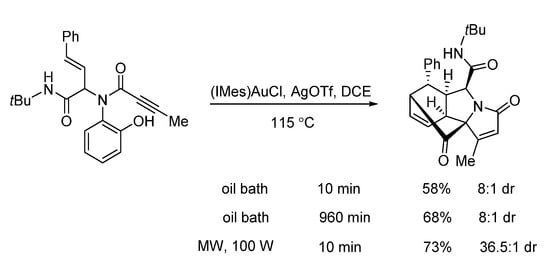
Scheme 16. Gold-triggered spirocarbocyclization under conventional and microwave heating.
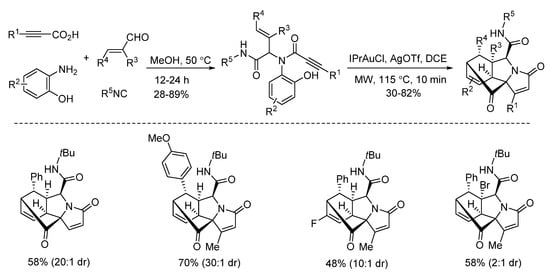
Scheme 17. Gold-triggered dearomative spirocarbocyclization/Diels–Alder reaction.
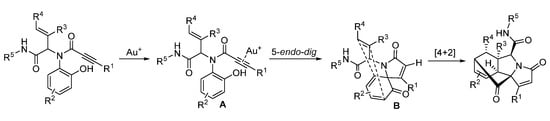
Scheme 18. Proposed mechanism for gold-catalyzed post-Ugi transformation.
4. Transition Metal-Free Catalysis
In 2012, a simultaneous deprotection and cyclization process of Ugi-azide adducts was developed by Hulme (Scheme 19). By using ethyl glyoxylate or phenylglyoxaldehydes, tricyclic tetrazolo-fused benzodiazepines and benzodiazepinones were prepared [11]. This microwave-assisted transformation could be completed in 10 min.
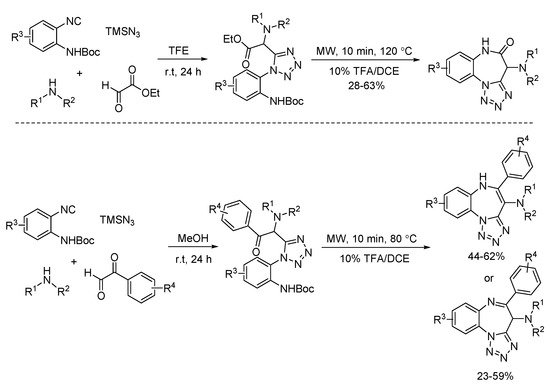
Scheme 19. Microwave-assisted deprotection and cyclization process.
Later on, Gámez-Montaño synthesized the xanthates through one-pot sequential Ugi-azide/N-acylation/SN2 reaction (Scheme 20). Under free radical conditions, intramolecular cyclization of xanthates delivered 3-tetrazolylmethyl-azepino[4,5-b]indol4-ones in good yields. Compared to conventional heating requiring 280 min, microwave irradiation could shorten the reaction time to 70 min [12]. Notably, based on a docking one, the final products showed the potential to inhibit 5-Ht6 protein.
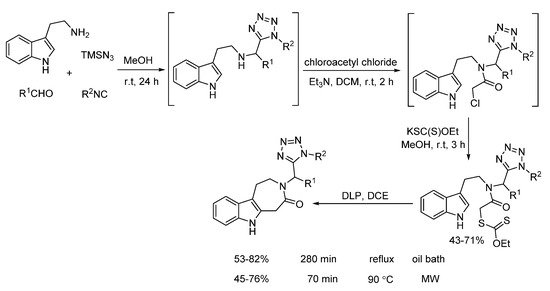
Scheme 20. Intramolecular cyclization of xanthates through radical process.
In 2014, El Kaim and Gámez-Montaño reported an efficient Pictet–Spengler reaction of Ugi-azide adducts and formaldehyde (Scheme 21). Under microwave heating, a series of 2-tetrazolylmethyl-2,3,4,9-tetrahydro1H-β-carbolines was synthesized in 73–83% yield in 5 h, while conventional heating gave 74–86% yield in 72 h, indicating that microwave irradiation remarkably shortens the reaction time of this transition metal-free process [13].
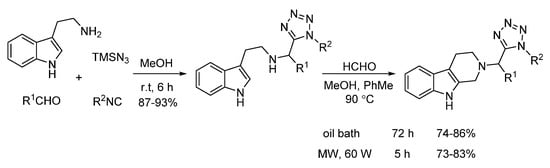
Scheme 21. Microwave-assisted Pictet–Spengler reaction of Ugi-azide adducts.
In 2018, Zhu’s group presented a one pot post-Ugi cyclization through nucleophilic substitution/deprotection/imine formation cascade (Scheme 22). By using microwave irradiation, different kinds of 2-azetidinones were rapidly synthesized in moderate yields [14]. The 2-azetidinones were screened against diverse cancer cell lines, showing good antitumor activities. Based on this method, the same group reported a similar microwave-assisted post-Ugi cyclization [15]. Through tandem deprotection/nucleophilic addition/nucleophilic substitution process, diverse benzimidazopyrazinones were prepared in a one-pot fashion with high yields (Scheme 23). Under the assistance of microwave heating, this transition metal-free protocol required only one purification procedure after three steps.
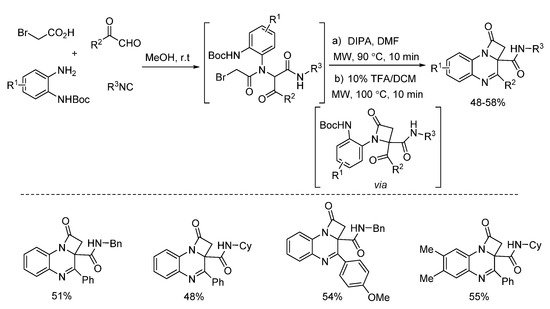
Scheme 22. Microwave-assisted synthesis of 2-azetidinones.
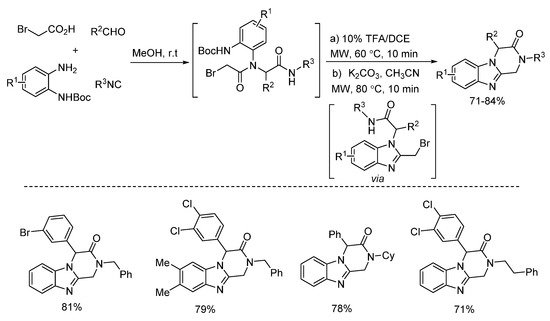
Scheme 23. Transition metal-free protocol for the preparation of benzimidazopyrazinones.
References
- Lei Zhang; Fei Zhao; Mingyue Zheng; Yun Zhai; Hong Liu; Rapid and selective access to three distinct sets of indole-based heterocycles from a single set of Ugi-adducts under microwave heating. Chemical Communications 2013, 49, 2894-2896, 10.1039/c3cc00111c.
- Lei Zhang; Fei Zhao; Mingyue Zheng; Yun Zhai; Jiang Wang; Hong Liu; Selective Synthesis of 5,6-Dihydroindolo[1,2-a]quinoxalines and 6,7-Dihydroindolo[2,3-c]quinolines by Orthogonal Copper and Palladium Catalysis. European Journal of Organic Chemistry 2013, 2013, 5710-5715, 10.1002/ejoc.201300667.
- Zhenghua Li; Laetitia Legras; Amit Kumar; Dipak D. Vachhani; Sunil K. Sharma; Virinder S. Parmar; Erik V. Van der Eycken; Microwave-assisted synthesis of 4H-benzo[f]imidazo[1,4]diazepin-6-ones via a post-Ugi copper-catalyzed intramolecular Ullmann coupling. Tetrahedron Letters 2014, 55, 2070-2074, 10.1016/j.tetlet.2014.02.023.
- Jianyu Shi; Jinlong Wu; Chengsen Cui; Wei-Min Dai; Microwave-Assisted Intramolecular Ullmann Diaryl Etherification as the Post-Ugi Annulation for Generation of Dibenz[b,f][1,4]oxazepine Scaffold. The Journal of Organic Chemistry 2016, 81, 10392-10403, 10.1021/acs.joc.6b01398.
- Gaofeng Feng; Jinlong Wu; Wei-Min Dai; Isolation and characterization of 2-alkylaminobenzo[b]furans. Evidence for competing O-arylation in Cu-catalyzed intramolecular amidation. Tetrahedron Letters 2007, 48, 401-404, 10.1016/j.tetlet.2006.11.084.
- Vijaya Gracias; Joel D. Moore; Stevan W. Djuric; Sequential Ugi/Heck cyclization strategies for the facile construction of highly functionalized N-heterocyclic scaffolds. Tetrahedron Letters 2004, 45, 417-420, 10.1016/j.tetlet.2003.10.147.
- Timothy P. Ribelin; Andrew S. Judd; Irini Akritopoulou-Zanze; Rodger F. Henry; Jeffrey L. Cross; And David N. Whittern; Stevan W. Djuric; Concise Construction of Novel Bridged Bicyclic Lactams by Sequenced Ugi/RCM/Heck Reactions. Organic Letters 2007, 9, 5119-5122, 10.1021/ol7023373.
- Vunnam Srinivasulu; Scott McN. Sieburth; Raafat El-Awady; Noor M. Kariem; Hamadeh Tarazi; Matthew John O’Connor; Taleb H. Al-Tel; Post-Ugi Cascade Transformations for Accessing Diverse Chromenopyrrole Collections. Organic Letters 2018, 20, 836-839, 10.1021/acs.orglett.7b03986.
- Liangliang Song; Guilong Tian; Anna Blanpain; Luc Van Meervelt; Erik V. Van der Eycken; Diversification of Peptidomimetics and Oligopeptides through Microwave‐Assisted Rhodium(III)‐Catalyzed Intramolecular Annulation. Advanced Synthesis & Catalysis 2019, 361, 4442-4447, 10.1002/adsc.201900550.
- Yi He; Thomas Narmon; Danjun Wu; Zhenghua Li; Luc Van Meervelt; Erik V. Van der Eycken; A gold-triggered dearomative spirocarbocyclization/Diels–Alder reaction cascade towards diverse bridged N-heterocycles. Organic & Biomolecular Chemistry 2019, 17, 9529-9536, 10.1039/c9ob01967g.
- Steven Gunawan; Muhammad Ayaz; Fabio De Moliner; Brendan Frett; Christine Kaiser; Nina Patrick; Zhigang Xu; Christopher Hulme; Synthesis of tetrazolo-fused benzodiazepines and benzodiazepinones by a two-step protocol using an Ugi-azide reaction for initial diversity generation. Tetrahedron 2012, 68, 5606-5611, 10.1016/j.tet.2012.04.068.
- Raul E. Gordillo-Cruz; Angel Rentería-Gómez; Alejandro Islas-Jácome; Carlos J. Cortes-García; Erik Díaz-Cervantes; Juvencio Robles; Rocío Gámez-Montaño; Synthesis of 3-tetrazolylmethyl-azepino[4,5-b]indol-4-ones in two reaction steps: (Ugi-azide/N-acylation/SN2)/free radical cyclization and docking studies to a 5-Ht6 model. Organic & Biomolecular Chemistry 2013, 11, 6470-6476, 10.1039/c3ob41349g.
- Laurent El Kaim; Rocío Gámez-Montaño; Luis E. Cárdenas-Galindo; Alejandro Islas-Jácome; Nancy V. Alvarez-Rodríguez; Synthesis of 2-Tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines by a One-Pot Ugi-Azide/Pictet–Spengler Process. Synthesis 2013, 46, 49-56, 10.1055/s-0033-1340051.
- Yong Li; Liu-Jun He; Jia Xu; Yong Ding; Zhi-Gang Xu; Jie Lei; Jiang-Ping Meng; Jin Zhu; Microwave-assisted facile synthesis of 2-azetidinone derivatives and evaluation of their antitumor activities. SCIENTIA SINICA Chimica 2018, 48, 751-758, 10.1360/n032017-00223.
- Gui-Ting Song; Yong Li; Jia Xu; Zhi-Gang Xu; Yong Ding; Jie Lei; Nicholas McConnell; Jin Zhu; Zhong-Zhu Chen; Microwave-assisted facile construction of quinoxalinone and benzimidazopyrazinone derivatives via two paths of post-Ugi cascade reaction. Molecular Diversity 2018, 23, 137-145, 10.1007/s11030-018-9855-y.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
799
Revisions:
3 times
(View History)
Update Date:
30 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




