Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Punniamoorthy Thiviya | -- | 2613 | 2022-05-24 06:13:31 | | | |
| 2 | Sirius Huang | Meta information modification | 2613 | 2022-05-25 02:39:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thiviya, P.; Gamage, A.S.; , .; Merah, O.; Madhujith, T. Functional Properties of Seaweed Proteins. Encyclopedia. Available online: https://encyclopedia.pub/entry/23270 (accessed on 07 February 2026).
Thiviya P, Gamage AS, , Merah O, Madhujith T. Functional Properties of Seaweed Proteins. Encyclopedia. Available at: https://encyclopedia.pub/entry/23270. Accessed February 07, 2026.
Thiviya, Punniamoorthy, Ashoka Sriyani Gamage, , Othmane Merah, Terrence Madhujith. "Functional Properties of Seaweed Proteins" Encyclopedia, https://encyclopedia.pub/entry/23270 (accessed February 07, 2026).
Thiviya, P., Gamage, A.S., , ., Merah, O., & Madhujith, T. (2022, May 24). Functional Properties of Seaweed Proteins. In Encyclopedia. https://encyclopedia.pub/entry/23270
Thiviya, Punniamoorthy, et al. "Functional Properties of Seaweed Proteins." Encyclopedia. Web. 24 May, 2022.
Copy Citation
Seaweeds contain several bioactive compounds, including polysaccharides, polyphenols, lipids, polyunsaturated fatty acids (PUFAs), sterols, proteins, dietary fiber, pigments, and vitamins. Several studies have revealed that seaweeds are an excellent source of various proteins (amino acids, peptides, phycobiliproteins, and lectins) with interesting biological properties.
seaweeds
macroalgae
bioactive peptides
seaweed proteins
1. Introduction
Functional foods can be defined as foods and food components that provide a health-promoting benefit beyond basic nutrition and energy [1]. “Let food be your medicine and medicine be your food” is a popular quote by the father of medicine, Hippocrates. Many studies have confirmed a direct relationship between diet and health, and the regular inclusion of functional ingredients in has an impact on the quality of life [2]. Seaweeds contain several bioactive compounds, including polysaccharides, polyphenols, lipids, polyunsaturated fatty acids (PUFAs), sterols, proteins, dietary fiber, pigments, and vitamins [3][4]. Several studies have revealed that the seaweeds are an excellent source of various proteins (amino acids, peptides, phycobiliproteins, and lectins) with interesting biological properties, such as antihypertensive, antioxidant, antidiabetic, anti-inflammatory, antitumoral, antiviral, and antimicrobial [5][6][7]. Table 1 summarizes the bioactive compounds and their functional properties for selected seaweeds.
Table 1. Seaweed protein exhibits potential bioactivities.
| Seaweed | Bioactive Compounds | Properties | References |
|---|---|---|---|
| Bryopsis spp. (green) | Cyclic depsipeptide |
Antimicrobial activity against Mycobacterium tuberculosis | [8] |
| Gracilariopsis lemaneiformis (red) | TGAPCR, FQIN [M(O)] CILR | Angiotensin-I-converting enzyme (ACE) inhibitory activity | [9] |
| Mazzaella japonica (red) | YRD, VSEGLD, TIMPHPR, GGPAT, SSNDYPI, SRIYNVKSNG, VDAHY, CPYDWV, YGDPDHY, NLGN, DFGVPGHEP |
ACE inhibitory activity | [10] |
| YRD, LDY, LRY, VY, LF, FY | ACE inhibitory activity | [11] | |
| Neopyropia yezoensis (formerly Porphyra yezoensis) (red) | Di- and tripeptides TPDSEAL |
ACE inhibition/antihypertensive activity Antimicrobial activity against Staphylococcus aureus |
[12] [13] |
| P. palmata (dulse) (red) | Peptides derived from phycobiliproteins: YRD, AGGEY, VYRT, VDHY, IKGHY, LKNPG, LDY, LRY, FEQDWAS |
ACE inhibition, antioxidant, | [14] |
| Alcalase, bromelain, and Promod-derived hydrolysates |
dipeptidyl peptidase IV (DPP-IV) inhibitory activities, | [15] | |
| Alcalase/Flavourzyme hydrolysates | Antihyperglycemic/antidiabetic potential | [16] | |
| Peptides: ILAP, LLAP, MAGVDHI |
DPP-IV inhibitory activities | [17] | |
| Papain hydrolysates: NIGK | Platelet-activating factor acetyl-hydrolase (PAF-AH) inhibitory peptides | [18] | |
| IRLIIVLMPILMA | Renin inhibitory activity | [19] | |
| SDITRPGGQM | Antioxidant | [20] | |
| P. dioica (red) | Peptides DYYKR, YLVA |
Antioxidant, ACE inhibition, DPP-IV inhibitory activities | [21] |
| P. columbina (formerly P. columbina) (red) | Peptides | ACE inhibitory, immunosuppressive, antioxidant properties | [22] |
| Saccharina longicruris (formerly Laminaria longicruris) (brown) | TITLDVEPSDTIDGVK, ISGLIYEETR, MALSSLPR, ILVLQSNQIR, ISAILPSR, IGNGGELPR, LPDAALNR, EAESSLTGGNGCAK, QVHPDTGISK |
Antibacterial activities | [23] |
| Sargassum pallidum (brown) | Dipeptides (aurantiamide, aurantiamideacetate, dia-aurantiamide) |
Antibiotic activity in vitro against S. aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa | [24] |
| Sargassum thunbergia (brown) | Iodo-amino acids | Possibly helps in human thyroid metabolism | [25] |
| U. rigida (green) | Peptides | ACE inhibition | [26] |
| U. pinnatifida (brown) | Di-, tri-, and tetrapeptides VYIY, AWFY, VW, IW, LW, YNKLKFYG, YKYY |
ACE inhibition/ antihypertensive activity, antioxidant | [27][28] |
| Boodlea coacta (green), Griffithsia spp. (red) |
Lectins griffithsin |
Antiviral effects against human immunodeficiency virus (HIV), Hepatitis C virus and SARS-CoV/ e SARS-CoV-2 by preventing the entry into the host cells | [29][30] |
| Caulerpa cupressoides (green) | Lectins | Antinociceptive and anti-inflammatory activities | [31] |
| C. fragile (green), Eucheuma serra (red) |
Lectins | Mitogenic activities, lipogenic activity | [32] |
| Mimica amakusaensis (formerly Eucheuma amakusaense) (red), Ulva spp. (formerly Enteromorpha spp.) (green) |
Lectins | Induce apoptosis, metastasis, and cell differentiation in cancer cells, antibiotic, anti-inflammatory, anti-HIV activity, and human platelet aggregation inhibition | [33] |
| N. yezoensis (formerly P. yezoensis) (red) | Taurine | Antioxidant | [34][35] |
| Saccharina angustata (formerly Laminaria angustata) (brown), Chondria armata (red) |
Laminine | Hypertensive effect, depress contraction of smooth muscles | [36] |
| C. crispus, Gelidium pusillum, Dasysiphonia japonica (formerly Heterosiphonia japonica), P. palmata (red) |
Phycobiliproteins | Antioxidant, antidiabetic, antitumor, anti-inflammatory, neuro-protective, and hepato-protective properties | [37] |
| Gracilaria tikvahiae, P. palmata (red) |
Phycobilliproteins (phycocyanins and allphycocyanins) |
Anti-inflammatory, liver-protecting, antiviral, antitumor, antiatherosclerosis, lipase activity inhibitor, serum lipid reducing agent, and antioxidant | [33] |
Abbreviation of amino acids as per Jones, 1999 [38]: A, Ala; R, Arg; N, Asn; D, Asp; C, Cys; Q, Gln; E, Glu; G, Gly; H, His; I, Ile; L, Leu; K, Lys; M, Met; F, Phe; P, Pro; S, Ser; T, Thr; W, Trp; Y, Tyr; V, Val.
2. Amino Acids
Amino acids are building blocks of polypeptides and proteins, and the amino acid composition varies with seaweed species. Amino acids serve as essential precursors for the synthesis of low molecular-weight substances (e.g., NO, polyamines, glutathione, creatine, carnitine, carnosine, thyroid hormones, serotonin, melanin, melatonin, and heme) with enormous physiological roles, including regulating nutrient transport and metabolism, cell-to-cell communication, gene expression, protein phosphorylation, antioxidative defense, immune function, reproduction, lactation, fetal and postnatal growth and development, tissue regeneration, neurotransmission, acid-base balance, homeostasis, intestinal microbial growth, and metabolism, among many others [39].
In general, glycine, alanine, arginine, proline, aspartic acid, and glutamic acid make up a larger portion, and cysteine, methionine, and tyrosine are found in lower concentrations in seaweeds [40]. Supplementation with amino acids has a beneficial effect on disease management, e.g., methionine for patients with multiple sclerosis; arginine has a neuroprotective effect after brain ischemia injury and in infertility; histidine improves insulin sensitivity in hyper-insulinemia; glycine alleviates liver and lung injury; tryptophan improves sleep disorders and depression [39][41]. Glutamic acid plays an important role in key physiological functions, including maintaining brain function and mental activity. Aspartic acid helps to initiate important metabolic pathways like the Krebs and urea cycles [40]. However, elevated amino acid levels and their products, such as ammonia, homocysteine, and asymmetric dimethylarginine, are pathogenic factors for neurological disorders, oxidative stress, and cardiovascular disease. Therefore, it is vital to maintain an optimal amino acid balance in the diet and circulation for whole-body homeostasis [39].
3. Peptides
Peptides that are 2–20 amino acids in length can be linear, cyclic, depsipeptides, dipeptides (carnosine, almazole D), tripeptides (glutathione), pentapeptides (galaximide), hexapeptides, oligopeptides, and phycobiliproteins [6][25]. These isolated bioactive peptides have hormone-like properties that are inactive within the parental proteins, but become activated upon release during fermentation or hydrolysis [33][42]. Based on their structural properties, amino acid composition, and sequences, they can display a wide range of biological functions, including antihypertensive (ACE inhibitory), antioxidant, antidiabetic (DPP-IV inhibitory, α-amylase inhibitory), appetite suppression, antitumoral, antimicrobial, antiviral, opioid agonistic, immunomodulatory, prebiotic, opioid, mineral binding, tyrosinase inhibitory, anticoagulatory, anti-thrombotic and hypocholesterolemic effects [6][42][43][44][45].
Hypertension is one of the major risk factors for cardiovascular disease (CVD) [43][46]. Renin and ACE are the two key enzymes in the renin-angiotensin system (RAS), which regulates peripheral blood pressure. ACE catalyzes the conversion of angiotensin-I to a potent vasoconstrictor, angiotensin-II, and degrades the vasodilator peptides bradykinin [26][47]. Thus, inhibition of ACE is one of the key therapeutic approaches in the management of hypertension (Figure 1) [43].

Figure 1. Mechanism of ACE inhibition and antihypertension.
To date, a number of ACE inhibitory or antihypertensive macroalgal peptide hydrolysates have been identified [46]. Paiva et al. revealed that ACE inhibitory peptides from U. rigida have potential therapeutic benefits for the prevention and/or treatment of hypertension and its related diseases [26]. ACE inhibitory peptides have also been reported in P. columbina (formerly P. columbina), P. palmata, N. tenera (formerly P. tenera), N. yezoensis (formerly P. yezoensis, S. chordalis, M. japonica (Rhodophyta), S. fusiforme (formerly H. fusiformis), U. pinnatifida (Phaeophyceae), Ulva prolifera (formerly Enteromorpha prolifera), and U. intestinalis (formerly E. intestinalis) (Chlorophyta) [11][22][23][27][43][46][48].
Furthermore, the recent outbreak of SARS-CoV-2 (or 2019-nCoV) responsible for the COVID-19 pandemic, enters host cells through an interaction between the spike viral protein and angiotensin-converting enzyme 2 (ACE 2) [49]. ACE inhibitory peptides with antiviral activity in edible seaweeds (U. pinnatifida, S. fusiforme, Porphyra spp.) could exert a protective effect against COVID-19 by reducing the dominance of the ACE/Ang II/ATR1 axis [50].
A few studies have reported the antidiabetic potential of seaweed protein/peptides that inhibit the α-amylase, α-glucosidase, and DPP-IV [16][17]. One therapeutic approach for type 2 diabetes mellitus (T2DM) management is to lower blood glucose levels by inhibiting the key enzymes involved in intestinal carbohydrate digestion (α-amylase and α-glucosidase). Two α-amylase inhibitory peptides have been identified in proteolytic enzyme hydrolysates of seaweed laver (Porphyra spp.) that can prevent postprandial hyperglycemia [51]. Another, newer, therapeutic approach for T2DM is to inhibit DPP IV as an insulin regulatory strategy. Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are the two incretin hormones that stimulate glucose-induced insulin secretion, inhibit postprandial glucagon release, and delay gastric emptying, which results in lower blood glucose level. DPP-IV inactivates GLP-1 and GIP, resulting in the loss of their insulinotropic potential in vivo. Hence, DPP-IV inhibitors prevent the degradation of GLP-1 and GIP and enhance its insulinotropic effects, and thus, can be used in the management of T2DM [17][52]. Figure 2 illustrates the simplified mechanism of DPP-IV inhibitors and antidiabetic activity.
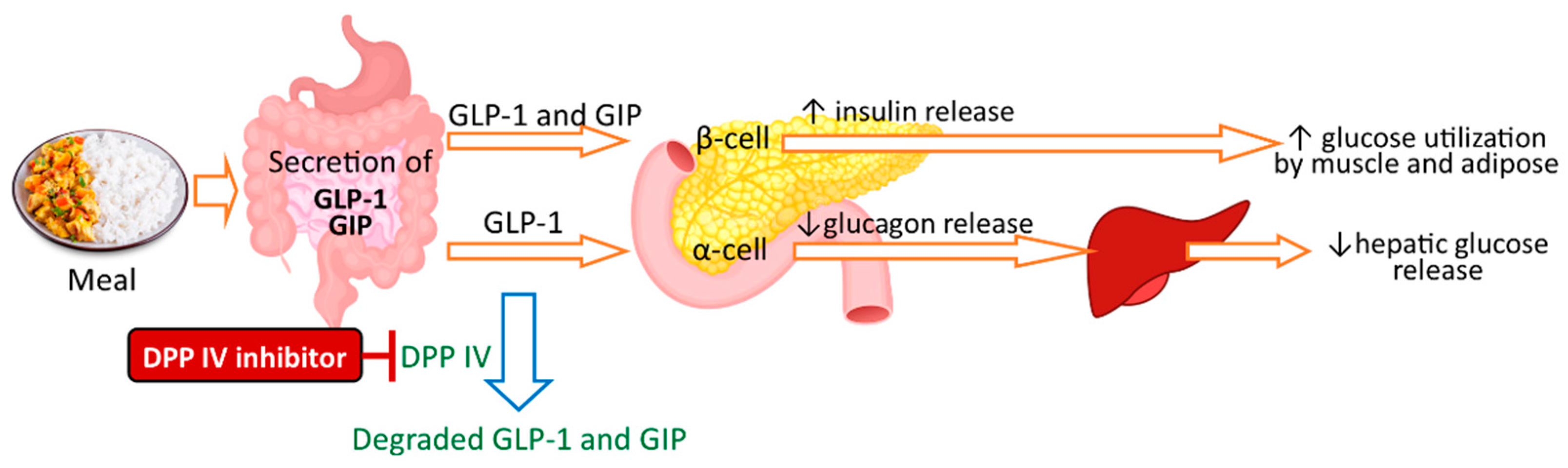
Figure 2. Antidiabetic activity of DPP-IV inhibitors.
Seaweeds can be a natural source of DPP-IV inhibitors. Studies have reported that protein hydrolysates of P. palmata have DPP-IV inhibitory activities that are useful for the management of T2DM [15][53] and obesity [15]. Oral administration of P. palmata protein hydrolysate derived from Alcalase and Flavourzyme reduced food intake by streptozotocin-induced diabetic mice and showed antihyperglycemic effects [16].
Reactive oxygen species (ROS) contribute to the development of chronic diseases, including cardiovascular diseases, cancer, diabetes mellitus, cataracts, and neurodegenerative disorders [20]. ROS includes free radical species, such as superoxide anions, hydroxyl radicals, and singlet oxygen, and non-radical species, such as hydrogen peroxide (H2O2), generated during the metabolic process [54]. The antioxidant activity of bioactive peptides is attributed to the hydrophobicity of valine, leucine, isoleucine, glycine, methionine, proline, and alanine and some aromatic amino acids (tyrosine, histidine, tryptophan, and phenylalanine) [20]. They exert a protective effect on the body by binding free radicals and other reactive oxygen compounds. Furthermore, the regulation of oxidative stress is an essential factor in tumor development and anticancer therapies [55]. Protein hydrolysates or peptides and amino acids exhibit multiple antioxidant properties. Two antioxidant peptides, such as carnosine and glutathione, generally present in high concentrations in animal muscle, have been found in seaweed [20]. Antioxidant peptides have been isolated from several species of macroalgae, including Scytosiphon lomentaria [56], Ecklonia cava, Sargassum coreanum (Phaeophyceae) [57], P. palmata [58], and P. columbina (formerly P. columbina) (Rhodophyta) [22]. Antioxidant and anticancer bioactivity have also been reported in Sri Lankan seaweed, and the highest value was reported for Caulerpa racemose [59]. N. yezoensis (formerly P. yezoensis), G. pusillum, and many other seaweed species have been studied for their antioxidant properties [35].
Antimicrobial peptides have been identified in S. longicruris (formerly L. longicruris) against S. aureus, and cyclic depsipeptide from Bryopsis spp. demonstrated activity against M. tuberculosis [8][23]. Protein hydrolysates from P. columbina (formerly P. columbina) also have immunosuppressive, antihypertensive, and antioxidant capacities [60].
Furthermore, inhibition of platelet-activating factor acetyl-hydrolase (PAF-AH) has been reported for peptides derived from P. palmata that could prevent high blood pressure and atherosclerosis [18]. PAF-AH plays an active role in atherosclerotic development and progression [18]. PAF-AH is thought to be involved in the generation of pro-inflammatory mediators, such as lysophosphatidylcholine (LPC) and oxidized non-esterified fatty acids (NEFA) [53][55]. In addition, macroalgae peptides from different species display many other biological activities (Table 1).
4. Lectins
Lectins and phycobiliproteins are two groups of functionally active proteins in seaweeds [61]. Lectins are proteins, glycoproteins, or hemagglutinin proteins that reversibly bind specific mono- or oligo-saccharides [31][33]. Lectins have been found in red and green algae, such as Eucheuma spp., Solieria filiformis, Enantiocladia duperreyi, Pterocladiella capillacea, Gracilaria cornea, Gracilaria ornate, Bryothamnion spp., M. amakusaensis (formerly E. amakusaense) (Rhodophyta), Ulva spp. (formerly Enteromorpha spp.), and C. fragile (Chlorophyta) [6][33][62]. Lectins are involved in numerous biological processes, such as host-pathogen interactions, intercellular communication, recognizing and binding carbohydrates, induction of apoptosis, metastasis, and cell differentiation in cancer cells [33][36]. These proteins also have other bioactive properties, including antibiotic, antibacterial, antifungal, anti-inflammatory, mitogenic, cytotoxic, antinociceptive, anticancer, fibroblast, human platelet aggregation inhibition, antiviral, and anti-human immunodeficiency virus (anti-HIV) activities [33][62][63][64][65].
Lectins from red algae Alsidium triquetrum (formerly Bryothamnion triquetrum), P. capillacea, Hypnea cervicornis, S. filiformis, and green seaweed C. cupressoides have demonstrated anti-inflammatory activities in different studies [66]. Lectin is the only seaweed protein reported as an antibacterial in the literature [23][61]. The lectins found in Alsidium seaforthii (formerly Bryothamnion seaforthii) and Hypnea musciformis show bactericidal activity, especially inhibiting the growth of S. aureus and P. aeruginosa [67]. Lectin extracted from red seaweed showed antibacterial activity against six pathogenic Gram-negative species, including Serratia marcescens, Salmonella typhi, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus spp., and P. aeruginosa [62]. The lectin isolated from B. seaforthii has a pro-healing property responsible for accelerating the healing of skin wounds [68]. Because of these antimicrobial properties, lectins are used to treat many pathologies such as cancer and chronic bacterial diseases, chronic otitis, tonsillitis, cystic fibrosis, periodontal diseases, and urinary tract infections [67].
Lectins have the ability to precipitate glycoprotein and agglutinate red blood cells [62][63]. Further, lectins have displayed antiviral effects against human immunodeficiency, hepatitis C, and SARS-CoV viruses, mainly by preventing entry of the virus into host cells, and thereby, their propagation [30]. Griffithsin, a highly potent broad-spectrum antiviral lectin from Griffithsia spp. has an antiviral effect against HIV [69], SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV) [70]. Lectins have also been widely studied for their antiviral activity against SARS-CoV-2 (or 2019-nCoV)since they can inhibit coronavirus infectivity by specifically binding to the spike glycoprotein. Glycoproteins, especially the spike protein of SARS-CoV-2, are involved in cell adhesion and invasion, morphogenesis, and modulation of immune response processes. This spike protein mediates viral adhesion through human ACE 2. Lectins that bind SARS-CoV-2 spike protein via their ability to recognize glycans can inhibit the adhesion of coronavirus and impair the initial steps of viral pathogenesis [71]. Many studies have highlighted lectins from seaweeds and their potential antiviral therapeutic activity against SARS-CoV and SARS-CoV-2 (COVID 19) [29][72]. Thus, seaweed lectins should be considered when developing new antiviral approaches because of their antiviral properties.
5. Phycobilliproteins
Phycobiliproteins are the only water-soluble algal pigments in red seaweeds [6]. Phycobiliproteins are the most abundant proteins in red seaweeds, representing nearly 50% of the total protein content [73]. Phycobiliproteins are grouped into the following four groups: phycoerythrin (purple), phycocyanin (blue), phycoerythrocyanins (purple), and allophycocyanin (bluish-green), whereas phycoerythrin is the main pigment [63][74]. Phycobiliproteins have been reported in many species, including Porphyra spp. [74], Gracilaria canaliculate (formerly Gracilaria crassa) [75], P. palmata [76], and G. tikvahiae [36]. Phycoerythrin has been reported in G. gracilis [77], Grateloupia turuturu [78], G. pusillum, and Rhodymenia pseudopalmata [63]. Furthermore, extraction of phycocyanin has been reported for C. crispus, G. gracilis, and Gelidium amansii with many bioactivities, including anticancer activity, anti-inflammatory effect, antioxidative, and anti-irradiative effects [79].
Phycobiliprotein has become popular for its biological activities, including antioxidant, ACE inhibitory, antitumoral, antidiabetic, immunomodulating, anti-inflammatory, liver-protecting, antiviral, anticancer, antiatherosclerosis, antihyperlipidemic activities, lipase activity inhibitor, serum lipid reducing agent, and obstructing absorption of environmental pollutants into the body [14][33][78][80]. Other than these, it is also beneficial for preventing or treating gastric ulcers and neurodegenerative diseases caused by oxidative stress (Alzheimer’s and Parkinson’s) due to their antioxidant effects [5][6].
Phycocyanin improves the immune system and has several other bioactivities, including in vitro anticancer activity, chemotherapy sensitiveness, photosensitized tumor suppressor activity, anti-inflammatory effects, antioxidative, anti-irradiative, and neuroprotective effects [79].
6. Free Amino Acids
The free amino acid fraction in seaweeds mainly consists of taurine, alanine, ornithine, citrulline, hydroxyproline, and aminobutyric acid [36]. Taurine content varies with the species. Red algae contains taurine in high concentrations, however it is rarely found in green and brown algae [81][82]. Seaweeds such as N. yezoensis (formerly P. yezoensis), N. tenera (formerly P. tenera), Gloiopeltis tenax, Gloiopeltis furcate, Gracilaria textorii, A. vermiculophyllum (formerly G. vermiculophylla) (Rhodophyta), U. pinnatifida, S. japonica (formerly L. japonica), and Sargassum confusum (Phaeophyceae) contain a high amount of taurine [34][82][83][84] and can be used in functional foods that contain naturally occurring taurine [81][82][85]. Taurine plays an important role in physiological functions such as bile-acid conjugation, retinal and neurological development, osmoregulation, antioxidant, a modulator of intracellular calcium level, and immune function [85]. In addition, taurine acts as an antioxidant and protects against the toxicity of various heavy metals, including lead and cadmium, by preventing their absorption in the stomach [33]. Taurine also has antihypertensive and hypocholesterolemic activities by reducing the secretion of serum lipids and apolipoprotein (very low-density lipoprotein, VLDL, and intermediate-density lipoproteins, IDL) [64][86].
In addition to taurine, macroalgae contain unusual amino acids, such as laminine, kanoids (kainic and domoic acid), and mycosporine-like amino acids with bioactivity [36][64]. Many macroalgae species, including Digenea simplex, C. armata, P. palmata, among others, contain kanoid amino acids (kainic and domoic acids), and extraction from D. simplex has been commercialized [32][36]. Kanoid amino acids are reported to have insecticidal, neuroexcitatory and anthelmintic properties [36]. In Japan, D. simplex and C. armata extracts contain kanoids and have been used for centuries as anthelmintic agents to treat ascariasis (a disease in humans caused by the parasitic roundworm). They also act as central nervous system stimulants and assist in neurophysiological disorders such as Alzheimer’s disease, Parkinson’s disease, and epilepsy. However, they become neurotoxins when safe levels are exceeded [64]. Laminine, a choline-like basic amino acid, has been isolated from S. angustata (formerly L. angustata) and C. armata, and can depress the contraction of excited smooth muscles and exert a transitory hypotensive effect [36].
References
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982.
- Tanna, B.; Mishra, A. Metabolites Unravel Nutraceutical Potential of Edible Seaweeds: An Emerging Source of Functional Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624.
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and Human Health. Nutr. Rev. 2014, 72, 205–216.
- Saikia, S.; Mahnot, N.K.; Sahu, R.K.; Kalita, J. Edible Seaweeds as Potential Source of Nutraceuticals. In Marine Niche: Applications in Pharmaceutical Sciences: Translational Research; Nathani, N.M., Mootapally, C., Gadhvi, I.R., Maitreya, B., Joshi, C.G., Eds.; Springer: Singapore, 2020; pp. 183–201. ISBN 9789811550171.
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96.
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301.
- Flórez-Fernández, N.; Torres, M.D.; Braz, L.; Grenha, A.; Loret, E.P.; Domínguez, H. Seaweed and Sea Anemones Proteins as a Source of New Pharmaceutical Active Principles. In Marine Niche: Applications in Pharmaceutical Sciences: Translational Research; Nathani, N.M., Mootapally, C., Gadhvi, I.R., Maitreya, B., Joshi, C.G., Eds.; Springer: Singapore, 2020; pp. 203–219. ISBN 9789811550171.
- Dmitrenok, A.; Iwashita, T.; Nakajima, T.; Sakamoto, B.; Namikoshi, M.; Nagai, H. New Cyclic Depsipeptides from the Green Alga Bryopsis Species; Application of a Carboxypeptidase Hydrolysis Reaction to the Structure Determination. Tetrahedron 2006, 62, 1301–1308.
- Deng, Z.; Liu, Y.; Wang, J.; Wu, S.; Geng, L.; Sui, Z.; Zhang, Q. Antihypertensive Effects of Two Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Gracilariopsis lemaneiformis (Rhodophyta) in Spontaneously Hypertensive Rats (SHRs). Mar. Drugs 2018, 16, 299.
- Kumagai, Y.; Kitade, Y.; Kobayashi, M.; Watanabe, K.; Kurita, H.; Takeda, H.; Yasui, H.; Kishimura, H. Identification of ACE Inhibitory Peptides from Red Alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231.
- Kitade, Y.; Miyabe, Y.; Yamamoto, Y.; Takeda, H.; Shimizu, T.; Yasui, H.; Kishimura, H. Structural Characteristics of Phycobiliproteins from Red Alga Mazzaella japonica. J. Food Biochem. 2018, 42, e12436.
- Qu, W.; Ma, H.; Pan, Z.; Luo, L.; Wang, Z.; He, R. Preparation and Antihypertensive Activity of Peptides from Porphyra Yezoensis. Food Chem. 2010, 123, 14–20.
- Jiao, K.; Gao, J.; Zhou, T.; Yu, J.; Song, H.; Wei, Y.; Gao, X. Isolation and Purification of a Novel Antimicrobial Peptide from Porphyra Yezoensis. J. Food Biochem. 2019, 43, e12864.
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32.
- McLaughlin, C.M.; Harnedy-Rothwell, P.A.; Lafferty, R.A.; Sharkey, S.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Macroalgal Protein Hydrolysates from Palmaria palmata Influence the ‘Incretin Effect’ in Vitro via DPP-4 Inhibition and Upregulation of Insulin, GLP-1 and GIP Secretion. Eur. J. Nutr. 2021, 60, 4439–4452.
- McLaughlin, C.M.; Sharkey, S.J.; Harnedy-Rothwell, P.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Twice Daily Oral Administration of Palmaria palmata Protein Hydrolysate Reduces Food Intake in Streptozotocin Induced Diabetic Mice, Improving Glycaemic Control and Lipid Profiles. J. Funct. Foods 2020, 73, 104101.
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Purification and Identification of Dipeptidyl Peptidase (DPP) IV Inhibitory Peptides from the Macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406.
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a Seaweed Derived Platelet Activating Factor Acetylhydrolase (PAF-AH) Inhibitory Hydrolysate, Synthesis of Inhibitory Peptides and Assessment of Their Toxicity Using the Zebrafish Larvae Assay. Peptides 2013, 50, 119–124.
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and Characterization of Bioactive Pro-Peptides with in Vitro Renin Inhibitory Activities from the Macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427.
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and Identification of Antioxidant Peptides from an Enzymatically Hydrolysed Palmaria palmata Protein Isolate. Food Res. Int. 2017, 100, 416–422.
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide Identification from a Porphyra Dioica Protein Hydrolysate with Antioxidant, Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Activities. Food Funct. 2019, 10, 3421–3429.
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive Properties of Peptides Obtained by Enzymatic Hydrolysis from Protein Byproducts of Porphyra Columbina. Food Res. Int. 2012, 49, 364–372.
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.-E.; Turgeon, S.L. Characterization of Antibacterial Activity from Protein Hydrolysates of the Macroalga Saccharina Longicruris and Identification of Peptides Implied in Bioactivity. J. Funct. Foods 2015, 17, 685–697.
- Liu, X.; Wang, C.-Y.; Shao, C.-L.; Wei, Y.-X.; Wang, B.-G.; Sun, L.-L.; Zheng, C.-J.; Guan, H.-S. Chemical Constituents from Sargassum Pallidum (Turn.) C. Agardh. Biochem. Syst. Ecol. 2009, 37, 127–129.
- Conde, E.; Balboa, E.M.; Parada, M.; Falqué, E. 4—Algal Proteins, Peptides and Amino Acids. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 135–180. ISBN 978-0-85709-512-1.
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Isolation and Characterization of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Ulva Rigida, C. Agardh Protein Hydrolysate. J. Funct. Foods 2016, 26, 65–76.
- Suetsuna, K.; Nakano, T. Identification of an Antihypertensive Peptide from Peptic Digest of Wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454.
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Wakame (Undaria pinnatifida) and Their Antihypertensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2002, 50, 6245–6252.
- Barre, A.; Van Damme, E.J.M.; Simplicien, M.; Le Poder, S.; Klonjkowski, B.; Benoist, H.; Peyrade, D.; Rougé, P. Man-Specific Lectins from Plants, Fungi, Algae and Cyanobacteria, as Potential Blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) Coronaviruses: Biomedical Perspectives. Cells 2021, 10, 1619.
- Cheung, R.C.F.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.; Dan, X.; Ng, T.B. Marine Lectins and Their Medicinal Applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773.
- Vanderlei, E.S.O.; Patoilo, K.K.N.R.; Lima, N.A.; Lima, A.P.S.; Rodrigues, J.A.G.; Silva, L.M.C.M.; Lima, M.E.P.; Lima, V.; Benevides, N.M.B. Antinociceptive and Anti-Inflammatory Activities of Lectin from the Marine Green Alga Caulerpa cupressoides. Int. Immunopharmacol. 2010, 10, 1113–1118.
- Smit, A.J. Medicinal and Pharmaceutical Uses of Seaweed Natural Products: A Review. J. Appl. Phycol. 2004, 16, 245–262.
- Mendis, E.; Kim, S.-K. Chapter 1—Present and Future Prospects of Seaweeds in Developing Functional Foods. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Medicinal Foods; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 1–15.
- Wang, F.; Guo, X.-Y.; Zhang, D.-N.; Wu, Y.; Wu, T.; Chen, Z.-G. Ultrasound-Assisted Extraction and Purification of Taurine from the Red Algae Porphyra yezoensis. Ultrason. Sonochem. 2015, 24, 36–42.
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314.
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597.
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound Assisted Methods for Enhanced Extraction of Phycobiliproteins from Marine Macro-Algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103.
- Jones, J.H. A Short Guide to Abbreviations and Their Use in Peptide Science. J. Pept. Sci. 1999, 5, 465–471.
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411.
- Černá, M. Chapter 24—Seaweed Proteins and Amino Acids as Nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 297–312.
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods 2020, 9, 1382.
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33.
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16.
- Samarakoon, K.; Jeon, Y.-J. Bio-Functionalities of Proteins Derived from Marine Algae—A Review. Food Res. Int. 2012, 48, 948–960.
- Pimentel, F.B.; Alves, R.C.; Harnedy, P.A.; FitzGerald, R.J.; Oliveira, M.B.P.P. Macroalgal-Derived Protein Hydrolysates and Bioactive Peptides: Enzymatic Release and Potential Health Enhancing Properties. Trends Food Sci. Technol. 2019, 93, 106–124.
- Hayes, M. Chapter 14—Seaweeds: A Nutraceutical and Health Food. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 365–387. ISBN 978-0-12-418697-2.
- Turner, J.M.; Kodali, R. Should Angiotensin-Converting Enzyme Inhibitors Ever Be Used for the Management of Hypertension? Curr. Cardiol. Rep. 2020, 22, 95.
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva Intestinalis. Mar. Drugs 2019, 17, 179.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8.
- Tamama, K. Potential Benefits of Dietary Seaweeds as Protection against COVID-19. Nutr. Rev. 2021, 79, 814–823.
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp). J. Agric. Food Chem. 2018, 66, 4872–4882.
- Singh, A.-K.; Jatwa, R.; Purohit, A.; Ram, H. Synthetic and Phytocompounds Based Dipeptidyl Peptidase-IV (DPP-IV) Inhibitors for Therapeutics of Diabetes. J. Asian Nat. Prod. Res. 2017, 19, 1036–1045.
- Harnedy, P.A.; FitzGerald, R.J. In Vitro Assessment of the Cardioprotective, Anti-Diabetic and Antioxidant Potential of Palmaria palmata Protein Hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803.
- Kim, E.-Y.; Choi, Y.H.; Nam, T.-J. Identification and Antioxidant Activity of Synthetic Peptides from Phycobiliproteins of Pyropia Yezoensis. Int. J. Mol. Med. 2018, 42, 789–798.
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive Peptides and Carbohydrates from Seaweed for Food Applications: Natural Occurrence, Isolation, Purification, and Identification. Algal Res. 2020, 48, 101909.
- Ahn, C.-B.; Jeon, Y.-J.; Kang, D.-S.; Shin, T.-S.; Jung, B.-M. Free Radical Scavenging Activity of Enzymatic Extracts from a Brown Seaweed Scytosiphon Lomentaria by Electron Spin Resonance Spectrometry. Food Res. Int. 2004, 37, 253–258.
- Heo, S.-J.; Park, E.-J.; Lee, K.-W.; Jeon, Y.-J. Antioxidant Activities of Enzymatic Extracts from Brown Seaweeds. Bioresour. Technol. 2005, 96, 1613–1623.
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-Enhanced Extraction of Antioxidant Ingredients from Red Algae Palmaria palmata. LWT—Food Sci. Technol. 2010, 43, 1387–1393.
- Lakmal, H.C.; Samarakoon, K.W.; Lee, W.; Lee, J.-H.; Abeytunga, D.T.U.; Lee, H.-S.; Jeon, Y.-J. Anticancer and Antioxidant Effects of Selected Sri Lankan Marine Algae. J. Natl. Sci. Found. Sri Lanka 2014, 42, 315–323.
- Cian, R.E.; Fajardo, M.A.; Alaiz, M.; Vioque, J.; González, R.J.; Drago, S.R. Chemical Composition, Nutritional and Antioxidant Properties of the Red Edible Seaweed Porphyra Columbina. Int. J. Food Sci. Nutr. 2014, 65, 299–305.
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642.
- Pangestuti, R.; Kim, S.-K. Chapter 6—Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140. ISBN 978-0-12-418697-2.
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Chapter Nine—Current Knowledge and Challenges in Extraction, Characterization and Bioactivity of Seaweed Protein and Seaweed-Derived Proteins. Adv. Bot. Res. 2020, 95, 289–326.
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Proteins, Peptides, and Amino Acids from Macroalgae. J. Phycol. 2011, 47, 218–232.
- Singh, R.S.; Walia, A.K. Lectins from Red Algae and Their Biomedical Potential. J. Appl. Phycol. 2018, 30, 1833–1858.
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; De Souza Lopes, J.L.; De Brito, T.V.; Das Chagas Vieira Júnior, F.; Sales, A.B.; Aragão, K.S.; Souza, M.H.L.P.; Dos Reis Barbosa, A.L.; et al. Lectin Obtained from the Red Seaweed Bryothamnion Triquetrum: Secondary Structure and Anti-Inflammatory Activity in Mice. Int. J. Biol. Macromol. 2018, 112, 1122–1130.
- Vasconcelos, M.A.; Arruda, F.V.S.; Carneiro, V.A.; Silva, H.C.; Nascimento, K.S.; Sampaio, A.H.; Cavada, B.; Teixeira, E.H.; Henriques, M.; Pereira, M.O. Effect of Algae and Plant Lectins on Planktonic Growth and Biofilm Formation in Clinically Relevant Bacteria and Yeasts. BioMed Res. Int. 2014, 2014, e365272.
- Gonzaga do Nascimento-Neto, L.; Carneiro, R.F.; Da Silva, S.R.; Da Silva, B.R.; Arruda, F.V.S.; Carneiro, V.A.; Do Nascimento, K.S.; Saker-Sampaio, S.; Da Silva, V.A.; Porto, A.L.F.; et al. Characterization of Isoforms of the Lectin Isolated from the Red Algae Bryothamnion Seaforthii and Its Pro-Healing Effect. Mar. Drugs 2012, 10, 1936–1954.
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and Characterization of Griffithsin, a Novel HIV-Inactivating Protein, from the Red Alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353.
- Lusvarghi, S.; Bewley, C.A. Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses 2016, 8, 296.
- Nascimento da Silva, L.C.; Mendonça, J.S.P.; de Oliveira, W.F.; Batista, K.L.R.; Zagmignan, A.; Viana, I.F.T.; dos Santos Correia, M.T. Exploring Lectin–Glycan Interactions to Combat COVID-19: Lessons Acquired from Other Enveloped Viruses. Glycobiology 2021, 31, 358–371.
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-Spectrum in Vitro Activity and in Vivo Efficacy of the Antiviral Protein Griffithsin against Emerging Viruses of the Family Coronaviridae. J. Virol. 2010, 84, 2511–2521.
- Dumay, J.; Morançais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Chapter Eleven—Phycoerythrins: Valuable Proteinic Pigments in Red Seaweeds. Adv. Bot. Res. 2014, 71, 321–343.
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33.
- Sudhakar, M.P.; Jagatheesan, A.; Perumal, K.; Arunkumar, K. Methods of Phycobiliprotein Extraction from Gracilaria Crassa and Its Applications in Food Colourants. Algal Res. 2015, 8, 115–120.
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-Inflammatory Effects of Dulse (Palmaria Palmata) Resulting from the Simultaneous Water-Extraction of Phycobiliproteins and Chlorophyll a. Food Res. Int. 2017, 100, 514–521.
- Nguyen, H.P.T.; Morançais, M.; Déléris, P.; Fleurence, J.; Nguyen-Le, C.T.; Vo, K.H.; Dumay, J. Purification of R-Phycoerythrin from a Marine Macroalga Gracilaria Gracilis by Anion-Exchange Chromatography. J. Appl. Phycol. 2020, 32, 553–561.
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.-Y.; Fleurence, J.; Bergé, J.-P. Ultrasound-Assisted Extraction of R-Phycoerythrin from Grateloupia Turuturu with and without Enzyme Addition. Algal Res. 2015, 12, 522–528.
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19.
- Huang, C.-H.; Chen, W.-C.; Gao, Y.-H.; Chen, G.-W.; Lin, H.-T.V.; Pan, C.-L. Enzyme-Assisted Method for Phycobiliproteins Extraction from Porphyra and Evaluation of Their Bioactivity. Processes 2021, 9, 560.
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino Acids, Fatty Acids, and Dietary Fibre in Edible Seaweed Products. Food Chem. 2007, 103, 891–899.
- Kawasaki, A.; Ono, A.; Mizuta, S.; Kamiya, M.; Takenaga, T.; Murakami, S. The Taurine Content of Japanese Seaweed. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 1105–1112.
- Fleurence, J.; Morançais, M.; Dumay, J. 9—Seaweed Proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2018; pp. 245–262. ISBN 978-0-08-100722-8.
- Hwang, E.-S.; Ki, K.-N.; Chung, H.-Y. Proximate Composition, Amino Acid, Mineral, and Heavy Metal Content of Dried Laver. Prev. Nutr. Food Sci. 2013, 18, 139–144.
- Bito, T.; Teng, F.; Watanabe, F. Bioactive Compounds of Edible Purple Laver Porphyra sp. (Nori). J. Agric. Food Chem. 2017, 65, 10685–10692.
- Mochizuki, H.; Takido, J.; Oda, H.; Yokogoshi, H. Improving Effect of Dietary Taurine on Marked Hypercholesterolemia Induced by a High-Cholesterol Diet in Streptozotocin-Induced Diabetic Rats. Biosci. Biotechnol. Biochem. 1999, 63, 1984–1987.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
25 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




