Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fanny Lassalle | -- | 2474 | 2022-05-25 16:52:25 | | | |
| 2 | Sirius Huang | -7 word(s) | 2467 | 2022-05-26 03:10:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lassalle, F.; , .; Staels, B.; Dupont, H.2. Human Monocyte Subsets and Cardiovascular Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/23373 (accessed on 07 February 2026).
Lassalle F, , Staels B, Dupont H2. Human Monocyte Subsets and Cardiovascular Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/23373. Accessed February 07, 2026.
Lassalle, Fanny, , Bart Staels, Hématologie 2021 Dupont. "Human Monocyte Subsets and Cardiovascular Diseases" Encyclopedia, https://encyclopedia.pub/entry/23373 (accessed February 07, 2026).
Lassalle, F., , ., Staels, B., & Dupont, H.2. (2022, May 25). Human Monocyte Subsets and Cardiovascular Diseases. In Encyclopedia. https://encyclopedia.pub/entry/23373
Lassalle, Fanny, et al. "Human Monocyte Subsets and Cardiovascular Diseases." Encyclopedia. Web. 25 May, 2022.
Copy Citation
Monocytes are known for their very important role in tissue homeostasis and the innate immune system. The repartition of the circulating monocyte subsets has been identified as a predictive prognosis marker in various cardiovascular diseases caused by atherosclerosis.
monocytes
monocyte subsets
transcatheter aortic valve replacement
aortic valve stenosis
1. Human Monocyte Heterogeneity
Monocytes are known for their very important role in tissue homeostasis and the innate immune system. Originating from bone marrow, they continuously enter the blood circulation, where they constitute 8 to 10% of the total leukocyte population in humans. During infection or damage, they are recruited into tissues where they rapidly differentiate into macrophages or dendritic cells to exert their role in inflammation, immune defense, phagocytosis or tissue repair [1].
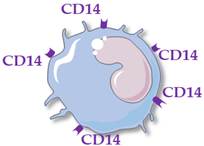
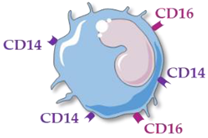
Human monocytes are now classified into three functionally different subsets, based on the expression of superficial cluster differentiation CD14 (a cell co-receptor for lipopolysaccharide) and CD16 (the low-affinity Fc receptor III for IgG), with different phenotypes and functions in homeostasis and diseases [2][3][4] (Table 1).
Table 1. Main characteristics of human circulating monocyte subsets. Abbreviations: cluster differentiation (CD), interleukin (IL), tumor necrosis factor (TNF).
| Monocyte Subsets |
Classical Monocytes |
Intermediate MONOCYTES |
Non-Classical Monocytes |
|---|---|---|---|
| Schematic representation |
 |
 |
 |
| Surface receptors |
CD14++ CD16− | CD14++ CD16+ | CD14+ CD16++ |
| Proportion of total monocytes | 85–90% | 5–10% | 5–10% |
| Main functions | Phagocytosis, tissue repair, inflammation, reactive oxygen species production | Antigen presentation, T-cell proliferation and stimulation, reactive oxygen species production, phagocytosis | Patrolling of endothelial cell integrity, clearance of dying endothelial cells, wound healing |
| Cytokine production |
IL-10, IL-6 | TNFα, IL-1β, IL-6 | TNFα, IL-1β, IL-6 |
Classical monocytes (CD14++ CD16−) are generally short-lived cells surviving for only one day. They are rapidly mobilized into infected or injured sites and are involved in diverse functions such as phagocytosis, infection control, inflammation regulation and tissue repair. Among the three monocyte subsets, they present the greatest migration capacity in tissues [5].
Non-classical monocytes (CD14+ CD16++) patrol endothelial cell integrity, clear dying endothelial cells, and protect vessel health. They survive for 7 days in humans and can be recruited into sites of vascular injury or infection or enter into the areas of inflammation, such as atherosclerotic plaques [6].
A third monocyte subset has also been described. They are referred to as intermediate monocytes (CD14++ CD16+), which are variously described as closely resembling either classical or non-classical monocytes. They express a high degree of MHC class II gene presentation, conferring them a predominant role in inducing T-cell proliferation and stimulation and are recruited at a later stage of inflammation. The classical (CD16−) and intermediate/non-classical (CD16+) monocytes represent 80–90% and 10–15% of total monocytes, respectively.
Although a large number of studies have focused on the differentiation of human monocyte subsets, the mechanisms regulating their recruitment into tissues and their functional and dynamic role in inflammation and immunity, there are still many aspects that need to be clarified. In this way, Cormican and Griffin [7], in a recent review about the gene expression analysis of monocyte subsets performed in the literature, pointed out conflicting results. The biggest inconsistencies remain in the production of proinflammatory cytokines by the different monocyte subsets. While Cros et al. [8] reported that classical monocytes produce high levels of reactive oxygen species (ROS) and intermediate monocytes have the highest production of TNFα and Il-1β but do not produce ROS, Wong et al. [9] showed that TNFα and Il-1β were essentially produced by non-classical monocytes with a low production of all cytokines for intermediate monocytes. Finally, Zawada et al. [10] attest that classical monocytes have the lowest production of ROS, produced the most by intermediate monocytes. It should be noted that the lack of standardization in the flow cytometry protocols for gating the different monocyte subsets can contribute to these discrepancies among studies. In any case, monocyte differentiation in these subsets seems to be tightly regulated, with the mechanisms still poorly understood.
Many studies report the close link between blood coagulation and the innate immune system, termed “thromboinflammation” or “immunothrombosis”. Indeed, activated monocytes express on their surface a tissue factor [11] that has an essential role in the coagulation cascade, by activating factor VII which initiates the coagulation in vivo, leading to the release of thrombin, able to convert fibrinogen into fibrin; to activate coagulation factor XIII, important for stabilizing the fibrin clot; to amplify the coagulation process by activating cofactors V and VIII and factor XI and to activate platelets. Moreover, recent studies have shown that monocytes were able to express coagulation factor XIII in response to stimulation by proinflammatory cytokines [12] and to secrete tissue-type plasminogen activator, a serine protease that converts plasminogen into plasmin leading to the degradation of fibrin clots, playing a key role in fibrinolysis [13]. Monocytes can be activated by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) and release tissue factor-bearing microvesicles [14]. Platelets and neutrophils also have a major role in immunothrombosis [15][16]. Many studies showed that an aberrant immunothrombosis process could contribute to thrombus formation in inflammatory diseases such as atherosclerosis [17]. In the AVS context, this process could be involved in disease progression and the hemostasis complications observed after TAVR.
2. Monocyte Subsets and Cardiovascular Diseases
Variations in the repartition of the monocyte subsets have been reported several times in diverse conditions, either as a protective mechanism or as taking part in the pathological process, such as in infections, cancers, autoimmune or inflammatory diseases [2], although the finer details of their involvement are not yet fully understood. Interestingly, the repartition of the circulating monocyte subsets has been identified as a predictive prognosis marker in various cardiovascular diseases caused by atherosclerosis such as coronary artery disease, stroke or peripheral arterial disease [18][19]. These studies frequently reported associations between an increase of intermediate monocyte levels and the severity or complications of the diseases [19][20][21][22]. For example, in a prospective cohort study of 951 subjects referred for elective coronary angiography, Rogacev et al. [23] showed that a higher level of intermediate monocytes at the inclusion was predictive of any adverse cardiovascular events (cardiovascular death, acute myocardial infarction or non-hemorrhagic stroke), after adjustment for confounders such as age, sex, diabetes, hypertension, smoking, high-density-lipoprotein cholesterol, CRP and total leukocyte count, with a mean follow-up of 3 years. Moreover, some studies highlighted that elevated intermediate monocyte levels play a key role in the growth and stability of atherosclerotic lesions [24][25].
3. Monocyte Subsets and Aortic Valve Stenosis
While data accumulate on the key role of monocytes/macrophages in AVS and the similarity in many aspects of AVS and atherosclerosis, very few studies have reported the circulating levels of total monocyte and of monocyte subsets in AVS patients. Shimoni et al. [26] compared a cohort of 54 patients with significant AVS (10 with moderate and 44 with severe AVS) to 33 patients with similar cardiovascular risk factors and no valvular disease. They observed that patients with AVS had increased levels of total circulating monocytes compared to controls with an inverse correlation between monocyte level and aortic valve area. Similarly, Efe et al. [27] observed in a cohort of 178 patients with a diagnosis of AVS (111 mild-to-moderate patients and 67 severe patients) and 139 age- and gender-matched without AVS controls, higher monocyte levels in severe AVS patients compared to mild-to-moderate patients and higher monocyte levels in mild-to-moderate AVS patients compared to controls. Moreover, they observed that the lymphocyte to monocyte ratio was lower in the group with severe AVS than in the group with mild-to-moderate AVS and lower in this group than in the control group; and that the lymphocyte to monocyte ratio was independently related to the severity of AVS (mean gradient). In both studies, they did not analyze the monocyte subsets. Hewing et al. [28] showed, in a cohort of 100 AVS patients compared to AVS free controls, that absolute levels of circulating intermediate monocytes were increased in severe AVS while absolute levels of circulating classical and non-classical monocyte subsets did not differ between both groups. Interestingly, the difference of intermediate monocyte levels between both groups was independent of age, sex, body mass index, low-density-lipoprotein cholesterol, N-terminal pro-B-type natriuretic peptide, New York Heart Association (NYHA) functional class and creatinine levels.
4. Monocyte Subsets and Transcatheter Aortic Valve Replacement
A few teams were interested in the variation of the monocyte subset levels before and after TAVR that may have been induced by the sudden change of hemodynamic conditions. At this time, only four studies reported these variations or associated the levels of a pre- or postprocedural subset with outcome after TAVR (Table 2). First, Hewing et al. [29] compared the monocyte subsets before aortic valve replacement versus 3 and 6 months after, in a cohort of 69 patients with severe AVS (44 TAVR and 25 surgery). They observed, in both groups (TAVR and surgery), no change in monocyte counts at 3 and 6 months after aortic valve replacement compared with baseline and a decrease of absolute intermediate monocyte levels at 6 months after surgery procedure and earlier after TAVR (at 3 and 6 months.) Absolute classical and non-classical monocytes remained stable in both groups as well as inflammatory markers (CRP, IL-6, TNF-α). Then, Neuser et al. [30] compared the monocyte subsets before TAVR versus day 4 to 7 after in a cohort of 57 patients. No difference in total absolute monocytes, classical and non-classical monocyte levels was observed, whereas they reported a decline of absolute intermediate monocyte levels after TAVR. Moreover, high levels of absolute intermediate monocytes prior to TAVR were associated with worse cardiac function and lower probability to reach an improvement in NYHA functional class 3 months after TAVR. In this study, CRP increased after TAVR but was not correlated with intermediate monocyte levels at any point. More recently, Pfluecke et al. [31] compared the three monocyte subsets on the day before, 24 h and 7 days after TAVR with the mortality at 3 months in a cohort of 120 severe AVS patients. They observed that non-classical and intermediate monocyte levels were higher before TAVR in patients who died within 3 months after TAVR compared to survivors. At 24 h and 7 days after TAVR, no significant difference was observed for the three monocyte subsets between survivors and non-survivors, except for classical monocytes the day after TAVR, higher in survivors. Interestingly, the intermediate monocyte level measured before TAVR remains an independent predictor for 3-month mortality, after adjustment with age, left ventricular ejection fraction, circulating CRP and IL-8 and CD11b-expression on monocytes, marker of cell activation. In this cohort, Cybularz et al. [32] investigated the association of frailty with monocyte subsets and observed higher absolute intermediate monocyte levels in 28 frail patients compared to not-frail patients. Moreover, intermediate monocyte levels were independent predictors for post-TAVR 6 months mortality after adjustment for frailty and CRP. Finally, Hoffmann et al. [33] compared the monocyte subsets before TAVR versus immediately, 24 h and 3 days after TAVR in a cohort of 129 patients. They observed a significant elevation of classical and intermediate monocytes at 24 h followed by an elevation of non-classical monocytes 3 days after TAVR. Moreover, they reported that levels of intermediate monocytes tended to be predictive of 12 month mortality and that non-classical monocytes measured immediately after TAVR were associated with 12 month all-cause mortality, even after the exclusion of those patients dying within the first 30 days after TAVR.
Table 2. Evolution of the circulating monocyte subsets (absolute values, compared to pre-transcatheter aortic valve replacement (TAVR)) in patients with aortic valve stenosis underwent TAVR. Age, Society of Thoracic Surgeons (STS) score and European system for cardiac operative risk evaluation (EuroSCORE II) are presented as mean ± standard deviation or median (interquartile ranges) according to available data. *: post-TAVR; =: stable; ↗: increase; ↘: decrease. Abbreviation: New York Heart Association (NYHA).
| Number; Origin of Patients | Age; Gender Proportion | STS Score (%) EuroSCORE II (%) |
Time of Blood Sampling |
Classical Monocytes | Intermediate Monocytes | Non-Classical Monocytes | Association with Outcomes | Reference |
|---|---|---|---|---|---|---|---|---|
| 44; Germany, single center |
80.2 ± 6.1; 50% male | 2.5 (1.4–3.9); 3.6 (2.3–5.7) |
Pre-TAVR | Not available | Hewing [29] | |||
| 3 months * | = | ↘ | = | |||||
| 6 months * | = | ↘ | = | |||||
| 57; Germany, single center |
83.3 ± 0.79; 47% male |
5.97 ± 0.39; 6.71 ± 0.65 | Pre-TAVR | High levels of intermediate monocytes pre-procedure associated with worse cardiac function and lower probability to reach an improvement in NYHA 3 months after TAVR | Neuser [30] | |||
| Day 4 to 7 * | = | ↘ | = | |||||
| 120; Germany, single center |
81; 33% male |
>4 | Pre-TAVR | No comparison between times | High levels of intermediate monocytes pre-procedure associated with 3-month mortality | Pfluecke [31] Cybularz [32] |
||
| 24 h * | ||||||||
| Day 7 * | ||||||||
| 129; Germany, single center |
83 (79–86); 76% male |
3.41 (2.45–4.94) 3.31 (2.31–6.04) |
Pre-TAVR | High levels of intermediate monocytes pre-procedure trended to be predictive of 12-month mortality and high levels of non-classical monocytes post-procedure associated with 12-month mortality | Hoffmann [33] |
|||
| 24 h * | ↗ | ↗ | ↗ | |||||
| Day 3 * | ↗ | ↗ | ↗ | |||||
It is very difficult to compare these studies as the times of study of the monocyte subset repartitions in the post-TAVR procedure as well as the follow-up periods and type of analyzed adverse events were not the same. However, all these studies reported an impact of the TAVR procedure on intermediate monocyte levels with an earlier increase in post-procedural and then a decrease under baseline a few days and months after TAVR.
The mechanisms involved in the modulation of circulating intermediate monocyte levels during a TAVR procedure remain speculative. The TAVR procedure results in sudden changes in wall shear stress and flow turbulence associated with a proinflammatory response. It can be hypothesized that increased levels of circulating intermediate monocytes quickly after TAVR are the result of interplay between significant hemodynamic disturbances and inflammation response. To go deeper into the comprehension of the shear stress impact on monocyte function, Baratchi et al. [34] recently compared the activation status of monocytes in patients with severe AVS before TAVR, i.e., under high shear stress, and after TAVR, i.e., normal shear stress. They showed that monocytes were more activated before TAVR in comparison with 1 month after TAVR, with a higher phagocytic activity, greater adhesive capacity and oxidized low-density-lipoprotein uptake and higher monocyte expression of proinflammatory cytokines (IL-6, interferon β1, TNFα). These results were confirmed in a microfluidic system recapitulating the shear conditions observed before and after TAVR. Interestingly, they identified the mechano-sensitive calcium channel receptor Piezo-1 as an essential mediator of the shear-stress responsive mechanoreceptor in human monocytes and observed that the expression of this receptor on monocytes is downregulated after TAVR. Thus, besides its hemodynamic benefits, a TAVR procedure also induces beneficial anti-inflammatory effects. Targeting Piezo-1 with pharmacological agents to inhibit monocyte activation may constitute a new therapeutic perspective in AVS. Interestingly, in all studies on monocyte subsets and TAVR, the authors reported an association between pre- or postprocedural levels of intermediate monocytes and TAVR deleterious complications at 3, 6 or 12 months. These results are in line with previous findings of higher major cardiovascular events depending on elevated intermediate monocyte levels [19] but these observational studies cannot answer the question whether the modulation of intermediate monocyte levels after TAVR represent a causal factor for outcomes or just a consequence of the procedure itself. Additional work is needed to understand how these modulations may influence later clinical events [18]. Moreover, one of the main limitations of these studies on TAVR patients is their small sample size. All findings should be confirmed in larger and longitudinal studies before considering intermediate monocyte levels as a usual risk marker and as a possible target for therapeutics to decrease AVS progression and the rate of complications associated with TAVR procedure. Finally, it should be noted that the impact of monocyte subsets on thrombosis and bleeding complications occurring after TAVR have not yet been described, although these complications are frequent in post-TAVR patients and monocytes are key players in the process of thromboinflammation.
References
- Ingersoll, M.A.; Platt, A.M.; Potteaux, S.; Randolph, G.J. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011, 32, 470–477.
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, e12883.
- Ziegler-Heitbrock, L.; Hofer, T.P. Toward a Refined Definition of Monocyte Subsets. Front. Immunol. 2013, 4, 23.
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460.
- Connaughton, E.P.; Naicker, S.; Hanley, S.A.; Slevin, S.M.; Eykelenboom, J.K.; Lowndes, N.F.; O’Brien, T.; Ceredig, R.; Griffin, M.D.; Dennedy, M.C. Phenotypic and functional heterogeneity of human intermediate monocytes based on HLA-DR expression. Immunol. Cell Biol. 2018, 96, 742–758.
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical Patrolling Monocyte Function in the Vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316.
- Cormican, S.; Griffin, M.D. Human Monocyte Subset Distinctions and Function: Insights from Gene Expression Analysis. Front. Immunol. 2020, 11, 1070.
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.-Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim Monocytes Patrol and Sense Nucleic Acids and Viruses via TLR7 and TLR8 Receptors. Immunity 2010, 33, 375–386.
- Wong, K.L.; Tai, J.J.-Y.; Wong, W.-C.; Han, H.; Sem, X.; Yeap, W.-H.; Kourilsky, P.; Wong, S.-C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31.
- Zawada, A.M.; Rogacev, K.S.; Rotter, B.; Winter, P.; Marell, R.-R.; Fliser, D.; Heine, G.H. SuperSAGE evidence for CD14++ CD16+ monocytes as a third monocyte subset. Blood 2011, 118, e50–e61.
- Maugeri, N.; Brambilla, M.; Camera, M.; Carbone, A.; Tremoli, E.; Donati, M.B.; Gaetano, G.D.; Cerletti, C. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J. Thromb. Haemost. 2006, 4, 1323–1330.
- Alshehri, F.S.M.; Whyte, C.S.; Tuncay, A.; Williams, M.-L.; Wilson, H.M.; Mutch, N.J. Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation. Int. J. Mol. Sci. 2021, 22, 6591.
- Seillier, C.; Hélie, P.; Petit, G.; Vivien, D.; Clemente, D.; Le Mauff, B.; Docagne, F.; Toutirais, O. Roles of the tissue-type plasminogen activator in immune response. Cell. Immunol. 2022, 371, 104451.
- Gaertner, F.; Massberg, S. Blood coagulation in immunothrombosis—At the frontline of intravascular immunity. Semin. Immunol. 2016, 28, 561–569.
- Darbousset, R.; Thomas, G.M.; Mezouar, S.; Frère, C.; Bonier, R.; Mackman, N.; Renné, T.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Tissue factor–positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 2012, 120, 2133–2143.
- Müller, I.; Klocke, A.; Alex, M.; Kotzsch, M.; Luther, T.; Morgenstern, E.; Zieseniss, S.; Zahler, S.; Preissner, K.; Engelmann, B. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003, 17, 476–478.
- Libby, P.; Simon, D.I. Inflammation and Thrombosis. Circulation 2001, 103, 1718–1720.
- Williams, H.; Mack, C.D.; Li, S.C.H.; Fletcher, J.P.; Medbury, H.J. Nature versus Number: Monocytes in Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 9119.
- Oh, E.S.; Na, M.; Rogers, C.J. The Association between Monocyte Subsets and Cardiometabolic Disorders/Cardiovascular Disease: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 640124.
- Zeynalova, S.; Bucksch, K.; Scholz, M.; Yahiaoui-Doktor, M.; Gross, M.; Löffler, M.; Melzer, S.; Tárnok, A. Monocyte subtype counts are associated with 10-year cardiovascular disease risk as determined by the Framingham Risk Score among subjects of the LIFE-Adult study. PLoS ONE 2021, 16, e0247480.
- Wildgruber, M.; Aschenbrenner, T.; Wendorff, H.; Czubba, M.; Glinzer, A.; Haller, B.; Schiemann, M.; Zimmermann, A.; Berger, H.; Eckstein, H.-H.; et al. The “Intermediate” CD14++ CD16+ monocyte subset increases in severe peripheral artery disease in humans. Sci. Rep. 2016, 6, 39483.
- Elchinova, E.; Teubel, I.; Roura, S.; Fernández, M.A.; Lupón, J.; Gálvez-Montón, C.; de Antonio, M.; Moliner, P.; Domingo, M.; Zamora, E.; et al. Circulating monocyte subsets and heart failure prognosis. PLoS ONE 2018, 13, e0204074.
- Rogacev, K.S.; Cremers, B.; Zawada, A.M.; Seiler, S.; Binder, N.; Ege, P.; Große-Dunker, G.; Heisel, I.; Hornof, F.; Jeken, J.; et al. CD14++ CD16+ Monocytes Independently Predict Cardiovascular Events. J. Am. Coll. Cardiol. 2012, 60, 1512–1520.
- Ozaki, Y.; Imanishi, T.; Hosokawa, S.; Nishiguchi, T.; Taruya, A.; Tanimoto, T.; Kuroi, A.; Yamano, T.; Matsuo, Y.; Ino, Y.; et al. Association of Toll-Like Receptor 4 on Human Monocyte Subsets and Vulnerability Characteristics of Coronary Plaque as Assessed by 64-Slice Multidetector Computed Tomography. Circ. J. 2017, 81, 837–845.
- Imanishi, T.; Ikejima, H.; Tsujioka, H.; Kuroi, A.; Ishibashi, K.; Komukai, K.; Tanimoto, T.; Ino, Y.; Takeshita, T.; Akasaka, T. Association of monocyte subset counts with coronary fibrous cap thickness in patients with unstable angina pectoris. Atherosclerosis 2010, 212, 628–635.
- Shimoni, S.; Meledin, V.; Bar, I.; Fabricant, J.; Gandelman, G.; George, J. Circulating CD14(+) monocytes in patients with aortic stenosis. J. Geriatr. Cardiol. JGC 2016, 13, 81–87.
- Efe, T.H.; Gayretli Yayla, K.; Yayla, C.; Ertem, A.G.; Cimen, T.; Erken Pamukcu, H.; Bilgin, M.; Erat, M.; Dogan, M.; Yeter, E. Calcific aortic stenosis and its correlation with a novel inflammatory marker, the lymphocyte/monocyte ratio. Rev. Port. Cardiol. 2016, 35, 573–578.
- Hewing, B.; Au, S.C.-D.; Ludwig, A.; Ellerbroek, R.; van Dijck, P.; Hartmann, L.; Grubitzsch, H.; Giannini, C.; Laule, M.; Stangl, V.; et al. Severe Aortic Valve Stenosis in Adults is Associated with Increased Levels of Circulating Intermediate Monocytes. J Cardiovasc. Transl. Res. 2017, 10, 27–34.
- Hewing, B.; Ellerbroek, R.; Au, S.; Stangl, V.; Dreger, H.; Laule, M.; Grubitzsch, H.; Knebel, F.; Baumann, G.; Ludwig, A.; et al. Levels of Circulating Intermediate Monocytes Decrease after Aortic Valve Replacement in Patients with Severe Aortic Stenosis. Thromb. Haemost. 2017, 117, 2346–2355.
- Neuser, J.; Galuppo, P.; Fraccarollo, D.; Willig, J.; Kempf, T.; Berliner, D.; Bauersachs, J.; Widder, J.D. Intermediate CD14++ CD16+ monocytes decline after transcatheter aortic valve replacement and correlate with functional capacity and left ventricular systolic function. PLoS ONE 2017, 12, e0183670.
- Pfluecke, C.; Wydra, S.; Berndt, K.; Tarnowski, D.; Cybularz, M.; Jellinghaus, S.; Mierke, J.; Ende, G.; Poitz, D.M.; Barthel, P.; et al. Mon2-monocytes and increased CD-11b expression before transcatheter aortic valve implantation are associated with earlier death. Int. J. Cardiol. 2020, 318, 115–120.
- Cybularz, M.; Wydra, S.; Berndt, K.; Poitz, D.M.; Barthel, P.; Alkouri, A.; Heidrich, F.M.; Ibrahim, K.; Jellinghaus, S.; Speiser, U.; et al. Frailty is associated with chronic inflammation and pro-inflammatory monocyte subpopulations. Exp. Gerontol. 2021, 149, 111317.
- Hoffmann, J.; Mas-Peiro, S.; Berkowitsch, A.; Boeckling, F.; Rasper, T.; Pieszko, K.; De Rosa, R.; Hiczkiewicz, J.; Burchardt, P.; Fichtlscherer, S.; et al. Inflammatory signatures are associated with increased mortality after transfemoral transcatheter aortic valve implantation. ESC Heart Fail. 2020, 7, 2597–2610.
- Baratchi, S.; Zaldivia, M.T.K.; Wallert, M.; Loseff-Silver, J.; Al-Aryahi, S.; Zamani, J.; Thurgood, P.; Salim, A.; Htun, N.M.; Stub, D.; et al. Transcatheter Aortic Valve Implantation Represents an Anti-Inflammatory Therapy via Reduction of Shear Stress–Induced, Piezo-1–Mediated Monocyte Activation. Circulation 2020, 142, 1092–1105.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
26 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




