Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eliza Gruczyńska-Sękowska | -- | 3850 | 2022-05-26 13:36:03 | | | |
| 2 | Rita Xu | Meta information modification | 3850 | 2022-05-27 03:30:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gruczyńska-Sękowska, E.; Koczoñ, P.; , .; Michorowska, S.; Tarnowska, K.; Kowalska, D.; Niemiec, T.; Lipińska, E. Selected Polymers - Structure, Properties and Food-Related Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/23433 (accessed on 03 March 2026).
Gruczyńska-Sękowska E, Koczoñ P, , Michorowska S, Tarnowska K, Kowalska D, et al. Selected Polymers - Structure, Properties and Food-Related Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/23433. Accessed March 03, 2026.
Gruczyńska-Sękowska, Eliza, Piotr Koczoñ, , Sylwia Michorowska, Katarzyna Tarnowska, Dorota Kowalska, Tomasz Niemiec, Edyta Lipińska. "Selected Polymers - Structure, Properties and Food-Related Applications" Encyclopedia, https://encyclopedia.pub/entry/23433 (accessed March 03, 2026).
Gruczyńska-Sękowska, E., Koczoñ, P., , ., Michorowska, S., Tarnowska, K., Kowalska, D., Niemiec, T., & Lipińska, E. (2022, May 26). Selected Polymers - Structure, Properties and Food-Related Applications. In Encyclopedia. https://encyclopedia.pub/entry/23433
Gruczyńska-Sękowska, Eliza, et al. "Selected Polymers - Structure, Properties and Food-Related Applications." Encyclopedia. Web. 26 May, 2022.
Copy Citation
Every application of a substance results from the macroscopic property of the substance that is related to the substance’s microscopic structure. For example, the forged park gate in your city was produced thanks to the malleability and ductility of metals, which are related to the ability of shifting of layers of metal cations, while fire extinguishing powders use the high boiling point of compounds related to their regular ionic and covalent structures. This also applies to polymers.
biopolymer
polysaccharide
food-related applications
enzyme immobilisation
starch
1. Introduction
Various applications of polymer-based materials, ranging from films and coatings for food and packaging, through to the delivery of health-promoting bioactive compounds, to food–body interactions manipulated via nanotechnology, have recently gained interest in fields such as nutrition, medicine, and pharmacology. The number of previous and recent reports call for an urgent need to compile and review the multiple structural aspects of these important materials with relation to their properties and both current and future applications [1][2][3][4].
2. Starch
2.1. Structure
Starch is a branched homopolymer of glucose, usually synthesized in plant amyloplasts to store the energy gained from photosynthesis. Starch comprises two types of polymers: amylose and amylopectin [2]. Amylose (Figure 1) is a linear molecule of glucose units linked by α 1-4 glycosidic bonds, slightly branched by α 1-6 linkages, while amylopectin (Figure 2) is a highly branched polymer consisting of relatively short branches of α 1-4 glycopyranose that are interlinked by α 1-6 glycosidic linkages approximately every 22 glucose units [3]. The larger molecule of amylopectin is branched non-randomly and this organized branching nature of amylopectin creates symmetry and a type of uniformity responsible for the crystallinity of the starch [4], while amylose is found in the amorphous lamella of the starch granule. Observed under a microscope, starch shows birefringence under polarized light, and the specific “Maltese cross” indicating a radial orientation of the macromolecules [3].
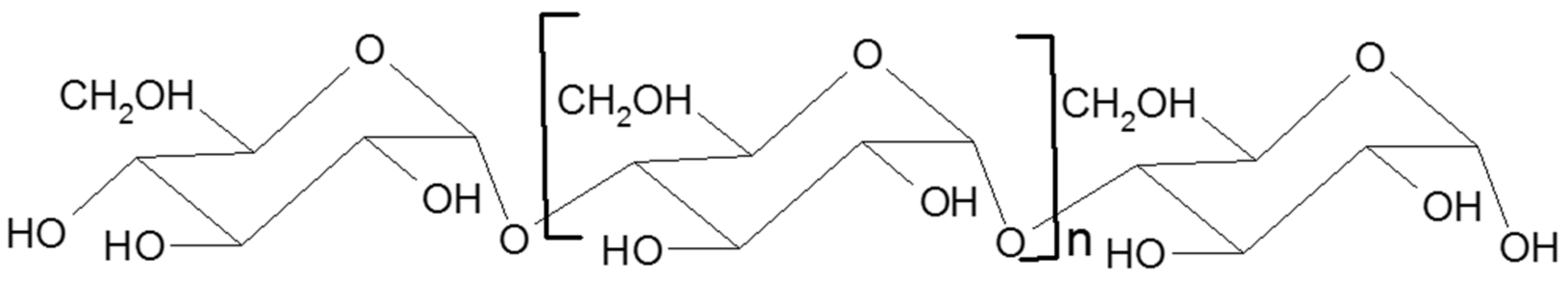
Figure 1. Schematic structure of amylose.
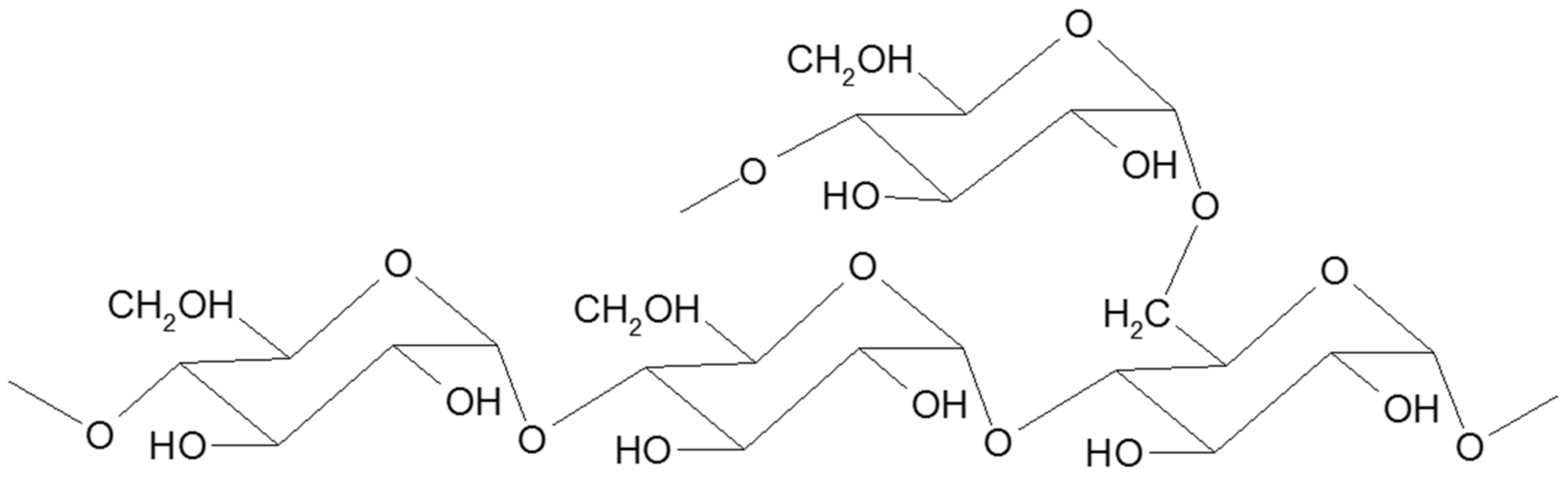
Figure 2. Schematic structure of amylopectin.
2.2. Properties and Applications
Starch is one of the most abundant biopolymers. It is completely biodegradable, renewable, and inexpensive (e.g., much cheaper than polyethylene). Moreover, it is colourless, but easy to colour; odourless, but easy to flavour; edible though tasteless; and easy to mix with other components [5].
Starch is the major source of energy in plants (up to about 90% dry matter). It is usually accumulated in tubers, seeds, and roots. Most starches for the industry are obtained from maize, wheat, cassava, potato, and the recently rediscovered amaranth and quinoa [5]. Starch is the main energy storage carbohydrate in wheat and constitutes approximately 60–75% of grain and 70–80% of flour. It has a vital role to play in human nutrition as it contributes to 90% of caloric intake in developing countries and more than 50% in the Western world [6].
A starch’s nutritive value is linked with the ease of its digestion in the small intestine. Its dietary value is defined by the content of rapidly digestible starch (RDS), slowly digestible starch (SDS), as well as resistant starch (RS). RDS causes a rapid and sharp postprandial rise in blood glucose levels and is therefore not recommended for people with diabetes and obesity. SDS is the fraction of starch that slowly but completely hydrolyses in the small intestine. Due to its stabilising effect on glucose level, it is physiologically more preponderant than RDS. Some benefits of consuming SDS include the prevention and treatment of type II diabetes because it promotes feeling of satiety through the metabolic response, namely postprandial low blood glucose and insulin levels [7].
RS, in turn, is defined as starch and its breakdown products that are not absorbed in the small intestine of a healthy human. RS in the diet exerts a positive effect on the human body as it stimulates the growth of beneficial microbiota and lowers colonic pH, postprandial blood glucose, and cholesterol levels. RS is ideal for use in ready-to-eat breakfast cereals, snacks, pasta, noodles, baked goods, and fried foods, and allows for food to be labelled as simply “starch with added nutraceutical benefits”. Its content in food can be increased by modifying processing conditions such as pH, temperature, number of heating and cooling cycles, and freezing and drying times. RS improves the tenderness of some products, resulting in a better mouthfeel than products made with traditional fibres. RS exhibits physiological properties that may reduce the risk of several diseases, including colon cancer and diabetes, and may be very useful in controlling diabetes and obesity. Products fortified with RS are willingly accepted by consumers for the unique physicochemical properties of RS and its bland taste. RS is for example retrograded starch, produced as a result of cooking and cooling of starchy foods, as well as amylase–lipid complexes produced during thermal processes of starch in the presence of lipids. The potential prebiotic effect of RS, resulting from its ability to be fermented by the colon microbiota, leads to the production of short-chain fatty acids. Chemical, physical, and enzymatic methods to produce SDS and RS with specific structures provide opportunities to tailor both fractions for health benefits [7][8].
Starches are classified based on the proportions of amylose and amylopectin: starches with 25–30% amylose and 70–75% amylopectin are called “normal” starches; starches with very high levels of amylopectin (98–99%) are named “waxy” starches; a third group contains starches with high amylose content (50–70%) and has no specific name [8].
The ratio of amylose to amylopectin in starch profoundly impacts its physico-chemical properties or starch-based industrial products’ features, which in turn affect their functionality and potential applications. Higher amylose content implies increased film strength, while the presence of highly branched amylopectin leads to the formation of films with poor mechanical strength, which needs to be enhanced by adding sorbitol, glycerol, or other plasticizers [9].
Ultrasound assisted (UA) treatment increases swelling power of sweet potato and cereal starches. The damage of the starch granules helps water to penetrate them easily, increasing the starch solubility. The longer the time of UA treatment, the lower the starch peak and final viscosities. Disintegration of the starch granules can cause the reduced pasting viscosities; hydration and disassociation of starch chains are caused by water penetrating through the pits and pores on granule surface. Ultrasound greatly increases the RDS content of gelatinised maize starch. A decrease in crystallinity index and damage to starch granules allows for easier penetration of the enzymes and can be responsible for the increase in the enzymatic hydrolysis. UA-treated gelatinized corn starch dispersions produce starch films with better transparency and a more cohesive structure than untreated ones. UA treatment destroys non-solubilised part of gelatinised starch and releases more amylose to form stronger film [1].
The development of other non-thermal techniques such as pulsed electric field (PEF) and cold plasma (CP) treatment have attracted more attention recently. Various field intensities of PEF applied to starch resulted in its narrow molecular size distribution suggesting that chain breakage did not occur in this process. CP treatment in turn only partially decomposed starch granules and did not form starch nanoparticles, whereas they were formed when the combination of CP with UA was used [1].
Unmodified starches, also known as native starches (NS), are insoluble in water, show inadequate thermal dissociation, and suffer readily from retrogradation and syneresis. Furthermore, the paste and gel of NSs are unstable under stress, at high temperature, and high pH. There are many disadvantages associated with NSs: unmodified sweet potato products which contain NS are unacceptably hard and have poor transparency, whereas cereal NSs make cohesive, rubbery, weak-bodied pastes and form unwanted gels while boiled. However, as NS contains ubiquitous hydroxyl groups and simple glycosidic bonds, it can be easily modified physically, chemically, enzymatically, or biotechnologically, or using a combination of these methods. The aim of the modifications is to incorporate specific features enhancing cooking properties, gel clarity, sheen, texture, adhesion, and film formation, increasing freeze–thaw stability or reducing syneresis, retrogradation, and gelling tendencies. Starch amphiphilicity, hydrophobicity, mechanical strength, and thermal stability are among the properties that can be improved as a result of starch modification [4]. The comparison of structure, properties and applications of unmodified and UA-treated starch is presented in Table 1 below.
Table 1. The comparison of structure, properties and applications of unmodified and UA-treated starch.
| Treatment | Structure | Property | Application |
|---|---|---|---|
| Unmodified | high amylose content | high mechanical strength | strong films |
| or | |||
| high amylopectin content |
low mechanical strength, unstable under stress, at high temp. and pH | films require addition of plasticisers | |
| UA | more amylose, less amylopectin |
higher strength, higher swelling, better solubility |
stronger, more transparent films |
| de-polymerisation | lower viscosity | ||
| more RDS | limited nutritional application |
The emulsion capacity of NS is somewhat low because of relatively large granule size (>1 μm) and low surface hydrophobicity. Dry heating or chemical modification can increase granule hydrophobicity but heat modification does not require any particular labelling when used in food applications, what makes it more attractive. However, the application of starch granules as emulsifiers is limited by the smallest possible granule size. In turn, nanocrystals of starch (size range 40–100 nm), obtained by NS hydrolysis, are very efficient stabilisers of continuous water emulsions [3].
Starch is commonly utilized in numerous industries such as food, paper, textile, chemical, and pharmaceutical, where it plays various roles such as adhesive, stabiliser, thickener, as well as bulking, gelling, and water holding agent [6]. Starch in various forms can be blended with conventional polymers to produce other bio-based polymers [9].
Starch is used to fabric biodegradable films which are translucent or transparent, colourless, flavourless, and tasteless. Improving mechanical-handling properties and structural integrity of food as well as acting as barriers to flavour compounds, gases, and water vapour blocking (WVB) are amongst traditional tasks of conventional packaging and need to be performed by edible starch-based films and coatings. Starch-based coatings can be successfully used for fresh fruit and vegetable packaging, e.g., the edible composite coating based on tapioca-starch with κ-carrageenan coating was applied onto fresh pumpkin to extend the shelf life. Corn starch-based coating with sunflower oil applied to Brussels sprouts allowed to reduce loss of moisture, polyphenols, and vitamin C, and to extend retention of colour and firmness. The effectiveness of emulsion coatings based on corn starch, methylcellulose, and soybean oil was noted in slowing down the hydration process of bakery products such as crackers in high relative humidity conditions. Similarly, the application of emulsified coatings from pea starch, whey protein isolate, and carnauba wax to walnuts and pine nuts confirmed that edible packaging can prevent the oxidation and hydrolytic rancidity of nuts. In addition, the coated nuts received higher sensory scores than the control group [10]. There are examples of starch use for meat and poultry packaging, e.g., starch-based film (with chitosan, alginate, and pectin) was used on fresh turkey [11].
Another currently explored novel application of starch is in the immobilisation of enzymes, proteins, or drugs to improve catalytic activity of enzymes used in food technology or to create more efficient drug delivery systems. The incorporation of excess hydroxyl groups into the starch backbone is necessary for numerous functional groups, such as polyamines, ester, ether, and carboxyl groups to be embedded, enabling starch to bind conveniently to ligands such as amino acids, proteins, and enzymes. Some examples of immobilisation using starch are presented below. The urease was successfully immobilised on starch molecule, i.e., Dialdehyde Porous Starch, by chemical and physical methods. The L-asparaginase was solidly immobilised on the poly(2-hydroxyethyl methacrylate)-starch (pHEMA-S) matrix by physical method and gained different benefits such as higher activity and enhanced storage and thermal stability. Complementary research was reported on immobilising L-asparaginase on poly(methyl methacrylate)-starch composites (pMMA-S) instead of (pHEMA-S) prepared by emulsion polymerisation technique. According to these results non-starch pMMA had not as good thermal and hydrophilic properties as the starch composites. Design of medicine delivery and biocatalyst immobilisation systems would not be possible without nanocarriers benefitting the sectors of food industry pharmacy, medicine, bioreactors, and biosensors. The immobilisation of lipase on the magnetic nanomaterials of dialdehyde starch was also performed. Immobilised lipase had storage stability and recycling rate at 82.5% and 53.6% of the native enzyme, respectively. The efficiency of the biocatalyst increased with an external magnet used to separate and purify final reaction product [12].
3. Pullulan
3.1. Structure
Pullulan (Figure 3) is a homo-polysaccharide consisting of maltotriose residues linked by α 1-6 glycosidic bonds and produced by the fungus Aureobasidium pullulans using starch as a substrate [13][14].
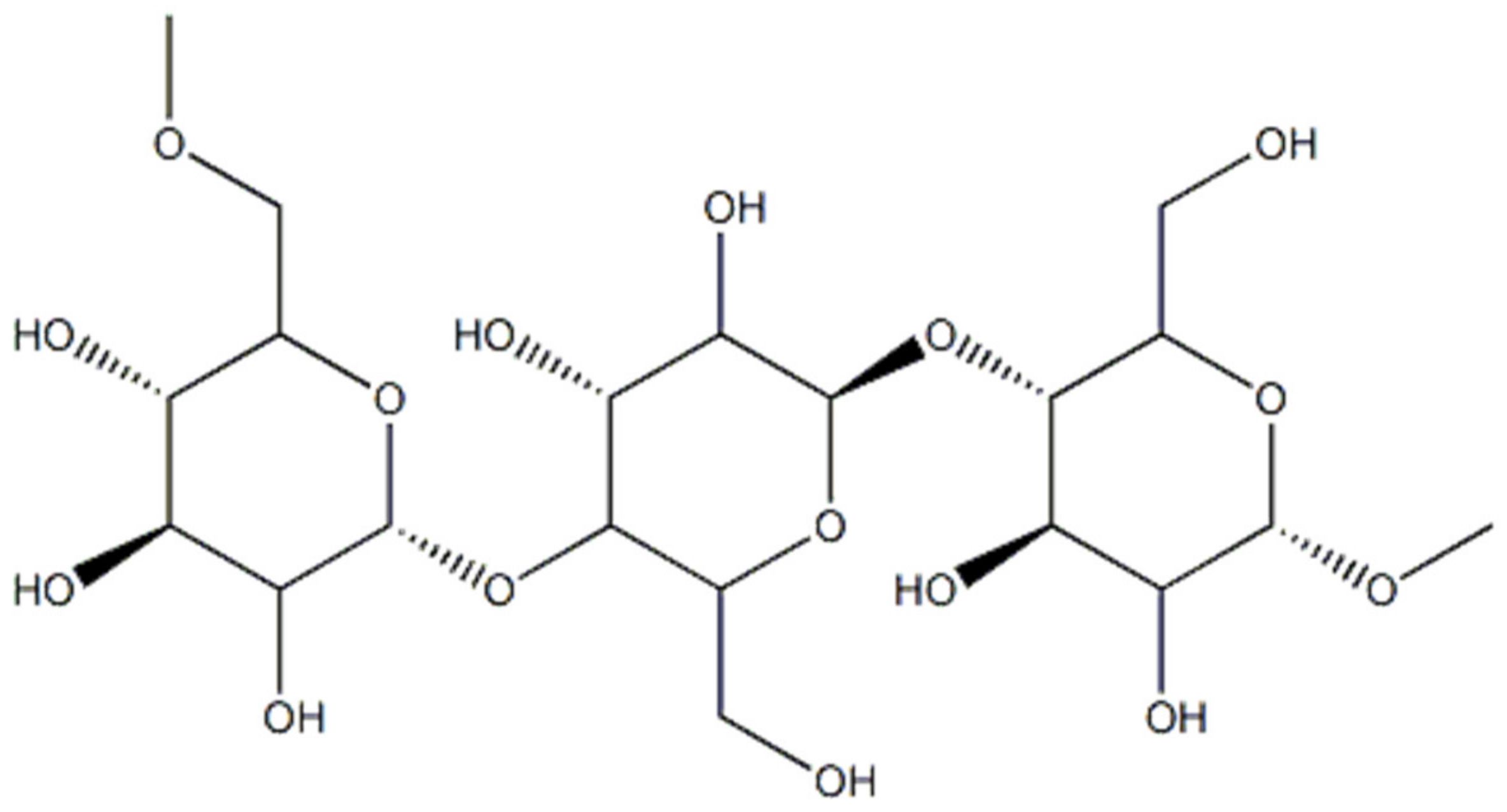
Figure 3. Representation of the pullulan structure.
3.2. Properties
Pullulan is highly soluble in water and displays unique film-forming properties. Films formed by pullulan being impermeable boundaries to gases and oils are perfect as edible coating materials. Furthermore, they are transparent, tasteless, odourless, and highly permeable to water [13]. Pullulan of food, pharmaceutical and cosmetic grades is listed on the United States Pharmacopoeia—National Formulary and Japanese Pharmacopoeia [15].
3.3. Applications
With its superior film-forming performance, good gas blocking characteristics, and desired mechanical properties, pullulan is widely used to manufacture biopolymer films [13][14].
While the application of pullulan brings numerous benefits, the high price has hindered its widespread use in industry. This disadvantage is overcome by mixing pullulan with other biopolymers, e.g., alginate, chitosan, cellulose derivatives, starch, and casein. As a result, pullulan films with improved physicochemical and mechanical properties were produced. From the above-mentioned polysaccharides, starch is cost-effective, renewable, and easily biodegradable [13]. Similarly, blending pullulan and carrageenan improves physical properties as they supplement each other’s shortcomings. This blend-film was further improved by the use of copper-sulfide nanoparticles and limonene as fillers (homogenously spread in the matrix film). This way, the mechanical characteristics of received film, namely tensile strength, elongation at break, and Young’s modulus, were profoundly improved, whilst the thermostability remained unchanged. The WVB was insignificantly enhanced, with hydrophobicity remaining unaltered. The films manufactured with the addition of various probiotic strains also exhibited high antimicrobial activity against pathogenic microorganisms, and thus they may be applied to monitor their growth in food [14].
Pullulan and its derivatives have numerous advantages, such as high solubility in water, homogenous dispersity, as well as non-toxicity and plasticity. Moreover, they are biodegradable, biocompatible, and thermally and chemically resistant. These features make pullulan ideal as a support for immobilisation of enzymes and anti-cancer drugs. In 1981 Hirohara et al. were granted the US Patent [16] for their novel approach to immobilisation of highly specific and exhibiting great catalytic activity enzymes by pullulan gel.
The immobilisation of Burkholderia cepacia lipase was performed on a chemically modified pullulan matrix that occurred to be a perfect carrier as the enlarged particle size consequently increased the adsorption surface for the lipase. As a result, the immobilised lipase shortened the reaction equilibrium time significantly. In another research, a green method of β-glucosidase covalent immobilisation was tested. Poly-aldehyde pullulan was applied as a cross-linking agent. Amino-tannic acid-modified Fe3O4 magnetic nanoparticles were used as a biocompatible nanoplatform. As a consequence, enzymatic activity of the enzyme was significantly improved [12].
4. Carrageenan
4.1. Structure
Carrageenans and agarans constitute nearly half of the algal dry matter and they are the major cell wall carbohydrates of marine red seaweeds of the Rhodophyceae class. As they are biocompatible, biodegradable, and low toxic their importance as components of pharmaceuticals and beauty products as well as in nutrition is growing [17].
The term carrageenan (CG) includes compounds from a class of heavy molecular weight linear sulfated carbohydrates containing α-D-galactopyranose and 3,6-anhydro-galactopyranose molecules linked together by α 1-3 and β 1-4 glycosidic bonds (Figure 4). CG is negatively charged and hydrophilic and its structure is similar to natural glycosaminoglycan.
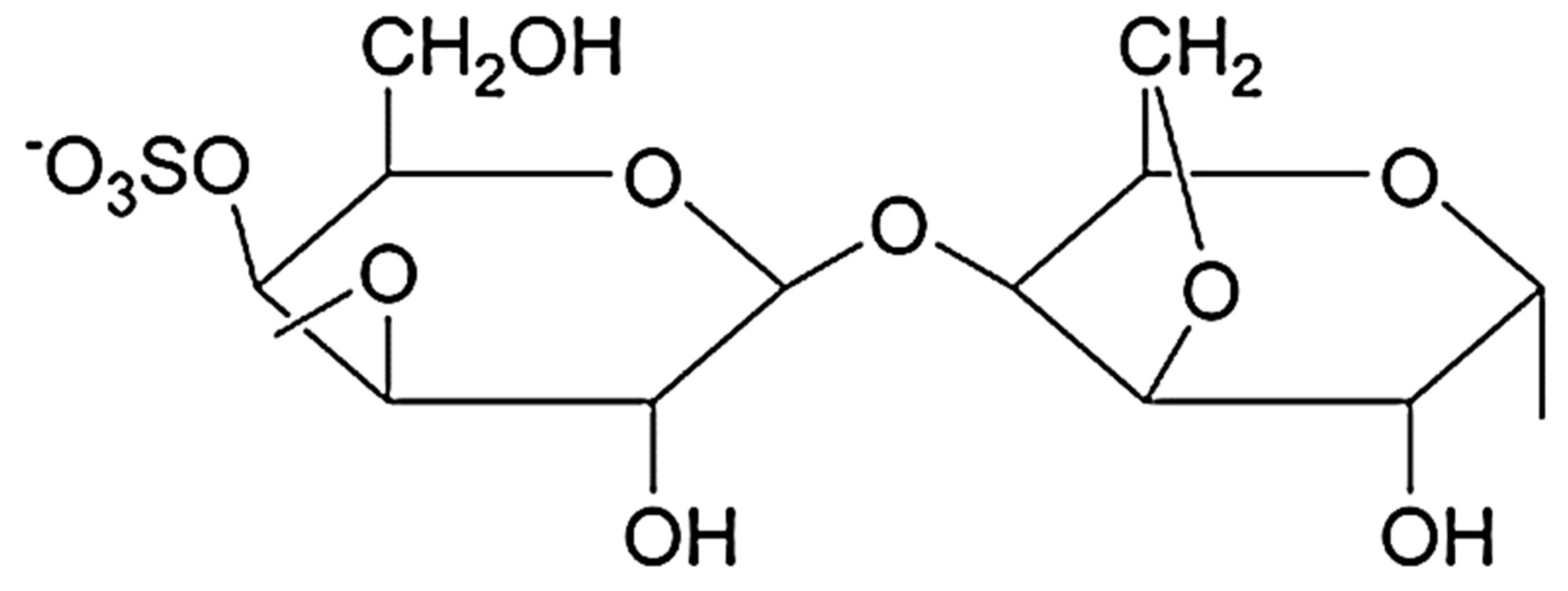
Figure 4. Representative of carrageenan structure (κ–CG).
Kappa-CG, ι-CG (iota), λ-CG (lambda), μ-CG (mu), ν-CG (nu), and θ-CG (theta) are six main kinds of carrageenans. Amongst them, κ-, ι- and λ- are the most important and commercially applicable types of CG. The amount and distribution of ester sulfate groups and the 3,6-anhydro-galactopyranose units in CGs affect primarily the properties of the compounds. Kappa-, ι- and λ-CG have 1, 2, and 3 negatively charged ester sulfate groups per dimer unit, respectively, but λ-CG has no 3,6-anhydro-D-galactopyranosyl linkage (Figure 5). Mu-, ν-, and θ-CG are biological derivatives of κ-, ι-, and λ-CG, respectively [14][18][19][20].
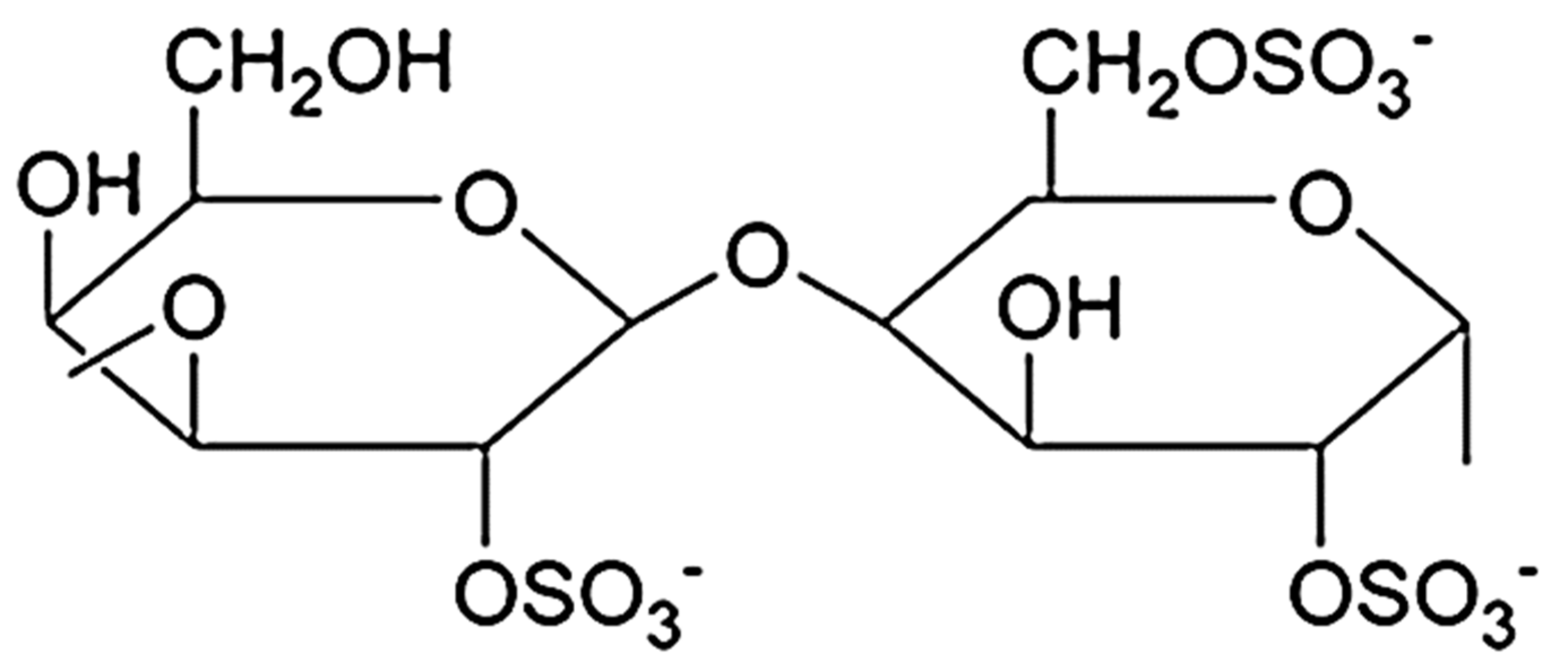
Figure 5. Chemical structure of the repeating unit of λ–CG.
4.2. Properties and Applications
All CGs are soluble in water but do not dissolve in organic media. The number of sulphate groups, and the resulting Na+ and K+ cation equilibrium strongly affects CG water solubility, viscosity, and gelling ability. Clinical studies confirmed CGs being very low or non-toxic, and causing no developmental malformations [18]. As per low toxicity, the safety of CG as food additive was analysed in a large number of studies and concluded that a CG daily dose of up to 75 mg/kg body weight in the human diet was safe [21].
Even a small amount (1% by weight) of curcumin added to CG functional films improved the surface hydrophobicity, swelling ratio, as well as UV-blocking and WVB properties. Additionally, the CG composite films gained antioxidant activity and some antibacterial properties, and could be applied in the form of coatings for active food packaging [22].
Similar results were found when κ-CG edible films were made with orange essential oil and trehalose. Such films could also be used for active food packaging, as blocking property of UV and visual light was greatly improved by the addition of orange essential oil and trehalose. Moreover, the packaging film gained meaningful antimicrobial activity against Staphyloccocus aureus, wherein the higher the content of κ-CG in the film, the higher the antimicrobial activity against all studied microorganisms [23].
In search of new applications, the effect of CG in yoghurt formulation was tested. It was found that although CG (similarly to xanthan gum) made skim yoghurt formulation more solid and viscous, but at the same time syneresis occurred and positive organoleptic features became worse [24].
Kappa-CG can be used to produce the composite hydrogel with methacrylate. Addition of κ-CG increased gel water uptake over 3 times which makes such a hydrogel classified as superabsorbent. It also significantly improved mechanical properties of hydrogel which, as a result, gained features useful for biotechnology and drug formulation or sustained drug release [17][19].
Carrageenan is also used by some manufacturers as gelling agent to produce toothpaste hydrogel. Adding limonene as filler improves mechanical properties of such a gel (this is the authors’ observation based on the local Polish dental hygiene product market).
The CGs antioxidant property was screened by scavenging assays of such reactive oxygen species (ROS) as 2,2-diphenyl-1-picrylhydrazyl radical as well as superoxide and hydroxyl radicals. Similarly, the CG capability of ROS cleaning could also be confirmed by the increase in superoxide dismutase, catalase, glutathione peroxidase, or Fe2+ chelating ability. For example, ι-CG from Solieria filiformis manifests antioxidant activity by preventing glutathione consumption and reducing levels of malondialdehyde and haemoglobin, while κ-CG shows outstanding capacities of free radicals scavenging (including all listed above) and Fe2+ chelating activity in vitro [17].
Sulfate groups in CGs can neutralize positive charges on the surface of host cells, thereby impeding viral uptake, penetration, and detachment, wherein the higher the number of sulphate residues and degree of polymerisation, the stronger the antiviral property. Various types of CG have various antiviral inhibitory activities against various viruses. For example, fully oxidized κ-CG was proven to have worse antiviral property against HSV-1 and HSV-2 (herpes simplex virus) than its partially oxidized counterpart. In addition, the combination of CG and griffithsin (a non-antiretroviral HIV entry inhibitor) indicated strong possibility of interfering with HIV-1, HSV-2, and HPV, which would significantly reduce the risk of sexually transmitted infections in women. This result indicates a potential of enhancing of CGs antiviral activities through drug combination therapy. It was observed that CGs helped to block the connection of virus to receptors of host cells as a result of CG’s affinities for glycoproteins of viruses [17][20].
Historical records dated as early as the 1830s mentioned that “carrageen” or “Irish moss” (known as not specified mixture of coexisting naturally marine red seaweeds, Chondrus crispus and Mastocarpus stellatus) was often applied as medicine. Even in modern Ireland it is still thought to have antiviral properties and to be efficient in dislodging mucus, and therefore it is used in traditional curative infusions against common bronchitis, infections, and chronic coughs. High iodine content and traditional presence of seaweed in the diet was believed to be related to initial low level of infection caused by newly emerging SARS-CoV-2 in Japan, specifically in Hokkaido. Admittedly, the number of these infections increased dramatically afterwards; however, it seems that seaweed helped Hokkaido population to reduce contagion in an initial period, though soon such additional actions as isolation or social distancing were required. Both children and adults with virus-originated cold, treated with a CG-based nasal spray had the disease duration shortened with faster removal of virus. It is worth mentioning that there is a commercially available ι-CG-based nasal spray named Bisolviral® sold by Sanofi Aventis [25].
In the past many reports confirmed both κ-CG and ι-CG activities against influenza A virus in vitro as well as positive therapeutic effects in vivo, while the antiviral potential of λ-CG was seldom examined. Therefore, the special attention was paid to check if λ-CG is able to protect against both influenza A and B viruses and/or SARS-CoV-2, as this compound has a higher sulfate groups number and is easier soluble in cold water than the other two CGs. An experimental study was run to prove whether λ-CG is active against various respiratory viruses, namely: influenza A and B viruses and SARS-CoV-2. The data revealed that not only did λ-CG suppress expression of viral proteins in invaded cells, but also inhibited the production of viral progeny in a quantity-proportional way. Taken together, these results suggested that λ-CG had significant antiviral potential against influenza A and B viruses in vitro. In vivo antiviral activity was assessed by observing body mass and lethality of infected mice for 15 days. The infected and untreated mice suffered from significant body mass loss and died not later than on 7th day, yet λ-CG administered as nasal spray at 5 mg/kg alleviated infection-induced body mass loss, resulting in a 60% survival rate. Firefly luciferase test using lysates of infected cells showed that λ-CG was capable of limiting infections of both SARS-CoV-2 and pseudoviruses derived from influenza A viral glycoproteins in a quantity-proportional way. Moreover, further examinations showed λ-CG reduced both the level of viral protein in cell lysate and the level of viral RNA in the supernatants. The above analyses unequivocally demonstrated the antiviral activity of λ-CG against SARS-CoV-2 [20].
Among numerous methods of chemical modification applicable to CGs carboxy-methylation seems to be the most commonly used, because it makes CG more soluble in water, what together with CG natural features of gel-forming and viscosity make it a very attractive compound for drug delivery and specifically for controlled drug release. Nevertheless, oral administration of CGs still risks unexpected immune reaction, harmful gastrointestinal (GI) effects, and other potentially unwanted organism’s responses [17]. The comparison of structure, properties and applications of unmodified and modified carrageenans is presented in Table 2 below.
Table 2. The comparison of structure, properties and applications of unmodified and modified carrageenans.
| Treatment | Structure | Property | Application |
|---|---|---|---|
| Unmodified | basic | water solubility, viscosity, and gelling ability depending on the number of sulphate groups | edible films, active food packaging |
| κ-CG | antimicrobial activity, high water uptake | composite superabsorbent gel | |
| κ-CG, ι-CG | high antioxidant activity | edible films, active food packaging, functional food | |
| κ-CG, ι-CG, λ-CG | high antiviral activity | antiviral drugs | |
| Carboxymethylation | additional carboxymethyl groups | higher water solubility | drug delivery/controlled release |
References
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508.
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920.
- Ashogbon, A.O. Dual modification of various starches: Synthesis, properties and applications. Food Chem. 2021, 342, 128325.
- Patel, A.R. Functional and engineered colloids from edible materials for emerging applications in designing the food of the future. Adv. Funct. Mater. 2018, 30, 1806809.
- Basiak, E.; Galus, S.; Lenart, A. Characterisation of composite edible films based on wheat starch and whey-protein isolate. Int. J. Food Sci. Technol. 2015, 50, 372–380.
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications-A review. Int. J. Food Sci. Technol. 2017, 52, 38–58.
- Piecyk, M.; Wołosiak, R.; Drużynska, B.; Worobiej, E. Chemical composition and starch digestibility in flours from Polish processed legume seeds. Food Chem. 2012, 135, 1057–1064.
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Starch digestibility: Past, present, and future. J. Sci. Food Agric. 2018, 100, 5009–5016.
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739.
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283.
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178.
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986.
- Kanmani, P.; Lim, S.T. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049.
- Roy, S.; Rhim, J.-W. Fabrication of copper sulfide nanoparticles and limonene incorporated pullulan/carrageenan-based film with improved mechanical and antibacterial properties. Polymers 2020, 12, 2665.
- Dulong, V.; Kouassi, M.-C.; Labat, B.; Le Cerf, D.; Picton, L. Antioxidant properties and bioactivity of Carboxymethylpullulan grafted with ferulic acid and of their hydrogels obtained by enzymatic reaction. Food Chem. 2018, 262, 21–29.
- Hirohara, H.; Nabeshima, S.; Fujimoto, M.; Nagase, T. Enzyme Immobilisation with Pullulan Gel. U.S. Patent 4,247,642, 27 January 1981.
- Jiang, J.-L.; Zhang, W.-Z.; Ni, W.-X.; Shao, J.-W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642.
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482.
- Pettinelli, N.; Rodríguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of chitosan, pectin or κ-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mater. Sci. Eng. C 2019, 96, 583–590.
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.-I.; Kim, C.W.; Lee, H.-R.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 821.
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16, e05238.
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302.
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—Structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332.
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104.
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
656
Revisions:
2 times
(View History)
Update Date:
27 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





