Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carolina Di Fabrizio | -- | 3474 | 2022-05-24 20:48:20 | | | |
| 2 | Catherine Yang | Meta information modification | 3474 | 2022-05-25 03:33:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Di Fabrizio, C.; Giorgione, V.; Khalil, A.; Murdoch, C. Oxidative Stress in Pregnancy. Encyclopedia. Available online: https://encyclopedia.pub/entry/23315 (accessed on 07 February 2026).
Di Fabrizio C, Giorgione V, Khalil A, Murdoch C. Oxidative Stress in Pregnancy. Encyclopedia. Available at: https://encyclopedia.pub/entry/23315. Accessed February 07, 2026.
Di Fabrizio, Carolina, Veronica Giorgione, Asma Khalil, Colin Murdoch. "Oxidative Stress in Pregnancy" Encyclopedia, https://encyclopedia.pub/entry/23315 (accessed February 07, 2026).
Di Fabrizio, C., Giorgione, V., Khalil, A., & Murdoch, C. (2022, May 24). Oxidative Stress in Pregnancy. In Encyclopedia. https://encyclopedia.pub/entry/23315
Di Fabrizio, Carolina, et al. "Oxidative Stress in Pregnancy." Encyclopedia. Web. 24 May, 2022.
Copy Citation
Human pregnancy can be affected by numerous pathologies, from those which are mild and reversible to others which are life-threatening. Oxidative stress is caused by an imbalance between production and accumulation of reactive oxygen species (ROS) in cells and tissues and the ability of a biological system to detoxify these reactive products. In pregnancy, ROS are generated mainly in the placenta, but xanthine oxidase (XO) are present in the vascular endothelium, and nitric oxide synthase (NOS) are also sources of ROS.
oxidative stress
pregnancy
preeclampsia
antioxidants
1. Oxidative Stress in Normal Pregnancies
As the placenta develops, it transits from a hypoxic environment to a more oxygenated setting. The period of placental development is characterised by a low grade of OS, increased circulating levels of oxidised low-density lipoproteins (LDL), and a reduction in total antioxidant capacity (TAC). A recent study by Mannaerts et al. [1] has shown that systemic inflammation also increases with the advance of pregnancy, thus activating maternal endothelial cells and increasing OS.
ROS are necessary for certain cellular functions, such as mitochondrial or endothelial functions, normally in low and stable levels. Redox signalling is pivotal in many physiological processes, whereby oxidative post-translational modifications induce changes in structural and functional characteristics of molecules, thus modifying signalling processes [2]. However, elevated levels of ROS, as observed in pathologic pregnancies, are associated with adverse outcomes, including tissue and mitochondrial damage and accelerated aging.
First Trimester
During the first 6–8 weeks of pregnancy, with the placenta not yet completely attached to maternal circulation, the uterine spiral arteries are blocked by intraluminal cytotrophoblast and a physiological and local hypoxia is generated. In this period, since the syncytiotrophoblast cells do not express the antioxidant mitochondrial superoxide dismutase (SOD), ROS levels are high, inducing the production of factors that regulate cell proliferation and angiogenesis, such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), which are abundantly expressed in the placenta [3].
The angiogenic signalling pathway is tightly regulated at various levels, such as a soluble Fms-like tyrosine kinase-1 (sFlt-1), acting as a decoy receptor to neutralize the pro-angiogenic effects from VEGF and PIGF. Maternal blood levels of sFlt-1 and PlGF have been analysed extensively [4] and are utilised as key biomarkers during pregnancy (see below).
Second trimester
Unblocking of the spiral arteries begins at 8 weeks and continues until 16 weeks of gestation. While spiral arteries lose their muscular layer and transform into a large vessel with low resistance, an increased oxygen tension is quickly imposed, bringing the associated risks of damage from OS. In non-pathological concentrations, OS stimulates cell proliferation. However, with premature opening (unplugging) of the spiral arteries, the placental growth is prejudiced since there is insufficient antioxidant defence by this time. A deficiency in the early development of the placenta restricts its growth, which can never be catch up [5].
From a chemical point of view, these periods of ischemia followed by reperfusion are associated with the conversion of xanthine dehydrogenase into xanthine oxidase, which is a potent source of superoxide free radicals, thus increasing OS. It has been demonstrated that xanthine oxidase activity is increased in the placenta of women affected by HDP. In addition, a lower production of NO is related to the peripheral vasoconstriction observed in preeclampsia [6].
Third Trimester
The third trimester is the period of maximum growth of both the placenta and the foetus, corresponding to a great increase in placental vascular and energetic requests. During this stage, the highly metabolic placenta relies on efficient mitochondrial function to produce the necessary energy. In this scenario, a hypovolemic state could progress to placental hypoperfusion and, therefore, to foetal growth restriction.
Cardiovascular System
During pregnancy, the maternal cardiovascular system adapts to the increased demand, resulting in an increase in cardiac output, stroke volume, heart rate, and plasma volume. The heart undergoes significant remodelling to keep up with the demand. The right heart volume is significantly higher and left ventricle mass increases by an average of 40% by the end of the pregnancy [7]. Despite all these modifications, in most cases, maternal blood pressure remains stable, compensated by generalized peripheral vasodilation.
This vascular change is largely driven by a higher production of NO in endothelial cells [8]. NO is a prime target for inactivation by superoxide and this free radical is increased in preeclamptic pregnancies, thus explaining the generalized peripheral vasoconstriction observed in this pathological state. This systemic vasoconstriction can be clinically observed in preeclampsia and acts as a major indicator of pregnancy complications. In the first trimester, uterine artery Doppler can be performed to assess the risk of preeclampsia. The uterine artery pulsatility index (PI), defined as the difference between peak systolic and end diastolic flow velocity, divided by the time-averaged flow velocity [9], provides a measure of uteroplacental perfusion. A high PI index suggests an increased risk of developing PE, FGR, and stillbirth. Examples of normal and abnormal uterine artery doppler waveform in the first trimester are shown in Figure 1. Moreover, maternal renal vasoconstriction favours hypertension and a modest reduction in glomerular filtration rate with a latter disruption of glomerular fenestrae, causing proteinuria.
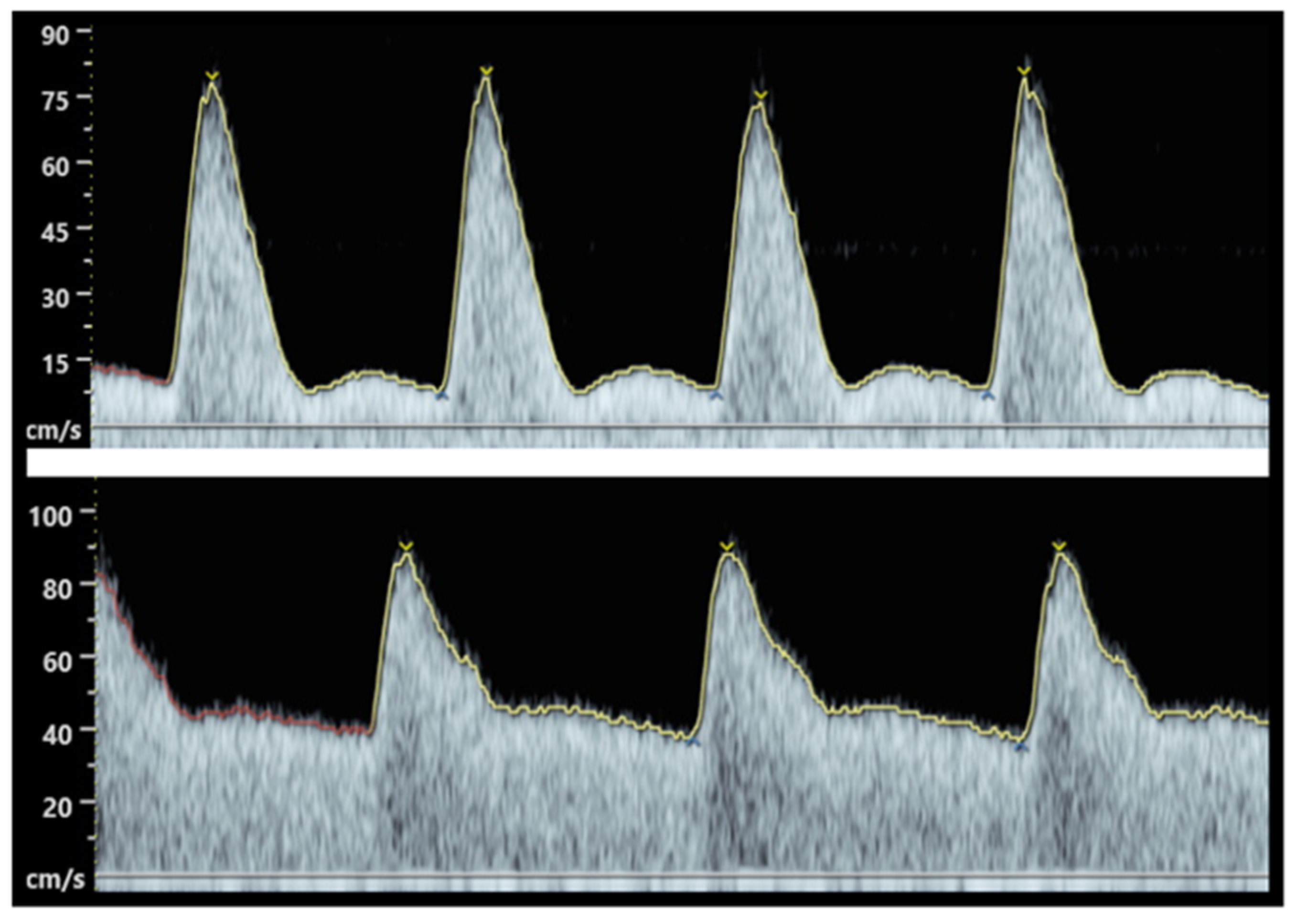
Figure 1. Abnormal (top) and normal (bottom) uterine artery Doppler waveform in the first trimester. While systolic velocities are similar, the lower waveform show a low resistance vessel with broader systolic peak, as well as a continuous diastolic flow, secondary to a complete remodelling of the spiral arteries. In contrast, the abnormal waveform (up) shows an artery with high resistance, as well as a sharp systolic peak, a flow reduction at the start of diastole (“notch”), and low diastolic velocities with poor blood flow.
2. Oxidative Stress in Pregnancy Pathologies and Its Biomarkers
Although the main focus of this entry is OS damage in pregnancy concerning the foetus and the mother, it is important to note that OS influences the entire reproductive lifespan of women and men. There has been a considerable amount of work regarding oxidative damage in sperm and oocytes. Male gametes are sensitive cells to the accumulation of damaged DNA, which can be induced by a wide variety of factors, such as diet [10], ionization, or even heavy metals [11][12]. Since DNA damage in the gametes could have serious consequences in reproduction [13], the sperm may also play a role in complicated pregnancies.
2.1. Oxidative Stress in Preeclampsia
Preeclampsia is a disease in which the mother develops high blood pressure after 20 weeks of pregnancy and presents with proteins in her urine, alterations in blood test, or clinical symptoms (such as severe headache, abdominal pain, or visual alterations). Clinically preeclampsia is defined by American College of Obstetricians and Gynaecologists (ACOG) (2013) as a “syndrome characterized by both new-onset of hypertension plus new-onset proteinuria ≥ 300 mg/24 h after 20 weeks of gestation or, in the absence of proteinuria, hypertension in association with thrombocytopenia, impaired liver function, renal insufficiency, pulmonary edema, or new-onset cerebral or visual disturbances’’ [14]. The only cure available for this clinical entity is delivery, which can represent a major disadvantage for the foetus if the pregnancy is not advanced, adding prematurity to a possible suboptimal development.
The aetiology of preeclampsia is not completely understood and has been previously described as a “2 stage-disease” [15]. During the first trimester, abnormal and asymptomatic placentation occurs, followed by a symptomatic maternal syndrome that carries an excess of antiangiogenic factors. The definition was later expanded to a “6-stage” disease [16], incorporating immunological factors (toleration of the mother to the semen of the father), abnormal placentation, and OS in the first 10 weeks, as well as placental damage and atherosis. See Figure 2 for an overview of the main events in the development of preeclampsia.
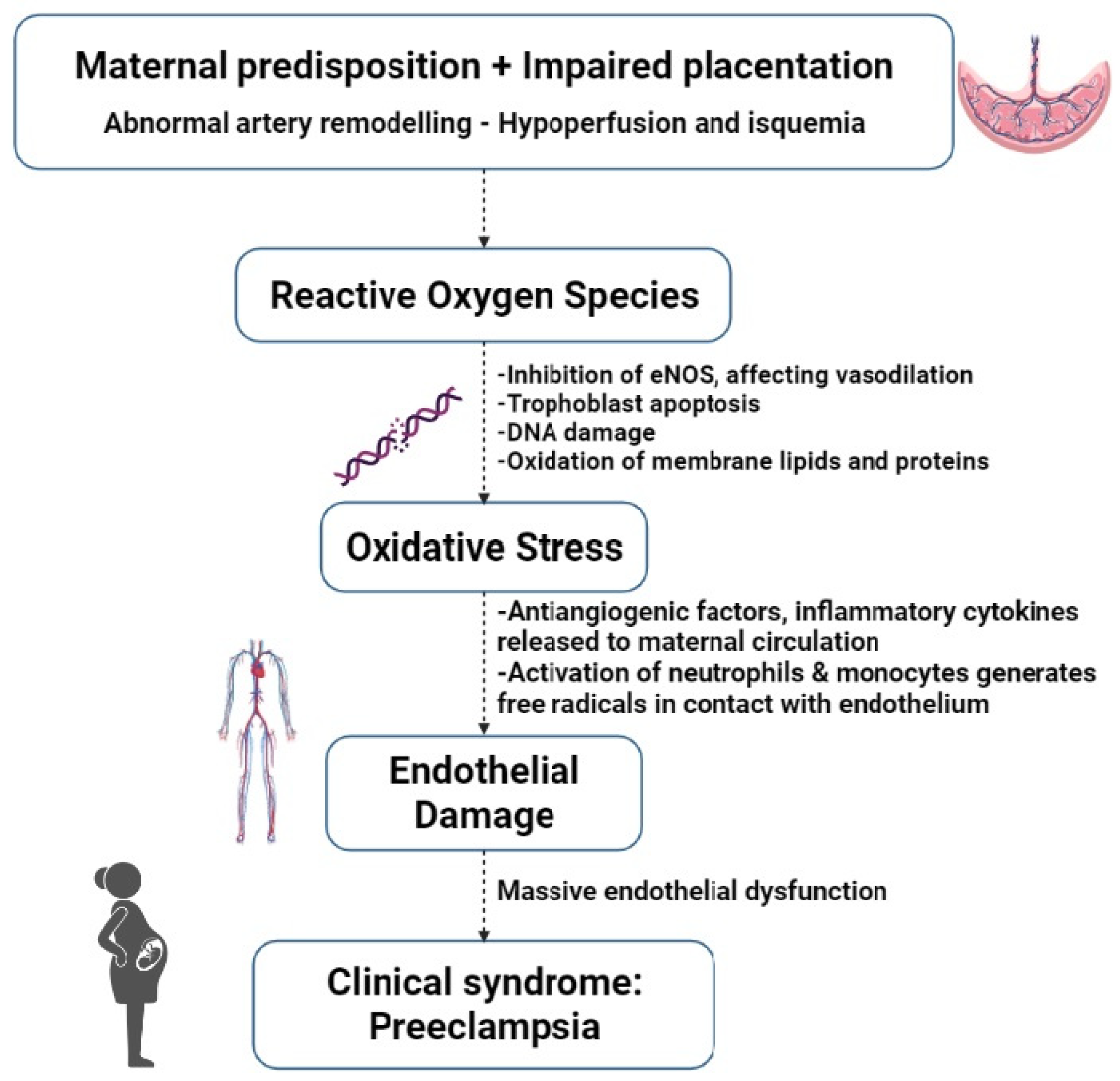
Figure 2. Main events in the development of preeclampsia. eNOS = endothelial nitric oxide synthase.
According to the timing of clinical onset, before or after 34 weeks of gestation, preeclampsia is classified as early-onset preeclampsia (EoPE) and late-onset preeclampsia (LoPE), respectively.
EoPE, also referred to as “placental preeclampsia”, is associated with a poor development of the cytotrophoblast early in pregnancy, leading to reduced transformation of spiral arteries. While this subtype is the less frequent (5% to 20% of all cases), its clinical impact is significant, since it can add prematurity to a foetus that has been developing in sub-optimal conditions if delivery becomes necessary. The threshold of foetal viability is around 24 to 26 weeks; however, babies born at this gestational age present high mortality and morbidity, as 50% of neonates that survive will suffer from moderate to severe neurological sequelae [17].
LoPE, also referred to as “maternal preeclampsia”, is associated with a mismatch between the increasing metabolic demands of the placenta–foetal duet and the normal maternal perfusion, contributing to a maternal predisposition to cardiovascular and metabolic pathologies. It represents more than 80% of all cases. Although foetuses at this point are already more developed, they are at risk of being either small or large for gestational age and they tend to have less adaptability and can rapidly deteriorate [18].
Despite the classification of EoPE and LoPE attributing the cause to the placenta and maternal, respectively, it is important to clarify that the combination of both maternal and placental factors can contribute to the development of both types of preeclampsia.
OS provides one explanation for the pathogenesis of preeclampsia since it leads to lipid peroxidation accompanied by endothelial dysfunction, i.e., a consequence of periods of ischemia–reperfusion generated from failed spiral artery remodelling during placentation. A vicious cycle of enhanced placental OS can allow the release of leukocytes, neutrophils, and cytokines from the placenta, as well as further ROS into the maternal blood circulation, resulting in a massive systemic endothelial dysfunction. In cases where great endothelial damage is observed, arterial compliance is lower and vascular resistance is higher [19]. Furthermore, as widely demonstrated in the Framingham Heart Study [20], arterial stiffness acts as a predictor of cardiovascular disease, cognitive impairment, and dementia.
Elevated ROS levels could also provide the cause for early elevation of antiangiogenic or decrease in angiogenic factors at a time when the placenta needs more vascular development. There is abundant evidence of higher serum and tissue concentrations of biomarkers for OS and systemic inflammation, as well as decreased concentrations of antioxidants, such as vitamin C and vitamin E in women with preeclampsia [21].
However, a major challenge in recent decades has been to establish reliable biomarkers for the early detection and prediction of pregnancy complications. Identifying a circulatory biomarker can have major clinical impacts, especially if they can provide early opportunities for clinical intervention. Unfortunately, to date, both predictive and diagnostic biomarkers have rarely been successful. Several molecules related to OS stress have been proposed, since there are direct chemical interactions between ROS and biological components in preeclampsia. Nevertheless, most of these biomarkers are diagnostic, meaning they are elevated by the time the disease is clinically present, thus with scarce predictive capacity.
Glutathione (GSH), one of the most prevalent antioxidants, can reduce unstable ROS and form oxidized GSH. Reduced GSH represents the most prevalent form of GSH in the organism and the ratio of reduced GSH and oxidized GSH can be used to demonstrate levels of OS. In a study conducted by Kharb et al. [22], women with preeclampsia were found to have significantly lower levels of reduced GSH in blood compared with healthy pregnant controls.
With regards to the lower levels of reduced GSH seen in preeclamptic patients, it is important to note that an association between the use of paracetamol (acetaminophen) and the depletion of GSH has been described [23]. Paracetamol is one of the most consumed drugs during pregnancy, not limited to hypertensive women. In fact, it has been published that more than 40% of pregnant women use paracetamol at least once in pregnancy [24]. Although it is feasible to speculate that paracetamol may be responsible for lower GSH levels, it is doubtful that such a chronic effect would be sustained. Further investigations are needed in order to elucidate the eventual correlation between this worldwide used drug and the antioxidant capacity during pregnancy.
In response to OS, protein carbonyls are generated and researchers have shown elevated levels of these molecules in plasma [25] and placentas [26][27] of preeclamptic women during the clinical stage. However, higher levels of protein carbonyls were also described in pregnancies complicated with GDM [28], making these molecules a poorly specific biomarker.
2.2. Oxidative Stress in Foetal Growth Restriction (FGR)
FGR or intra-uterine growth restriction (IUGR) is a common pregnancy pathology in which the foetus is unable to achieve its genetically determined potential size and weight. Growth restricted foetuses are under the tenth centile of estimated foetal weight compared to standard weights at a given gestational age, affecting 10–15% of pregnancies [29]. They carry a higher risk of long-term complications, such as poor cognitive performance, growth retardation, as well as lifelong risk of cardiovascular disease and metabolic syndrome.
Since the main cause is placental dysfunction, it has been associated with elevated OS. Placentas from pregnancies with FGR have a significantly decreased expression of genes involved in mitochondrial function and oxidative phosphorylation but higher markers of OS [30]. Additionally, elevated levels of oxidized low-density lipoproteins (Ox-LDL) in placental tissue have been shown in patients with preeclampsia and FGR. A major issue with utilising oxidative stress biomarkers for pregnancy complications is that labour activates oxidative stress in the placenta [31], which can lead to wrong conclusions. Care needs to be taken when interpreting results from placenta and blood taken post-delivery.
Studies that have monitored blood levels of ROS during pregnancies with FGR have shown an increased level of MDA—a breakdown product of lipid peroxidation [32], as well as reduced plasma TAC [33] both in maternal plasma, placental tissue [34], and cord blood in the infants [35].
Pregnancy-associated plasma protein-A (PAPP-A) is a protein also related to FGR. It is synthesized by the decidua and measurable in maternal blood in early pregnancy. One of its functions is to cleave IGFBP-4, an insulin growth factor (IGF) inhibitor, thus augmenting the activity of IGFs. Lower levels of PAPP-A are associated with an increased risk for FGR.
2.3. Oxidative Stress in Gestational Diabetes Mellitus (GDM)
GDM is usually a transient hyperglycaemic state brought on by pregnancy and linked to insulin dysregulation. Clinically, it is defined as “carbohydrate intolerance of variable severity with onset or first recognition during pregnancy” [36], and it is estimated that, worldwide, one in seven pregnant women may suffer from hyperglycaemia, which in 85% of cases corresponds to GDM (WHO, 2016). However, GDM and type 2 diabetes share a common pathogenesis related to insulin resistance or β-cell dysfunction. It represents an important gestational disorder and a precursor for lifetime disease, since up to half of women with a history of GDM will develop type 2 diabetes five to ten years after delivery [37].
Following NICE Guidelines [38], pregnant patients are offered a 75 g 2 h oral glucose tolerance test (OGTT) at 24 to 28 weeks. GDM is diagnosed if the woman has either a fasting plasma glucose level of >5.6 mmol/L or a 2 h plasma glucose level of >7.8 mmol/L.
Being closely related to obesity, a new term has emerged in recent years: “Diabesity”, considered a modern epidemic, which indicates the coexistence of both diabetes and obesity [6]. In the last three decades, prevalence of GDM has increased ostensibly in all countries despite the income levels (WHO, 2016).
As in preeclampsia, increased ROS and lower plasma antioxidant capacity occur in association with an altered maternal metabolic environment, and GDM is frequently associated with systemic and chronic inflammation. It has been observed that insulin resistance reduces mitochondrial respiration [39] and that human umbilical cord mesenchymal stromal cells from patients with GDM have premature senescence phenotypes and mitochondrial dysfunction.
The increased levels of ROS are associated with non-enzymatic glycation of macromolecules, producing “advanced glycation end products” (AGEs), which can lead to further OS, inflammatory, and thrombotic reactions, thus playing a role in the development of maternal and neonatal complications [40]. Elevated levels of AGEs are also observed in patients with preeclampsia [41].
Moreover, hyperglycaemia has been found to upregulate NADPH oxidase, whose primary role is to generate ROS [42]. As shown by Leloup et al. [43], more than a decade ago, glucose has the capacity to induce ROS and H2O2 production in isolated rat islets of Langerhans. Furthermore, insulin secretion can be induced by mitochondrial ROS. It has been demonstrated that incubation of trophoblast from normal placentas, with glucose at a concentration similar to in vivo hyperglycaemic levels, also generates a rise in MDA [30].
Other molecules involved in OS and GDM are F2-isoprostanes, formed mostly by the peroxidation of arachidonic acid. They have an important role in organ vasoconstriction, including in the kidney and the placenta, and can be detected in the plasma and urine of diabetic and preeclamptic pregnant women. Kapustin et al. [44] recently described increased levels of isoprostanes in patients with GDM, thus adding more information to what has already been published by Walsh [45], who had demonstrated higher levels of isoprostanes in preeclamptic placentas.
3. Oxidative Stress in Common Risk Factors for Complicated Pregnancies
OS plays an important role, not only in preeclampsia and GDM, but in several disorders which represent risk factors for complicated pregnancy and subsequent (and possible pre-) comorbidities, i.e., type 2 diabetes, renal failure, and cardiovascular disease.
3.1. Endothelial Dysfunction
Vascular endothelium consists of a single but complex layer of epithelial cells covering the interior surface of blood vessels. This organ is responsible for controlling the passage of molecules in and out of the bloodstream; has paracrine and autocrine functions; and plays a role in inflammatory cell adherence, anticoagulation, and angiogenesis. Endothelial dysfunction is a key event in the development of vascular diseases and it is enhanced by OS, leading to cardiac failure, peripheral artery disease, diabetes mellitus, and stroke [30]. It is a state characterized by vasoconstriction, inflammation, and prothrombotic tendency.
On a chemical pathway, excessive production of ROS induces oxidation of tetrahydrobiopterin (BH4), a cofactor of endothelial NOS, which produces NO from l-arginine. In consequence, lower levels of BH4 results in less generation of NO [46]. Moreover, the deficient generation of NO causes systemic vasoconstriction, leading to hypertension, hypoperfusion, and ischemia. In addition, ROS modifies the intracellular influx of calcium, resulting in interstitial oedema, haemoconcentration, and ischemia, as well as further production of ROS, creating a self-perpetuating cycle [30].
Furthermore, IGF-1 is synthesized by several tissues and, after binding with its receptor (IGF-1R), becomes an important mediator of cell growth and differentiation, as well as an antiapoptotic factor in endothelial cells. IGF-1 enhances NO production by eNOS and upregulates antioxidant enzymes, such as glutathione peroxidase (GPX) and superoxide dismutase (SOD). IGF-1-infused animals show lower vascular superoxide levels and higher levels of vascular eNOS and NO [47].
3.2. Obesity
The World Health Organization recognizes obesity as a global epidemic. In 2014, approximately 13% of the world’s population was classified as obese, defined as “abnormal or excessive fat accumulation that presents a risk to health. A body mass index (BMI) over 25 kg/m2 is considered overweight and over 30 kg/m2 is obese” (WHO, 2016). The same organization in 2011 estimated that the female prevalence of overweight and obesity was as high as 77% in the United States, 69% in South Africa, 37% in France, and 32% in China. It is an increasingly common complication in pregnancies, related to the lifestyles of modern society and changes in dietary habits.
This situation also carries major economic consequences. In 2000, Galtier-Dereure et al. [48] described that the average cost of hospital prenatal care was five times higher in mothers who were overweight before pregnancy than in normal-weight control women.
Obese women are 2–3 times more likely to develop preeclampsia [39] since metabolic factors related to obesity (lipids, insulin, glucose, and leptin) enhance placental and endothelial dysfunction. It has been proposed that obesity induces a state of mitochondrial dysfunction and OS [49]. Furthermore, NO increases blood flow by relaxing the smooth vascular muscle and abdominal, and central obesity leads to an imbalanced production of fat-derived metabolic products, hormones, and adipokines that predispose to a state endothelial dysfunction, activating NADPH oxidase [50]. The FINNPEC cohort [51], a cross-sectional case control study in Finland from 2008 to 2011, confirmed that preeclamptic women had increased pre-pregnancy BMI, demonstrating the close relation between obesity and adverse pregnancy outcomes.
Obesity also modifies the immune system. The uterine environment of obese women shows natural killer cells overexpressing the Decorin gene, which codifies a protein that promotes the apoptosis of proliferative trophoblasts [52].
3.3. Advanced Maternal Age (AMA)
Defined as pregnancy among women aged 35 years or older, it has become more prevalent in modern society due to women postponing motherhood, resulting in an advanced maternal age at first pregnancy. In 2013, women aged 35 years or over were responsible for 20% of births in England while women 40 years or over were responsible for 4% of births in England, compared to 6% and 1%, respectively, in 1980 [53].
Older women are more likely to suffer from previous pathological conditions, such as obesity, high blood pressure, or insulin resistance, placing them at high risk for pregnancy complications. It has long been suggested that ageing per se is associated with increased inflammation and ROS. As Odame Anto et al. [54] highlighted, women with AMA (35–45 years old) presented higher levels of sFlt-1 at 28–32 weeks and after birth than pregnant women of optimal childbearing age (20–29 years old). Conversely, levels of PIGF, TAC, and PIGF: sFlt-1 ratio were significantly lower in the older group.
References
- Mannaerts, D.; Faes, E.; Cos, P.; Briede, J.J.; Gyselaers, W.; Cornette, J.; Gorbanev, Y.; Bogaerts, A.; Spaanderman, M.; Van Craenenbroeck, E.; et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS ONE 2018, 13, e0202919.
- Lee, M.Y.; Griendling, K.K. Redox Signaling, Vascular Function, and Hypertension. Antioxid. Redox Signal. 2008, 10, 1045–1059.
- Huang, Q.T.; Zhang, M.; Zhong, M.; Yu, Y.H.; Liang, W.Z.; Hang, L.L.; Gao, Y.F.; Huang, L.P.; Wang, Z.J. Advanced glycation end products as an upstream molecule triggers ROS-induced sFlt-1 production in extravillous trophoblasts: A novel bridge between oxidative stress and preeclampsia. Placenta 2013, 34, 1177–1182.
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.-L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161.
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49.
- Maia, L.B.; Moura, J.J.G. Putting xanthine oxidoreductase and aldehyde oxidase on the NO metabolism map: Nitrite reduction by molybdoenzymes. Redox Biol. 2018, 19, 274–289.
- Melchiorre, K.; Sharma, R.; Khalil, A.; Thilaganathan, B. Maternal Cardiovascular Function in Normal Pregnancy. Hypertension 2016, 67, 754–762.
- Staff, A.; Zeisler, H.; Llurba, E.; Chantraine, F.; Vatishe, M.; Sennströmf, M.; Olovssong, M.; Brennecke, S.; Stepani, H.; Allegranza, D.; et al. Angiogenic factors and prediction of adverse pregnancy outcomes in suspected preeclampsia: The PROGNOSIS study. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2017, 7, 56–57.
- Nicolaides, K.; Rizzo, G.; Hecher, K.; Ximenes, R. Doppler in Obstetrics; Fetal Medicine Foundation: London, UK, 2002.
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568.
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4198.
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular effects of copper on the reproductive system of mytilus galloprovincialis. Mol. Reprod. Dev. 2019, 86, 1357–1368.
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires—A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci. 2020, 21, 6710.
- American College of Obstetricians and Gynecologists. Practice Bulletin. Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260.
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799.
- Redman, C. The six stages of pre-eclampsia. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2014, 4, 246.
- Peral, J.H.; Rodríguez, S.M.; Ayala, A.U.; González-Mesa, E.; Garcia, E.S. Manejo perinatal en el límite de la viabilidad. Propuestas de abordaje en un hospital terciario. Prog. Obstet. Ginecol. 2013, 56, 65–72.
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403.
- Perry, H.; Gutierrez, J.; Binder, J.; Thilaganathan, B.; Khalil, A. Maternal arterial stiffness in hypertensive pregnancies with and without small-for-gestational-age neonate. Ultrasound Obstet. Gynecol. 2020, 56, 44–50.
- Cooper, L.L.; Mitchell, G.F. Incorporation of Novel Vascular Measures into Clinical Management: Recent Insights from the Framingham Heart Study. Curr. Hypertens. Rep. 2019, 21, 19.
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876.
- Kharb, S. Low whole blood glutathione levels in pregnancies complicated by preeclampsia and diabetes. Clin. Chim. Acta 2000, 294, 179–183.
- Nuttall, S.L.; Khan, J.N.; Thorpe, G.H.; Langford, N.; Kendall, M.J. The impact of therapeutic doses of paracetamol on serum total antioxidant capacity. J. Clin. Pharm. Ther. 2003, 28, 289–294.
- Allegaert, K.; van den Anker, J.N. Perinatal and neonatal use of paracetamol for pain relief. Semin. Fetal Neonatal Med. 2017, 22, 308–313.
- Zusterzeel, P.L.M.; Mulder, T.P.J.; Peters, W.H.M.; Wiseman, S.A.; Steegers, E.A.P. Plasma protein carbonyls in nonpregnant, healthy pregnant and preeclamptic women. Free Radic. Res. 2000, 33, 471–476.
- Vanderlelie, J.; Venardos, K.; Clifton, V.L.; Gude, N.M.; Clarke, F.M.; Perkins, A.V. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005, 26, 53–58.
- Padmini, E.; Lavanya, S.; Uthra, V. Preeclamptic placental stress and over expression of mitochondrial HSP70. Clin. Chem. Lab. Med. 2009, 47, 1073–1080.
- Llurba, E.; Gratacós, E.; Martín-Gallán, P.; Cabero, L.; Dominguez, C. A comprehensive study of oxidative stress and antioxidant status in preeclampsia and normal pregnancy. Free Radic. Biol. Med. 2004, 37, 557–570.
- Rashid, C.S.; Bansal, A.; Simmons, R.A. Oxidative Stress, Intrauterine Growth Restriction, and Developmental Programming of Type 2 Diabetes. Physiology 2018, 33, 348–359.
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496.
- Burton, G.J.; Yung, H.-W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta 2009, 30, 43–48.
- Karowicz-Bilińska, A.; Suzin, J.; Sieroszewski, P. Evaluation of oxidative stress indices during treatment in pregnant women with intrauterine growth retardation. Med. Sci. Monit. 2002, 8, CR211-6.
- Cuffe, J.S.; Xu, Z.C.; Perkins, A.V. Biomarkers of oxidative stress in pregnancy complications. Biomark. Med. 2017, 11, 295–306.
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Maiahy, T.J. Concept and connotation of oxidative stress in preeclampsia. J. Lab. Physicians 2018, 10, 276–282.
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, Ö.; Durak, İ. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecol. Obstet. Investig. 2007, 64, 187–192.
- Tamás, G.; Kerényi, Z. Gestational diabetes: Current aspects on pathogenesis and treatment. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S400–S411.
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr. Diabetes Rep. 2016, 16, 7.
- National Institute for Health and Care Excellence (NICE). Diabetes in Pregnancy: Management from Preconception to the Postnatal Period; NICE Guidelines: London, UK, 2020; Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 12 March 2022).
- Hebert, J.F.; Myatt, L. Placental mitochondrial dysfunction with metabolic diseases: Therapeutic approaches. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 165967.
- Sisay, M.; Edessa, D.; Ali, T.; Mekuria, A.N.; Gebrie, A. The relationship between advanced glycation end products and gestational diabetes: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0240382.
- Chen, W.; Zhang, Y.; Yue, C.; Ye, Y.; Chen, P.; Peng, W.; Wang, Y. Accumulation of Advanced Glycation End Products Involved in Inflammation and Contributing to Severe Preeclampsia, in Maternal Blood, Umbilical Blood and Placental Tissues. Gynecol. Obstet. Investig. 2017, 82, 388–397.
- Xia, L.; Wang, H.; Munk, S.; Kwan, J.; Goldberg, H.J.; Fantus, I.G.; Whiteside, C.I. High glucose activates PKC-ζ and NADPH oxidase through autocrine TGF-β 1 signaling in mesangial cells. Am. J. Physiol.-Ren. Physiol. 2008, 295, F1705–F1714.
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.L.; Ktorza, A.; Casteilla, L.; Pénicaud, L. Mitochondrial Reactive Oxygen Species Are Obligatory Signals for Glucose-Induced Insulin Secretion. Diabetes 2009, 58, 673–681.
- Kapustin, R.; Chepanov, S.; Kopteeva, E.; Arzhanova, O. Maternal serum nitrotyrosine, 8-isoprostane and total antioxidant capacity levels in pre-gestational or gestational diabetes mellitus. Gynecol. Endocrinol. 2020, 36 (Suppl. S1), 36–42.
- Walsh, S.W. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000, 14, 1289–1296.
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165354.
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.-Y.; Delafontaine, P. IGF-1, oxidative stress and atheroprotection. Trends Endocrinol. Metab. 2010, 21, 245–254.
- Galtier-Dereure, F.; Boegner, C.; Bringer, J. Obesity and pregnancy: Complications and cost. Am. J. Clin. Nutr. 2000, 71, 1242S–1248S.
- Sen, S.; Herlihy, M.; Hacker, M.; Mcelrath, T.; Cherkerzian, S.; Oken, E.; Meydani, S. BMI-based Prenatal Vitamins to Ameliorate Oxidative Stress in Obese Pregnant Women: A Randomized Controlled Trial (P11-135-19). Curr. Dev. Nutr. 2019, 3 (Suppl. S1), nzz048-P11.
- Fortuño, A.; Bidegain, J.; Baltanás, A.; Moreno, M.U.; Montero, L.; Landecho, M.F.; Beloqui, O.; Díez, J.; Zalba, G. Is leptin involved in phagocytic NADPH oxidase overactivity in obesity? Potential clinical implications. J. Hypertens. 2010, 28, 1944–1950.
- Jääskeläinen, T.; Heinonen, S.; Hämäläinen, E.; Pulkki, K.; Romppanen, J.; Laivuori, H. Impact of obesity on angiogenic and inflammatory markers in the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) cohort. Int. J. Obes. 2019, 43, 1070–1081.
- Hoch, D.; Gauster, M.; Mouzon, S.H.; Desoye, G. Diabesity-associated oxidative and inflammatory stress signalling in the early human placenta. Mol. Asp. Med. 2019, 66, 21–30.
- Oficce for National Statistics, Birth Summary Tables. England and Wales. 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2020 (accessed on 30 March 2022).
- Odame Anto, E.; Owiredu, W.K.B.A.; Sakyi, S.A.; Turpin, C.A.; Ephraim, R.K.D.; Fondjo, L.A.; Obirikorang, C.; Adua, E.; Acheampong, E. Adverse pregnancy outcomes and imbalance in angiogenic growth mediators and oxidative stress biomarkers is associated with advanced maternal age births: A prospective cohort study in Ghana. PLoS ONE 2018, 13, e0200581.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
25 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




