
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paula Garcia-Oliveira | -- | 3648 | 2022-05-25 16:46:35 | | | |
| 2 | Vivi Li | -6 word(s) | 3642 | 2022-05-26 05:04:08 | | |
Video Upload Options
Extra virgin olive oil (EVOO) is one of the most distinctive ingredients of the Mediterranean diet. There are many properties related to this golden ingredient, from supreme organoleptic characteristics to benefits for human health. EVOO contains in its composition molecules capable of exerting bioactivities such as cardio protection, antioxidant, anti-inflammatory, antidiabetic, and anticancer activity, among others, mainly caused by unsaturated fatty acids and certain minor compounds such as tocopherols or phenolic compounds. EVOO is considered the highest quality vegetable oil, which also implies a high sensory quality. The organoleptic properties related to the flavor of this valued product are also due to the presence of a series of compounds in its composition, mainly some carbonyl compounds found in the volatile fraction, although some minor compounds such as phenolic compounds also contribute.
1. Introduction
2. EVOO’s Flavor
2.1. Flavor Compounds
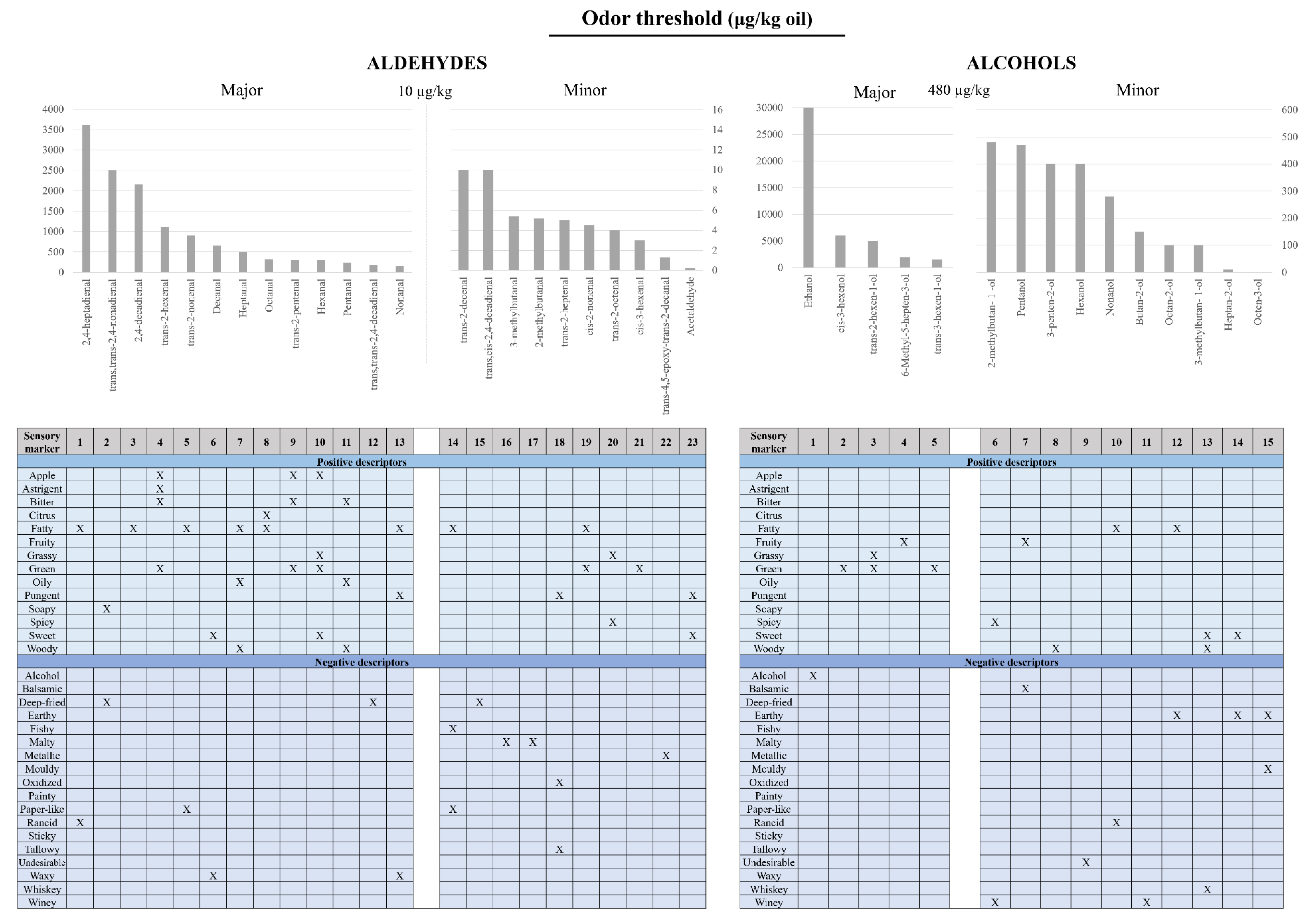
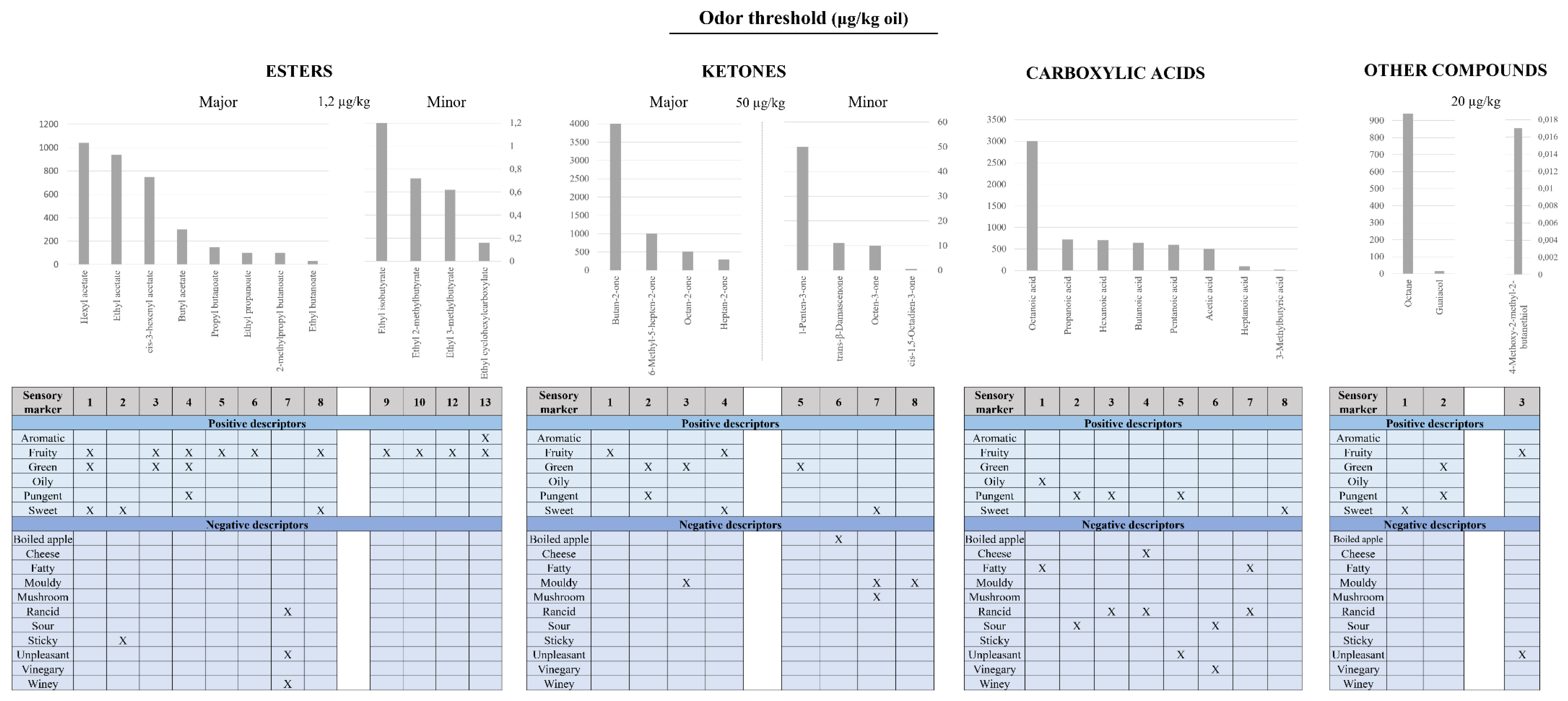
2.2. Influencing Factors in EVOO’s Flavor Compounds
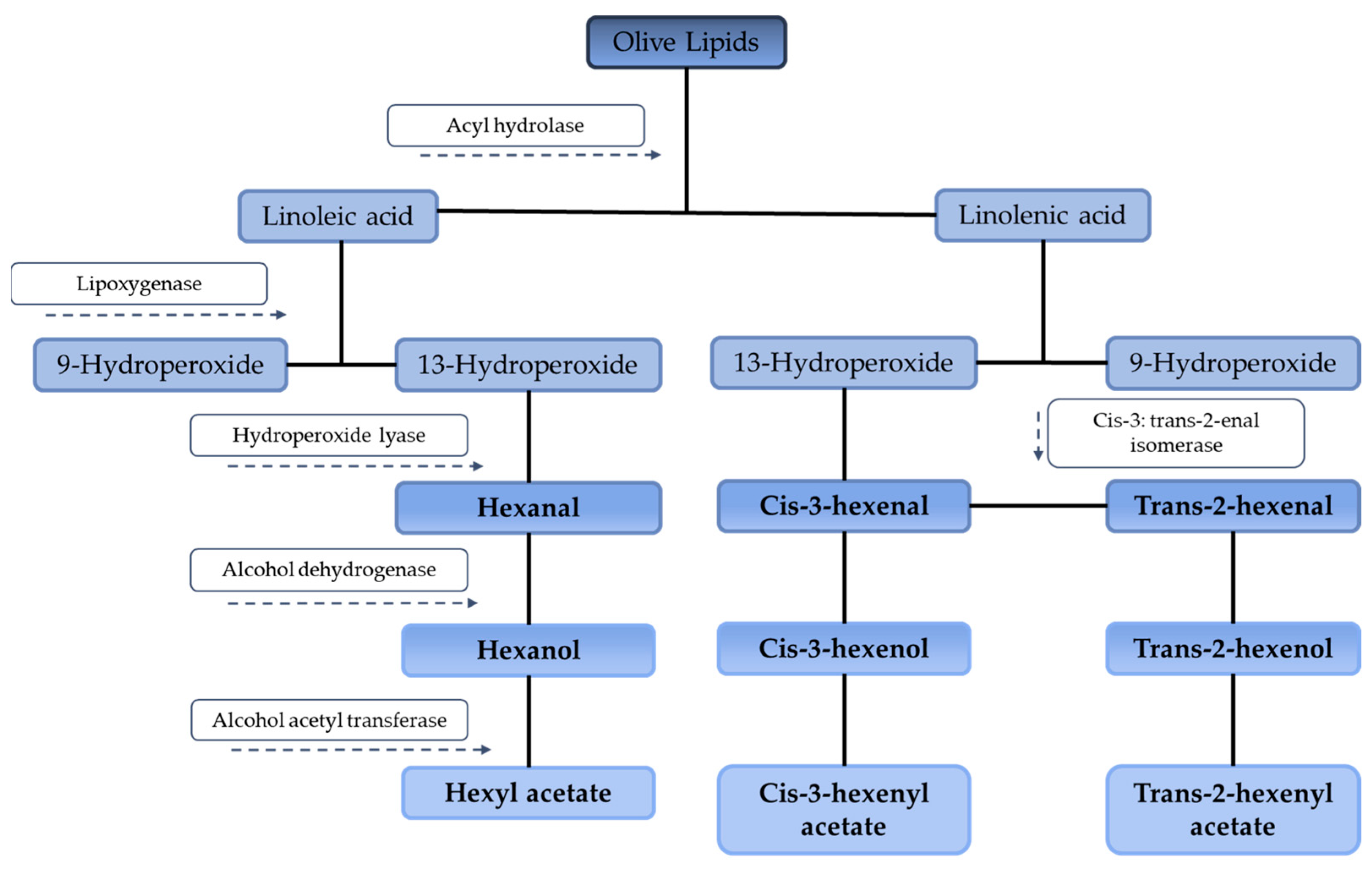
3. Degradation of EVOO’s Organoleptic Properties
References
- Hachicha Hbaieb, R.; Kotti, F.; García-Rodríguez, R.; Gargouri, M.; Sanz, C.; Pérez, A.G. Monitoring endogenous enzymes during olive fruit ripening and storage: Correlation with virgin olive oil phenolic profiles. Food Chem. 2015, 174, 240–247.
- Jiménez Herrera, B.; Carpio Dueñas, A. La Cata De Aceites: Aceite De Oliva Virgen Caracterêsticas Organolépticas Y Análisis Sensorial; de Andalucía, J., Instituto de Investigación y Formación Agraria y Pesquera, Consejería de Agricultura y Pesca, Eds.; Secretaria General Técnica (Andalucía): Andalucía, Spain, 2002; ISBN 9788484742715.
- International Olive Council. Trade Standard Applying to Olive Oils and Olive-Pomace Oils. IOC Stand. Methods Guides 2015, 15, 1–17.
- International Olive Council. World Olive Encyclopaedia; International Olive Oil Council: Madrid, Spain, 1996; ISBN 9788401618819.
- Fragaki, G.; Spyros, A.; Siragakis, G.; Salivaras, E.; Dais, P. Detection of extra virgin olive oil adulteration with lampante olive oil and refined olive oil using nuclear magnetic resonance spectroscopy and multivariate statistical analysis. J. Agric. Food Chem. 2005, 53, 2810–2816.
- Clodoveo, M.L.; Camposeo, S.; Amirante, R.; Dugo, G.; Cicero, N.; Boskou, D. Research and Innovative Approaches to Obtain Virgin Olive Oils with a Higher Level of Bioactive Constituents. In Olive and Olive Oil Bioactive Constituents; Academic Press: Cambridge, MA, USA; AOCS Press: Amsterdam, The Netherlands, 2015; pp. 179–215. ISBN 9781630670429.
- Alu, M.H.; Rababah, T.; Alhamad, M.N. Application of Olive Oil as Nutraceutical and Pharmaceutical Food: Composition and Biofunctional Constituents and Their Roles in Functionality, Therapeutic, and Nutraceutical Properties. In Soft Chemistry and Food Fermentation; Grumezescu, A., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2017; pp. 265–298. ISBN 9780128114124.
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153.
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and. Adv. Nutr. 2017, 8, 17–26.
- Serreli, G.; Deiana, M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants 2018, 7, 170.
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776.
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014.
- Santis, S.D.; Cariello, M.; Piccinin, E.; Sabb, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085.
- Ros, A.D.; Masuero, D.; Riccadonna, S.; Brki, K.; Mulinacci, N.; Mattivi, F.; Luki, I. Complementary Untargeted and Targeted Metabolomics for Di ff erentiation of Extra Virgin Olive Oils of Di ff erent Origin of Purchase Based on Volatile and Phenolic Composition and sensory quality. Molecules 2019, 24, 2896.
- Haddada, F.M.; Manai, H.; Daoud, D.; Fernandez, X.; Lizzani-Cuvelier, L.; Zarrouk, M. Profiles of volatile compounds from some monovarietal Tunisian virgin olive oils. Comparison with French PDO. Food Chem. 2007, 103, 467–476.
- Abbatangelo, M.; Núñez-Carmona, E.; Duina, G.; Sberveglieri, V. Multidisciplinary approach to characterizing the fingerprint of Italian EVOO. Molecules 2019, 24, 1457.
- Inarejos-garcía, A.M.; Santacatterina, M.; Salvador, M.D.; Fregapane, G.; Gómez-alonso, S. PDO virgin olive oil quality—Minor components and organoleptic evaluation. Food Res. 2010, 43, 2138–2146.
- Albuquerque, S.; Costa, H.S.; Oliveira, M.B.P.P. An Overview of Portuguese Olive Oils and Table Olives with Protected Designation of Origin. Eur. J. Lipid Sci. Technol. 2019, 121, 1–21.
- Barbieri, S.; Bendini, A.; Toschi, T.G. Recent Amendment to Product Specification of Brisighella PDO (Emilia-Romagna, Italy): Focus on Phenolic Compounds and Sensory Aspects. Eur. J. Lipid Sci. Technol. 2019, 121, 1–7.
- Zampounis, V.; Kontothanasis, K.; Christopoulou, E. Olive Oils from Greece. In Olive Oil Sensory Science; Monteleone, E., Langstaff, S., Eds.; Wiley-Blackwell: Chichester, UK, 2014; pp. 269–287. ISBN 9781118332528.
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286.
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–77. ISBN 9780444640574.
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833.
- Pereira, A.G.; Fraga, M.; Garcia-Oliveira, P.; Carpena, M.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Barros, L.; Ferreira, I.C.F.R.; Prieto, M.A.; Simal-Gandara, J. Management of Wine Aroma Compounds: Principal Basis and Future Perspectives; IntechOpen: London, UK, 2020; ISBN 9781839625763.
- Kanavouras, A.; Hernandez-Munoz, P.; Coutelieris, F.A. Packaging of olive oil: Quality issues and shelf life predictions. Food Rev. Int. 2006, 22, 381–404.
- Zhu, H.; Wang, S.C.; Shoemaker, C.F. Volatile constituents in sensory defective virgin olive oils. Flavour Fragr. J. 2016, 31, 22–30.
- Kesen, S.; Kelebek, H.; Sen, K.; Ulas, M.; Selli, S. GC-MS-olfactometric characterization of the key aroma compounds in Turkish olive oils by application of the aroma extract dilution analysis. Food Res. Int. 2013, 54, 1987–1994.
- Amanpour, A.; Kelebek, H.; Kesen, S.; Selli, S. Characterization of Aroma-Active Compounds in Iranian cv. Mari Olive Oil by Aroma Extract Dilution Analysis and GC–MS-Olfactometry. J. Am. Oil Chem. Soc. 2016, 93, 1595–1603.
- Tanouti, K.; Serghini-Caid, H.; Sindic, M.; Wathelet, J.-P.; Bouseta, A.; Elamrani, A. Volatile Compounds, Profiles of Virgin Olive Oils Produced In the Eastern Morocco: Oxidative Stability and Sensory Defects. J. Food Res. 2012, 1, 194–206.
- Pedan, V.; Popp, M.; Rohn, S.; Nyfeler, M.; Bongartz, A. Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules 2019, 24, 2041.
- Angerosa, F. Sensory Quality of Olive Oils. In Handbook of Olive Oil; Springer Internation Publisher: Berlin/Heidelberg, Germany, 2000; pp. 355–392.
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660.
- Cecchi, T.; Alfei, B. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME-GC-MS: Newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem. 2013, 141, 2025–2035.
- Sánchez, J.; Salas, J.J. Biogenesis of Olive Oil Aroma. In Handbook of Olive Oil; Springer Internation Publisher: Berlin/Heidelberg, Germany, 2000; pp. 79–99.
- Gargouri, O.D.; Rouina, Y.B.; Mansour, A.B.; Flamini, G.; Rouina, B.B.; Bouaziz, M. Comparative study of oil quality and aroma profiles from tunisian olive cultivars growing in saharian oasis using chemometric analysis. J. Oleo Sci. 2016, 65, 1033–1044.
- Reiners, J.; Grosch, W. Odorants of Virgin Olive Oils with Different Flavor Profiles. J. Agric. Food Chem. 1998, 46, 2754–2763.
- Zhou, Q.; Liu, S.; Liu, Y.; Song, H. Comparative analysis of volatiles of 15 brands of extra-virgin olive oils using solid-phase micro-extraction and solvent-assisted flavor evaporation. Molecules 2019, 24, 1512.
- Malheiro, R.; Rodrigues, N.; Pereira, J.A. Olive Oil Phenolic Composition as Affected by Geographic Origin, Olive Cultivar, and Cultivation Systems. In Olive and Olive Oil Bioactive Constituents; Academic Press: Cambridge, MA, USA; AOCS Press: Amsterdam, The Netherlands, 2015; pp. 93–121. ISBN 9781630670429.
- Gómez-Rico, A.; Fregapane, G.; Salvador, M.D. Effect of cultivar and ripening on minor components in Spanish olive fruits and their corresponding virgin olive oils. Food Res. Int. 2008, 41, 433–440.
- Youssef, N.B.; Zarrouk, W.; Carrasco-Pancorbo, A.; Ouni, Y.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Daoud, D.; Zarrouk, M. Effect of olive ripeness on chemical properties and phenolic composition of chétoui virgin olive oil. J. Sci. Food Agric. 2010, 90, 199–204.
- Lozano-Sánchez, J.; Bendini, A.; Quirantes-Piné, R.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Monitoring the bioactive compounds status of extra-virgin olive oil and storage by-products over the shelf life. Food Control 2013, 30, 606–615.
- Genovese, A.; Caporaso, N.; De Luca, L.; Paduano, A.; Sacchi, R. Influence of Olive Oil Phenolic Compounds on Headspace Aroma Release by Interaction with Whey Proteins. J. Agric. Food Chem. 2015, 63, 3838–3850.
- Manai, H.; Mahjoub-Haddada, F.; Oueslati, I.; Daoud, D.; Zarrouk, M. Characterization of monovarietal virgin olive oils from six crossing varieties. Sci. Hortic. (Amsterdam) 2008, 115, 252–260.
- Connor, D.J.; Gómez-del-Campo, M.; Rousseaux, M.C.; Searles, P.S. Structure, management and productivity of hedgerow olive orchards: A review. Sci. Hortic. (Amsterdam) 2014, 169, 71–93.
- Sanz, C.; Belaj, A.; Sánchez-Ortiz, A.; Pérez, A.G. Natural variation of volatile compounds in virgin olive oil analyzed by HS-SPME/GC-MS-FID. Separations 2018, 5, 24.
- Lukic, I.; Zanetic, M.; Spika, M.J.; Lukic, M.; Koprivnjak, O.; Bubola, K.B. Complex interactive effects of ripening degree, malaxation duration and temperature on Oblica cv. virgin olive oil phenols, volatiles and sensory quality ˇ anetic. Food Chem. 2017, 232, 610–620.
- Lukić, I.; Krapac, M.; Horvat, I.; Godena, S.; Kosić, U.; Bubola, K.B. Three-factor approach for balancing the concentrations of phenols and volatiles in virgin olive oil from a late-ripening olive cultivar. LWT Food Sci. Technol. 2018, 87, 194–202.
- Koprivnjak, O.; Procida, G.; Zelinotti, T. Changes in the volatile components of virgin olive oil during fruit storage in aqueous media. Food Chem. 2000, 70, 377–384.
- Oueslati, I.; Krichene, D.; Manaï, H.; Taamalli, W.; Zarrouk, M.; Flamini, G. Monitoring the volatile and hydrophilic bioactive compounds status of fresh and oxidized Chemlali virgin olive oils over olive storage times. Food Res. Int. 2018, 112, 425–433.
- Mildner-Szkudlarz, S.; Jeleń, H.H. The potential of different techniques for volatile compounds analysis coupled with PCA for the detection of the adulteration of olive oil with hazelnut oil. Food Chem. 2008, 100, 751–761.
- Giuffrè, A.M.; Capocasale, M.; Macrì, R.; Caracciolo, M.; Zappia, C.; Poiana, M. Volatile profiles of extra virgin olive oil, olive pomace oil, soybean oil and palm oil in different heating conditions. LWT 2020, 117, 108631.
- Meenu, M.; Cai, Q.; Xu, B. A critical review on analytical techniques to detect adulteration of extra virgin olive oil. Trends Food Sci. Technol. 2019, 91, 391–408.
- Nerín, C.; Tovar, L.; Salafranca, J. Behaviour of a new antioxidant active film versus oxidizable model compounds. J. Food Eng. 2008, 84, 313–320.
- Otero-Pazos, P.; Sendón, R.; Martínez, I.; Aurrekoetxea, G.P.; Angulo, I.; Rodríguez-Bernaldo de Quirós, A. Evaluation of oxygen absorber system effectiveness in butter containers. CyTA J. Food 2018, 16, 205–212.
- Robards, K.; Kerr, A.F.; Patsalides, E.; Korth, J. Headspace gas analysis as a measure of rancidity in corn chips. J. Am. Oil Chem. Soc. 1988, 65, 1621–1626.
- Pastorelli, S.; Valzacchi, S.; Rodriguez, A.; Simoneau, C. Solid-phase microextraction method for the determination of hexanal in hazelnuts as an indicator of the interaction of active packaging materials with food aroma compounds. Food Addit. Contam. 2006, 23, 1236–1241.
- Kanavouras, A.; Hernandez-Münoz, P.; Coutelieris, F.A. Shelf life predictions for packaged olive oil using flavor compounds as markers. Eur. Food Res. Technol. 2004, 219, 190–198.
- Cavalli, J.F.; Fernandez, X.; Lizzani-Cuvelier, L.; Loiseau, A.M. Characterization of volatile compounds of French and Spanish virgin olive oils by HS-SPME: Identification of quality-freshness markers. Food Chem. 2004, 88, 151–157.
- Serrano, A.; De la Rosa, R.; Sanchez-Ortiz, A.; Cano, J.; Perez, A.G.; Sanz, C.; Arias-Calderon, R.; Velasco, L.; Leon, L. Chemical components influencing oxidative stability and sensorial properties of extra virgin olive oil and effect of genotype and location on their expression. LWT-Food Sci. Technol. 2021, 136, 110257.
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Dynamic headspace/GC-MS to control the aroma fingerprint of extra-virgin olive oil from the same and different olive varieties. Food Control 2012, 25, 684–695.
- Fullana, A.; Carbonell-Barrachina, A.A.; Sidhu, S. Comparison of volatile aldehydes present in the cooking fumes of extra virgin olive, olive, and canola oils. J. Agric. Food Chem. 2004, 52, 5207–5214.
- Halvorsen, B.L.; Blomhoff, R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr. Res. 2011, 55, 1654–1661.




