| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alberto Orfao | + 4898 word(s) | 4898 | 2020-09-30 08:29:06 | | | |
| 2 | Bruce Ren | Meta information modification | 4898 | 2020-10-12 06:38:41 | | | | |
| 3 | Bruce Ren | Meta information modification | 4898 | 2020-10-13 09:37:53 | | |
Video Upload Options
Cancer dissemination and distant metastasis most frequently require the release of tumor cells into the blood circulation, both in solid tumors and most hematological malignancies, including plasma cell neoplasms. However, detection of blood circulating tumor cells in solid tumors and some hematological malignancies, such as the majority of mature/peripheral B-cell lymphomas and monoclonal gammopathies, has long been a challenge due to their very low frequency. In recent years, the availability of highly-sensitive and standardized methods for the detection of circulating tumor plasma cells (CTPC) in monoclonal gammopathies, e.g., next-generation flow cytometry (NGF), demonstrated the systematic presence of CTPC in blood in virtually every smoldering (SMM) and symptomatic multiple myeloma (MM) patient studied at diagnosis, and in the majority of patients with newly-diagnosed monoclonal gammopathies of undetermined significance (MGUS). These methods set the basis for further detailed characterization of CTPC vs. their bone marrow counterpart in monoclonal gammopathies, to investigate their role in the biology of the disease, and to confirm their strong impact on patient outcome when measured both at diagnosis and after initiating therapy.
1. Introduction
Plasma cell neoplasms are an heterogenous group of end-stage antibody-producing B-cell (i.e., plasma cell) disorders [1][2]. They are characterized by an expansion of tumor plasma cells (PC), typically inside bone marrow (BM) [3], with or without involvement of peripheral blood and/or other (extramedullary) tissues such as bone, soft tissues or skin [4]. In most monoclonal gammopathy patients, tumor PC produce and release an abnormal protein (i.e., monoclonal component) that is detectable in the patient’s blood and/or urine. Monoclonal gammopathy of undetermined significance (MGUS) is the most common plasma cell neoplasm [5] and affects 3.2% of adults >50 years, and 5.3% >70 years [6]. Despite the fact that most MGUS cases will never undergo malignant transformation, previous studies have shown that MGUS is a precursor stage of multiple myeloma (MM) [7][8]. In line with this observation, malignant transformation of MGUS occurs in around 1% of patients per year [7][8]. This transformation is characterized by an increase in BM PC infiltration (≥10% BM PC) and serum monoclonal protein (≥30 g/L), and it may present in the absence of clinical symptoms (i.e., smoldering MM (SMM) [9] or as symptomatic MM [10] with evidence of underlying organ dysfunction and/or predominant BM involvement. However, in a few MM patients, extramedullary tissue lesions with limited BM infiltration occurs (i.e., macrofocal MM) [11][12]. Similarly, solitary plasmacytoma is a localized plasma cell neoplasm in which tumor PC are confined to extramedullary sites (i.e., bone or extraosseous) [13]. MM patients might also evolve to the most aggressive plasma cell neoplasm subtype, known as plasma cell leukemia (PCL), which is characterized by massive blood involvement (>2 × 109 circulating tumor cells/L) [14]. In some cases, the monoclonal (most frequently lambda) light chain protein might deposit in distinct tissues and organs, affecting their function and giving rise to the so-called light chain amyloidosis, even in the presence of low numbers of tumor PC in BM and other tissues [15].
Circulating tumor plasma cells (CTPC) have long been detected in the blood of PCL patients [16][17][18][19][20][21][22], as well as in a significant fraction of MM [19][20][21][22], and to a lesser extent in MGUS cases [23]. From a clinical point of view, the presence and number of CTPC has been proven to have both diagnostic and prognostic implications in MGUS [23], SMM [24][25][26] and in symptomatic MM [27][28][29][30][31] patients. In addition, detection of CTPC has proven useful for (closer) minimally-invasive monitoring of MM after therapy [32][33][34][35]. Despite this, the reported frequency of MM and MGUS cases with detectable CTPC varies significantly, depending on the specific methodology used and its sensitivity and specificity. Thereby, usage of highly-sensitive and standardized techniques for the detection and quantitation of CTPC becomes critically important[35]. Here, we provide a detailed review of the currently available techniques for the detection of CTPC in patients with plasma cell neoplasms, their biological features, pathogenic role, and clinical relevance, with special focus on MGUS and MM patients.
2. Detection of Circulating Tumor Plasma Cells
During recent decades, different methods have been developed and used for the detection of CTPC (Graphical abstract [35] and Figure 1). Conventional cytology was first used (in combination with complete blood counts obtained in an automated hematology analyzer) for the identification of circulating PC in blood smears of patients diagnosed with monoclonal gammopathy. These counts already proved critical for the differential diagnosis between MM and PCL [36]. In addition, they confirmed the presence of variable PC counts in a minor fraction (17%) of all MM patients , which (frequently) cannot be accurately discriminated from normal/reactive plasmablasts [37] due to both the limited number of nucleated cells evaluated (i.e., <500 cells) and their morphological similarities [38], particularly among patients that show low numbers of circulating PC. Because of these limitations and the clear clinical utility of CTPC detection and quantitation in blood, conventional immunocytochemistry-based approaches were subsequently adopted. The latter technique allowed for (more specific) assessment of greater numbers of clonal PC in the blood of MM and MGUS patients based on restricted light chain expression by tumor PC. In order to further increase the sensitivity and specificity of the above techniques, several different conventional flow cytometry and next generation flow cytometry (NGF) procedures , together with polymerase chain reaction (PCR)-based, e.g., allele-specific oligonucleotide (ASO) quantitative PCR (qPCR) [39] and next generation sequencing (NGS) [40] techniques, were subsequently developed and tested. In addition to differences in the sensitivity reported for the above flow vs. molecular techniques, the results obtained so far are also influenced by the type of material analyzed (e.g., inclusion of nucleic acid from non-viable cells in molecular techniques) and/or the way patients were selected for analysis (e.g., inclusion of patients that reached no response or partial response together with complete response cases). The specific advantages and limitations of each of these techniques, together with their most relevant features are listed in Table and described below in more detail.
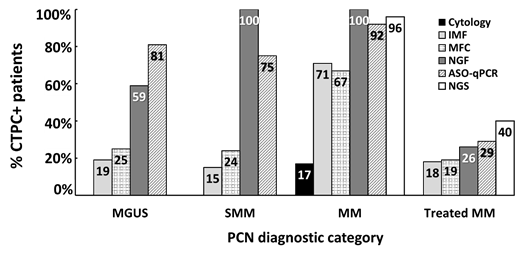
Figure 1. Frequency of newly-diagnosed PCN and treated MM patients with detectable CTPC in blood as assessed by distinct methods. ASO-qPCR, allele-specific oligonucleotide quantitative real-time polymerase chain reaction; CTPC, circulating tumor plasma cells; IMF, immunofluorescence microscopy; MFC, multiparameter flow cytometry; MM, multiple myeloma; NGS, next generation sequencing; MGUS, monoclonal gammopathy of undetermined significance; NGF, next generation flow; PCN, plasma cell neoplasm; SMM, smoldering MM. Data summarized from the following references in the literature [41][42][43].
Table 1. Most frequently used methods to detect circulating tumor plasma cells in patients diagnosed with plasma cell neoplasms.
|
Cytology |
IMF |
MFC (<8 colors) |
NGF |
ASO-qPCR |
NGS |
|
|
Availability |
High |
Limited |
High |
High |
Intermediate |
Limited |
|
Applicability |
≈100% |
≈100% |
≈100% |
≈100% |
42%-75% |
80-95% |
|
Sensitivity |
<10-2 |
<10-4 |
≤10-4 |
≤2×10-6 |
≤10-5-10-6 |
≤10-6 |
|
Specificity |
Limited |
Limited |
High |
High |
High |
High |
|
Quantitative |
Yes (high counts) |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Diagnostic sample |
Not required |
Not required |
Not required |
Not required |
Mandatory |
Mandatory |
|
Global sample analysis |
Yes |
No |
Yes |
Yes |
No |
No |
|
Time |
<2h |
>4h |
2-3h |
3-4h |
3-4 weeks |
≥7 days |
|
Fresh sample |
Needed |
Needed |
Needed (<36h) |
Needed (<36h) |
Not needed |
Not needed |
|
Sample pre-treatment* |
No |
Yes |
No |
No |
Yes |
Yes |
* Sample pre-treatment includes density gradient MNC- or magnetic/FACS- isolation. ASO-qPCR, allele-specific oligonucleotide quantitative real-time polymerase chain reaction; FACS, fluorescence activated cell sorting; IMF, immunofluorescence microscopy; MFC, multiparameter flow cytometry; MNC, mononuclear cells; NGF, next generation flow; NGS, next generation sequencing.
Table 2. Advantages and disadvantages of the most frequently used methods for detection of circulating tumor plasma cells in patients diagnosed with plasma cell neoplasms.
|
|
Cytology |
IMF |
MFC |
NGF |
ASO-qPCR |
NGS |
|
Availability |
High |
Low |
High |
High |
Intermediate |
Limited |
|
Applicability |
≈100% |
≈100% |
≈100% |
≈100% |
42% to75% |
80%-90% |
|
Sensitivity |
<10-2 |
<10-4 |
≤10-4 |
≤2×10-6 |
≤10-5 - 10-6 |
≤1×10-6 |
|
Specificity |
Limited |
Limited |
High |
High |
High |
High |
|
Standardized |
Yes |
No |
Ongoing |
Yes |
Yes |
Ongoing |
|
Quantitative |
Yes (high counts) |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Diagnostic sample |
Not required |
Not required |
Not required |
Not required |
Mandatory |
Mandatory |
|
Global sample analysis |
Yes |
No |
Yes |
Yes |
No |
No |
|
Time to results |
<2h |
4h |
2-3h |
3-4h |
3-4 weeks |
≥7 days |
|
Fresh sample |
Yes |
Yes |
Yes (<36h) |
Yes (<36h) |
No |
No |
|
Sample pre-treatment* |
No |
Yes |
No |
No |
Yes |
Yes |
|
Data analysis / interpretation |
Subjective |
Slightly subjective |
Slightly subjective |
More objective |
Slightly subjective |
More objective |
|
CTPC detection principle |
DFN |
Ig light-chain restriction |
DFN and LAIP |
DFN and LAIP |
Patient-specific IGH-V(D)J gene rearrangements |
Patient-specific IGH-V(D)J gene rearrangements¥ |
|
Additional biological characterization of CTPC |
No |
No |
Yes |
Yes |
No |
Yes |
|
Prognostic factor in MGUS |
NT |
Yes |
NT |
Yes |
NT |
NT |
|
Prognostic factor in SMM |
NT |
Yes |
Yes |
Limited |
NT |
NT |
|
Prognostic factor in MM |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Relative Cost |
Low |
High |
Intermediate |
Intermediate |
Intermediate |
High |
* Sample pre-treatment includes density gradient MNC- or magnetic/FACS- isolation.
¥ Including also potentially analysis of Ig light gene rearrangements.
ASO-qPCR, allele-specific oligonucleotide quantitative real-time polymerase chain reaction; CTPC, circulating tumor plasma cells; DFN, different from normal; FACS, fluorescence activated cell sorting; Ig, immunoglobulin; IGH, Ig heavy chain; IMF, immuno-fluorescence microscopy; LAIP, leukemia associated immunophenotype; MGUS, monoclonal gammopathy of undetermined significance; MFC, multiparameter flow cytometry; MM, multiple myeloma; MNC, mononuclear cells; NGF, next generation flow; NGS, next generation sequencing; NT, not tested; SMM, smoldering MM.
2.1. Circulating Tumor Plasma Cell Detection in Blood Smears by Conventional Cytology
Conventional cytology is a simple, fast and inexpensive approach for (expert-based subjective) identification of CTPC with a sensitivity of ≥1% (i.e., 10−2) of all nucleated cells in blood, which is available at virtually every clinical diagnostics laboratory worldwide (Table 2). The presence of CTPC by cytomorphology has long been associated with increased PC proliferation and more aggressive disease, which is observed (per definition) in PCL and in a small fraction of MM cases that present with high tumor load (≥5% of CTPC) and show a significantly poorer outcome -median overall survival (OS) rates of 1.1 years vs. 4.1 years for other MM cases with <5% or undetectable levels of CTPC at diagnosis, respectively - (Table 3). Thus, conventional cytomorphology remains the basis for the diagnosis of PCL . In addition, it is of great clinical utility for the identification of MM patients that show ≥2% CTPC by Wright–Giemsa cytology at diagnosis (14.1% of untreated MM patients), who (compared to MM patients with undetected CTPC in blood) display a poorer outcome both in terms of progression free survival (PFS) (median PFS of 17 months vs. 24 months, respectively) and OS rates (median OS of 25 months vs. 45 months, respectively) [29]. Altogether, these results indicate that conventional cytology is an easy and fast approach for the detection of (high numbers) of CTPC in the blood of MM patients, particularly in cases presenting with PCL-like laboratory findings (e.g., leukocytosis and elevated serum levels of lactate dehydrogenase) and in PCL patients. In contrast, conventional cytology is less useful among MGUS and SMM patients who usually present with low CTPC counts in blood. In fact, the absence of CTPC by cytomorphology should be interpreted with caution because of the limited sensitivity of the technique (Table 2).
Table 3. Prognostic impact of circulating tumor plasma cells on newly diagnosed and treated plasma cell neoplasms patients as assessed by distinct techniques.[44][45]
|
Methodology |
Diagnosis |
Treated |
|
|||||
|
MGUS |
SMM |
MM |
MM |
References |
||||
|
TTP/PFS |
TTP |
OS |
PFS |
OS |
PFS |
OS |
||
|
Cytology |
NT |
NT |
NT |
NT |
1.1 vs 4.1ya |
NT |
NT |
[36] |
|
IMF |
138m vs NRb |
12 vs 57mc |
49 vs 148mb |
NT |
2.4 vs 4.5yd |
6.2 vs 22.5me |
NT |
[41] |
|
MFC |
NT |
10m vs NRb |
NT |
25 vs 43mb (TTNT*) |
54 vs 89mb |
15.1m vs 29.6mb |
41m vs NRb |
[22] |
|
NGF |
31m vs NRf |
25% vs 0% at 2y (p>0.05)g |
NT |
22m vs NRg |
67% vs 0% at 2yg |
9 vs 46mb |
NT |
[21] |
|
ASO-qPCR |
NT |
NT |
NT |
26 vs 66mb |
53 vs 66m (p>0.05)b |
4 vs 15mb |
17 vs 52mb |
[44] |
|
NGS |
NT |
NT |
NT |
22.6 vs 47.5mh 26.7 vs 41.3mi |
>55mh,i |
NT |
NT |
[45] |
*TTNT, defined as time from diagnosis to next therapy due to documented relapse or progression of disease. ASO-qPCR, allele-specific oligonucleotide quantitative real-time polymerase chain reaction; IMF, immunofluorescence microscopy; m, months; MFC, multiparametric flow cytometry; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; NGF, next generation flow; NGS, next generation sequencing; NR, not reached; NT, not tested; OS, overall survival; PFS, progression-free survival; SMM, smoldering MM; TTP, time to progression; y; years. a ≥5% vs. <5% CTPC; b CTPC+ vs. CTPC-; c >5000 vs. ≤5000 CTPC/µL; d ≥4% vs. <4% CTPC; e ≥0.2 × 106 vs. <0.2 × 106 CTPC/L; f ≥0.058 vs. <0.058 CTPC/µL; g ≥0.1 vs. <0.1 CTPC/μL; h high vs. low expression levels of the CENPF gene; i high vs. low expression levels of the LGALS1 gene.
3.2. Fluorescence Microscopy
For decades now, fluorescence microscopy-based analysis of immuno-stained blood-derived mononuclear cells has been recurrently applied for the detection of CTPC in the blood of MGUS and MM patients, based on Ig light-chain restriction on tumor vs. normal PC . Briefly, this approach is based on the evaluation of anti-human light chain immunofluorescence staining patterns of density gradient isolated mononuclear cells from blood by fluorescence microscopy. Overall, this technique improves (by more than one log) the sensitivity of conventional cytology with the ability to detect one clonal PC among 10,000 mononuclear cells (sensitivity of 10−4) (Table 2). From a clinical point of view, the presence of CTPC by fluorescence microscopy is associated with disseminated disease [46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64], which is found in 19% to 29% of MGUS cases, 25% [24] to 50% of SMM patients and in 71% of untreated MM cases according to the literature. From a prognostic perspective, MM patients presenting with ≥4% CTPC in blood show significantly shorter median survival rates (2.4 vs. 4.5 years for MM patients with lower or undetected CTPC) (Table 3). In addition, the presence of CTPC in the blood of SMM patients has also been associated with shorter time to progression (TTP) rates compared to CTPC-negative SMM patients (median TTP of 9 vs. 30 months, respectively). This is even more evident among SMM cases presenting with higher CTPC counts (>5000 × 106 CTPC/L or >5% cytoplasmic Ig-positive CTPC/mononuclear cells) who show median time to progression rates of 12 vs. 57 months for other SMM patients with undetected or lower CTPC numbers. Similarly, MGUS patients that have CTPC by fluorescence microscopy also show a more adverse prognosis vs. CTPC-negative MGUS patients (median progression free survival of 138 months vs. not reached, respectively). Of note, recent CTPC detection techniques based on specific pre-analytical PC-enrichment procedures such as the CELLSEARCH® platform developed by Menarini-Silicon Biosystems (Castel Maggiore, Italy), have proven to increase the frequency of patients that show CTPC in blood to >85% of all MGUS, SMM and MM cases studied at diagnosis [65]. In addition, preliminary results suggest that this latter technology might also be of potential clinical utility among treated MM patients who reach complete response, because those patients that had higher CTPC counts in blood (≥100 CTPC/4 mL or ≥ 25 CTPC/mL) displayed a more adverse prognosis compared to patients with lower numbers of CTPC [49].
Despite the clinically relevant information provided by fluorescence microscopy (and other imaging-based approaches), this technique still has several important limitations that hamper its routine use in many laboratories [20,122]. These mainly include: i) the relatively limited sensitivity reached, ii) the fact that fluorescence microscopy is a laborious and time-consuming technique, iii) the need for a pre-enrichment step to isolate mononuclear cells with high potential for uncontrolled (selective) cell loss that might specifically affect CTPC at the same time it discards potentially relevant information on residual (non-mononuclear) hematologic blood cells , and iv) the need for a fluorescence microscope (or more complex instrumentation) usually not available for routine diagnostics in many haemato-oncology laboratories (Table 2).
3.3. Conventional Multiparameter Flow Cytometry
Flow cytometry has long been recognized as a well-suited methodology for the enumeration of CTPC in blood of plasma cell neoplasms patients . This technique is an easy, fast (<4 h), affordable and worldwide available approach which has been extensively used to identify, characterize and count CTPC in the blood of patients with plasma cell neoplasms. However, the lack of standardized protocols (i.e., very heterogeneous antibody panels have been used and variable numbers of cells analyzed per sample), the highly variable sensitivity levels reached for detection of CTPC (which translate into variable frequencies of CTPC-positive patients), together with the need for fresh (<24–48 h) samples, have limited the reproducibility of results, and thereby, its broader use and applicability (Table 2). Despite these limitations, flow cytometry has shown that the presence and number of CTPC in blood (both at diagnosis and after therapy) has important clinical implications in MM, and to a lesser extent, also in MGUS, and SMM patients [49].
Early multiparameter flow cytometry studies in MGUS based on very limited numbers of markers -e.g., three-color antibody combinations of CD45, CD38, and cytoplasmic (cy)Igκ or cyIgλ identified CTPC in the blood of 25% of cases with a median (range) of 0.3% (0.06%–0.97%)- CTPC from blood-derived mononuclear cells. However, these results could not be confirmed in subsequent studies using more sensitive approaches based on seven-color flow cytometry and analysis of ≥106 cells that reported counts of <0.0035% of CTPC in the blood of the majority of MGUS patients (93%) at diagnosis [49].
Similarly, in the few SMM-based flow cytometry studies reported in the literature, remarkably different frequencies of CTPC in blood were observed depending on the specific approach used. Thus, in an early three-color flow cytometry study in a small cohort of SMM patients, CTPC were identified in 3/8 cases (37.5%) . Conversely, a more recent report on a larger series of 100 SMM patients studied at diagnosis using six-color cytometry based on staining of approximately 150,000 mononuclear cells for CD45, CD19, CD38, CD138, cyIgκ and cyIgλ, identified CTPC in blood of only 24% of the patients . Of note, the detection of CTPC by multiparameter flow cytometry predicted shorter time to progression rates from SMM to MM (median TTP of 10 months vs. not reached for CTPC-positive vs. CTPC-negative patients) .
In contrast to MGUS and SMM, more studies have investigated the frequency and clinical implications of CTPC in the blood of MM patients by multiparameter flow cytometry, both at diagnosis and after starting therapy. Thus, CTPC have been identified by multiparameter flow cytometry in 50% to 75% of newly-diagnosed MM cases [19,20,22,28,31], depending on the number of markers and the specific antibody panels used , the number of cells analyzed [22,28,31], and the sample preparation protocol . Importantly, these studies showed that the rate of CTPC-positive cases in blood among untreated MM patients increases approximately 1.4 fold from density gradient mononuclear cell isolation-based approaches to whole blood flow cytometry protocols , potentially due to specific loss of CTPC during mononuclear cell isolation procedures. Additionally, in these studies, absolute CTPC counts by multiparameter flow cytometry in the blood of MM patients measured at diagnosis varied between 2.5–3 CTPC/µL .
From a prognostic point of view, higher CTPC counts in blood as detected by multiparameter flow cytometry (regardless of the specific threshold proposed) are systematically associated with an independent adverse prognostic impact among newly-diagnosed MM [49]. Thus, the presence of ≥0.0035% (vs. <0.0035%) CTPC in the blood of untreated MM patients translated into a worse outcome, with lower three-year time to progression (65% vs. 34%) and overall survival (52% vs. 90%) rates, respectively, independently of the therapeutic regimen used or the presence of adverse cytogenetics as defined by the International Myeloma Working Group criteria -i.e., Revised International Staging System (R-ISS)- [49]. Similarly, inferior overall survival (OS) rates were found in MM patients with ≥400 CTPC/150,000 mononuclear cells at diagnosis (median OS of 32 months vs. not reached for cases with <400 CTPC/150,000 mononuclear cells) . In line with these findings, a recent study on newly-diagnosed MM shows that the presence of ≥5 CTPC/µL of blood is associated with a significantly shorter time to next therapy (TTNT) and OS rates, compared with cases showing lower (<5 CTPC/µL) or undetected CTPC in blood (median TTNT of 21, 28 and 43 months, respectively, and median OS of 46, 76 and 89 months, respectively ). Based on these results, the authors suggest that R-ISS I and R-ISS II MM patients presenting with ≥5 CTPC/µL in blood at diagnosis might display a similarly dismal prognosis to R-ISS III MM patients.
Several flow cytometry-based studies also recognized the (adverse) prognostic impact of CTPC in treated MM patients, where decreasing frequencies of CTPC are associated with a progressively better response to therapy [33,116,125,126]. Overall, six-color flow cytometry (or higher) detected CTPC in 18.7% to 19.3% of treated MM patients prior to stem cell mobilization for autologous stem cell transplantation [51]. In MM patients who reached complete response prior to stem cell mobilization for autologous stem cell transplantation, the frequency of CTPC-positive patients ranges from 0% to 14%[50][51] , depending on the specific multiparametric flow cytometry approach used (e.g., for three- to six-color cytometry, the number of cells analyzed [50], and the sample preparation technique (e.g., staining of mononuclear cells) vs. erythrocyte-lysed whole blood [51]). In contrast, a significantly greater frequency (approximately 64%) of CTPC-positive cases was found among relapsed MM patients at the time of stem cell collection . In line with these findings, a poorer outcome (with significantly shorter time to progression, progression free survival and/or overall survival rates) of treated MM patients showing CTPC in the blood by multiparameter flow cytometry has been recurrently confirmed by several groups, independently of the therapeutic regimen administered and the depth of clinical response achieved [50][51] (Table 3).
3.4. Next Generation Flow Cytometry (NGF)
NGF approaches have been recently developed for minimal/measurable residual disease (MRD) monitoring in the BM of treated MM patients [52]. Early NGF studies already demonstrated a significantly greater sensitivity (sensitivity of ≤ 2x10−6) and reproducibility for NGF vs. classical (8–10-color) flow cytometry [52] (Table 2). This is mostly due to: i) evaluation of significantly greater numbers of cells per sample (i.e., ≥10 × 106 cells) achieved via ammonium chloride-based bulk-lysis of blood samples prior to antibody staining; ii) an optimized two eight-color tube antibody combination; and iii) usage of computer-assisted software tools for more objective and reproducible automated data analysis (i.e., the INFINICYT software from Cytognos Sl, Salamanca, Spain) [52].
More recently, NGF has also been applied for the detection of CTPC in the blood of MGUS, solitary plasmacytoma, SMM and MM (including a small group of macrofocal MM) patients. In this latter study, NGF showed that the presence of CTPC in blood is a sign of systemic disease with significantly lower rates of CTPC among patients with localized vs. disseminated diagnostic subtypes of monoclonal gammopathy: ≤25% in solitary plasmacytoma and macrofocal MM cases vs. 59% in MGUS and 100% in both SMM and MM patients. Overall, these are unprecedently high frequencies of CTPC-positive cases vs. those previously reported using other (less sensitive, i.e., flow) approaches . More interestingly, the number of CTPC in blood progressively increased from MGUS (median CTPC count of 0.008 CTPC/μL) to SMM (median of 0.16 CTPC/μL) and MM (median of 1.9 CTPC/μL) patients. Noteworthy, a cutoff of 0.058 CTPC/μL was able to discriminate MGUS from MM patients with high accuracy (80% sensitivity and 80% specificity) .
From a prognostic point of view, the number of CTPC detected by NGF also proved to efficiently discriminate between MGUS cases with high (≥0.058 CTPC/μL) vs. low (<0.058 CTPC/μL) risk of progression to MM (median time to progression of 31 months vs. not reached, respectively), and to identify newly-diagnosed MM patients with reduced (≥0.1 CTPC/μL) vs. prolonged (<0.1 CTPC/μL) progression free survival (PFS) and overall survival (OS) (median PFS of 22 months vs. not reached and 75% OS of 17 months vs. not reached, respectively). Interestingly, MM patients who had CTPC counts at diagnosis similar to those of MGUS (<0.1 CTPC/μL) displayed a significantly favorable long-term outcome, independent of their response to therapy (e.g., complete response and BM MRD status). In contrast, in this study, the number of CTPC found in blood by NGF did not show a significant impact on the outcome of SMM patients, probably due to the limited of number of cases analyzed(Table 3).
Recently, the presence of CTPC in the blood of MM patients has also been evaluated by NGF after starting therapy. Thus, results in a large cohort of 137 real-world MM patients treated outside of clinical trials demonstrated CTPC by NGF in 26% of treated MM cases, including 17% of cases who had achieved complete response/stringent complete response, a significantly greater percentage than previously shown by conventional multiparameter flow cytometry approaches. As expected, all treated MM patients who had CTPC in the blood by NGF were also MRD-positive in the BM, while the remaining two-thirds of BM MRD-positive cases did not show blood involvement by CTPC. Despite the lower sensitivity of detection of CTPC in blood vs. BM MRD, persistence of CTPC after treatment by NGF was associated with five-fold reduced progression free survival rates compared to CTPC-negative patients (median PFS of 9 vs. 46 months, respectively), independent of the patient’s tumor cytogenetics, the response achieved (complete response/stringent complete response vs. non-complete response) including the BM MRD status, and the treatment phase at which the presence of CTPC in blood had been assessed . Interestingly, sequential blood CTPC monitoring by NGF predicted better long-term outcomes than single time-point assessments. Thus, treated MM patients who were persistently CTPC-negative in blood (i.e., CTPC −/−) or turned negative after a first positive result (i.e., CTPC +/−), had significantly superior progression free survival rates at two years (92.5%) compared to cases with positivity for CTPC in the last NGF analyses (CTPC −/+ or CTPC +/+ cases) (41%).
Altogether, these findings demonstrate that NGF is a highly-sensitive technique that allows identification and quantitation of CTPC in blood at diagnosis in the majority of MGUS cases and virtually all SMM and MM cases . In addition, it provides valuable prognostic information in both patient groups and represents a new (minimally invasive) surrogate biomarker for BM MRD-positivity among treated MM patients .
3.5. Molecular (ASO-qPCR and Next Generation Sequencing) Techniques
Although molecular techniques cannot strictly detect entire (tumor) cells, including CTPC, quantitation of some unique genetic (e.g., DNA) tumor markers such as patient-specific IGH-V(D)J gene rearrangements of CTPC has been long proven to closely reflect the tumor cell load [53]. This is in contrast with techniques that measure the M-component (e.g., immunofixation and/or mass spectrometry) [55][56], because the serum levels of the monoclonal protein produced by the tumor PC depend on several parameters other than the number of CTPC, such as the tumor load in other tissues (e.g., BM), the (highly variable) amount of protein produced by individual tumor PC among different plasma cell neoplasms patients, and its half-life [57]. So far, most studies in which molecular techniques have been used for CTPC detection in plasma cell neoplasms have focused on MM patients evaluated at diagnosis and/or after starting therapy [58]. Of note, virtually all these studies used ASO-qPCR , whereas a few NGS-based studies have been more recently reported .
ASO-qPCR is a highly specific and sensitive (<10−5 to 10−6) molecular method for the detection and quantification of CTPC, based on the identification and follow-up of patient-specific Ig heavy chain (IGH) complementary determining region 3 (CDR3) gene rearrangements in the blood of MM patients . Thus, ASO-qPCR-based studies have shown the presence of CTPC in the blood in 67% to 93% of MM patients studied at diagnosis (the calculated number of CTPC detected ranged from 0.001% to 1.0% of all white blood cells). In turn, another preliminary ASO-qPCR-based study identified CTPC in 81% of MGUS cases, although the authors could not ensure that these patients were not in an advanced stage of the disease (with a constant increase of the monoclonal component) [19] (Figure 1). Similarly to NGF, the presence of CTPC by ASO-qPCR has been reported in the blood of between 24% of treated MM cases who are incomplete responders [113] to 73% of relapsed patients. From a prognostic point of view, detection of CTPC by ASO-qPCR has been associated with impaired survival rates both in newly-diagnosed MM patients (median progression free survival of 26 months vs. 66 months for CTPC negative cases, respectively), and in MM cases studied after three months of high-dose therapy plus autologous stem cell transplantation (median progression free survival of 4 months vs. 15 months, and median overall survival of 17 months vs. 52 months for CTPC-positive vs. CTPC-negative cases, respectively) (Table 3). However, data on the potential diagnostic and prognostic implications of CTPC detection by ASO-PCR in both MGUS and SMM patients is still limited and remains to be investigated.
As an advantage, ASO-qPCR and other (e.g., NGS) molecular techniques do not require fresh samples, since they can use stored blood-derived nucleic acids to evaluate CTPC. In contrast, they have a more limited applicability (range: 42% to 95% of cases) in MGUS, MM and other plasma cell neoplasms [54][59][62][63][64]. Additionally, these molecular PCR-based techniques require analysis of a baseline (e.g., diagnostic or relapse) sample to identify the patient-specific IGH rearrangement(s) [60][61] (Table 2). For this purpose, a pre-enrichment/purification of tumor PC via isolation of mononuclear cells through density gradient centrifugation or via magnetic and fluorescence activated cell sorting (FACS) of e.g., CD138+ PC is frequently required, even when diagnostic BM samples are available.
Next generation sequencing of IGH-V(D)J is a more sensitive (<10−6) and applicable (88%–95%) molecular technique than ASO-qPCR [60][61] (Table 2). Thus, analysis of blood leukocyte DNA samples identified the clonotypic V(D)J rearrangements of CTPC by NGS in between 71% and 78% of MM cases at baseline (e.g., before starting therapy) and in 40% of treated MM patients (who mostly reached partial response to therapy) . Nonetheless, in one study, the frequency of CTPC in blood increased to 96% of newly-diagnosed MM patients when RNA was used instead of DNA to detect tumor IGH-V(D)J gene rearrangements . However, to date, molecular analysis by next generation sequencing of blood-derived CTPC has preferentially focused on the molecular characterization of purified CTPC in order to better understand the biology of the disease [62][63][64]. In this regard, a recent study based on single-cell RNA sequencing reported that overexpression of CENPF and LGALS1 genes in CTPC from MM patients was associated with reduced progression free survival rates [119] (Table 3). In contrast, the clinical impact of next generation sequencing-based analysis of CTPC in MGUS, SMM and other plasma cell neoplasms currently remains unknown and deserves further investigation [62].
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390, doi:10.1182/blood-2016-01-643569.
- McKenna R.W Kyle R.A Kuehi W.M, Grogan T.M, Harris N.L, C.R.. Plasma cells neoplasms. In World Health Organization Calssification of Tumours of Haematopoietic and Lymphoid Tissue IARC; Lyon, 2008; Vol. 4th, pp. 200–208 ISBN 9789283224310.
- Palumbo, A.A.K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060, doi:10.1016/S0140-6736(14)60493-1.
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.-V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Prim. 2017, 3, 17046, doi:10.1038/nrdp.2017.46.
- Kyle, R.A.; Vincent Rajkumar, S. Monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2006, 134, 573–589, doi:10.1111/j.1365-2141.2006.06235.x.
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Offord, J.R.; Dispenzieri, A.; Katzmann, J.A.; Melton, L.J. Prevalence of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2006, 354, 1362–1369, doi:10.1056/NEJMoa054494.
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 2009, 113, 5412–5417, doi:10.1182/blood-2008-12-194241.
- Weiss, B.M.; Abadie, J.; Verma, P.; Howard, R.S.; Kuehl, W.M. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 2009, 113, 5418–5422, doi:10.1182/blood-2008-12-195008.
- Kyle, R.A.; Remstein, E.D.; Therneau, T.M.; Dispenzieri, A.; Kurtin, P.J.; Hodnefield, J.M.; Larson, D.R.; Plevak, M.F.; Jelinek, D.F.; Fonseca, R.; et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N. Engl. J. Med. 2007, 356, 2582–2590, doi:10.1056/NEJMoa070389.
- Kyle, R.A.; Child, J.A.; Anderson, K.; Barlogie, B.; Bataille, R.; Bensinger, W.; Bladé, J.; Boccadoro, M.; Dalton, W.; Dimopoulos, M.; et al. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: A report of the International Myeloma Working Group. Br. J. Haematol. 2003, 121, 749–757, doi:10.1046/j.1365-2141.2003.04355.x.
- Katodritou, E.; Kastritis, E.; Gatt, M.E.; Cohen, Y.C.; Avivi, I.; Pouli, A.; Lalayianni, A.; Lavi, N.; Delibasi, S.; Kyrtsonis, M.-C.; et al. Real-World Data on Incidence, Clinical Characteristics and Outcome of Patients with Macrofocal Multiple Myeloma (MFMM) in the Era of Novel Therapies: A Study of the Greco-Israeli Collaborative Myeloma Working Group. Blood 2018, 132, 3295–3295, doi:10.1182/blood-2018-99-114106.
- Fan, J.; Hou, J.; Du, J.; Jin, L.; Peng, L.; Chen, B.; Xi, H.; Zhang, H.; Jiang, H.; Zhou, F.; et al. Macrofocal Multple Myeloma Is a Particular Subgroup of Multiple Myeloma. Blood 2015, 126, 1855 LP-1855, doi:https://doi.org/10.1182/blood.V126.23.1855.1855.
- Oriol, A. Multiple myeloma with extramedullary disease. Adv. Ther. 2011, 28, 1–6, doi:10.1007/s12325-011-0079-0.
- Kyle, R.A.; Maldonado, J.E.; Bayrd, E.D. Plasma cell leukemia. Report on 17 cases. Arch. Intern. Med. 1974, 133, 813–818, doi:10.1001/archinte.133.5.813.
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654, doi:10.1016/S0140-6736(15)01274-X.
- Evans, L.A.; Jevremovic, D.; Nandakumar, B.; Buadi, F.K.; Dispenzieri, A.; Dingli, D.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P.; Go, R.S.; et al. Utilizing Multiparametric Flow Cytometry to Identify Patients with Primary Plasma Cell Leukemia at Diagnosis. Blood 2019, 134, 4334–4334, doi:10.1182/blood-2019-126777.
- Gundesen, M.T.; Lund, T.; Moeller, H.E.H.; Abildgaard, N. Plasma Cell Leukemia: Definition, Presentation, and Treatment. Curr. Oncol. Rep. 2019, 21, 1–10, doi:10.1007/s11912-019-0754-x.
- Fernández De Larrea, C.; Kyle, R.A.; Durie, B.G.M.; Ludwig, H.; Usmani, S.; Vesole, D.H.; Hajek, R.; San Miguel, J.F.; Sezer, O.; Sonneveld, P.; et al. Plasma cell leukemia: Consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 2013, 27, 780–791, doi:10.1038/leu.2012.336.
- Billadeau, D.; Van Ness, B.; Kimlinger, T.; Kyle, R. a; Therneau, T.M.; Greipp, P.R.; Witzig, T.E. Clonal circulating cells are common in plasma cell proliferative disorders: a comparison of monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and active myeloma. Blood 1996, 88, 289–296.
- Nowakowski, G.S.; Witzig, T.E.; Dingli, D.; Tracz, M.J.; Gertz, M.A.; Lacy, M.Q.; Lust, J.A.; Dispenzieri, A.; Greipp, P.R.; Kyle, R.A.; et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 2005, 106, 2276–2279, doi:10.1182/blood-2005-05-1858.
- Sanoja-Flores, L.; Flores-Montero, J.; Garcés, J.J.; Paiva, B.; Puig, N.; García-Mateo, A.; García-Sánchez, O.; Corral-Mateos, A.; Burgos, L.; Blanco, E.; et al. Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 2018, 8, 117, doi:10.1038/s41408-018-0153-9.
- Gonsalves, W.I.; Jevremovic, D.; Nandakumar, B.; Dispenzieri, A.; Buadi, F.K.; Dingli, D.; Lacy, M.Q.; Hayman, S.R.; Kapoor, P.; Leung, N.; et al. Enhancing the R‐ISS classification of newly diagnosed multiple myeloma by quantifying circulating clonal plasma cells. Am. J. Hematol. 2020, 95, 310–315, doi:10.1002/ajh.25709.
- Kumar, S.; Rajkumar, S.V.; Kyle, R.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Lust, J.A.; Gertz, M.A.; Greipp, P.R.; Witzig, T.E. Prognostic value of circulating plasma cells in monoclonal gammopathy of undetermined significance. J. Clin. Oncol. 2005, 23, 5668–5674, doi:10.1200/JCO.2005.03.159.
- Witzig, T.E.; Kyle, R.A.; O’Fallon, W.M.; Greipp, P.R. Detection of peripheral blood plasma cells as a predictor of disease course in patients with smouldering multiple myeloma. Br. J. Haematol. 1994, 87, 266–272, doi:10.1111/j.1365-2141.1994.tb04908.x.
- Bianchi, G.; Kyle, R.A.; Larson, D.R.; Witzig, T.E.; Kumar, S.; Dispenzieri, A.; Morice, W.G.; Rajkumar, S. V High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 2013, 27, 680–5, doi:10.1038/leu.2012.237.
- Gonsalves, W.I.; Rajkumar, S.V.; Dispenzieri, A.; Dingli, D.; Timm, M.M.; Morice, W.G.; Lacy, M.Q.; Buadi, F.K.; Go, R.S.; Leung, N.; et al. Quantification of circulating clonal plasma cells via multiparametric flow cytometry identifies patients with smoldering multiple myeloma at high risk of progression. Leukemia 2017, 31, 130–135, doi:10.1038/leu.2016.205.
- Witzig, T.E.; Gertz, M.A.; Lust, J.A.; Kyle, R.A.; O’Fallon, W.M.; Greipp, P.R. Peripheral blood monoclonal plasma cells as a predictor of survival in patients with multiple myeloma. Blood 1996, 88, 1780–7.
- Gonsalves, W.I.; Rajkumar, S.V.; Gupta, V.; Morice, W.G.; Timm, M.M.; Singh, P.P.; Dispenzieri, A.; Buadi, F.K.; Lacy, M.Q.; Kapoor, P.; et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high- risk myeloma. Leukemia 2014, 28, 2060–2065, doi:10.1038/leu.2014.98.
- An, G.; Qin, X.; Acharya, C.; Xu, Y.; Deng, S.; Shi, L.; Zang, M.; Sui, W.; Yi, S.; Li, Z.; et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann. Hematol. 2015, 94, 257–264, doi:10.1007/s00277-014-2211-0.
- Granell, M.; Calvo, X.; Garcia-Guiñón, A.; Escoda, L.; Abella, E.; Martínez, C.M.; Teixidó, M.; Teresa Gimenez, M.; Senín, A.; Sanz, P.; et al. Prognostic impact of circulating plasma cells in patients with multiple myeloma: Implications for plasma cell leukemia definition. Haematologica 2017, 102, 1099–1104, doi:10.3324/haematol.2016.158303.
- Bae, M.H.; Park, C.-J.; Kim, B.H.; Cho, Y.-U.; Jang, S.; Lee, D.-H.; Seo, E.-J.; Yoon, D.H.; Lee, J.-H.; Suh, C. Increased circulating plasma cells detected by flow cytometry predicts poor prognosis in patients with plasma cell myeloma. Cytometry B. Clin. Cytom. 2018, 94, 493–499, doi:10.1002/cyto.b.21606.
- Rawstron, A.C.; Owen, R.G.; Davies, F.E.; Johnson, R.J.; Jones, R. a; Richards, S.J.; Evans, P. a; Child, J. a; Smith, G.M.; Jack, a S.; et al. Circulating plasma cells in multiple myeloma: characterization and correlation with disease stage. Br. J. Haematol. 1997, 97, 46–55.
- Dingli, D.; Nowakowski, G.S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Rajkumar, S.V.; Greipp, P.R.; Litzow, M.R.; Gastineau, D.A.; Witzig, T.E.; et al. Flow cytometric detection of circulating myeloma cells before transplantation in patients with multiple myeloma : a simple risk stratification system. Blood 2006, 107, 3384–3388, doi:10.1182/blood-2005-08-3398.Supported.
- Gonsalves, W.I.; Morice, W.G.; Rajkumar, V.; Gupta, V.; Timm, M.M.; Dispenzieri, A.; Buadi, F.K.; Lacy, M.Q.; Singh, P.P.; Kapoor, P.; et al. Quantification of clonal circulating plasma cells in relapsed multiple myeloma. Br. J. Haematol. 2014, 167, 500–505, doi:10.1111/bjh.13067.
- Sanoja-Flores, L.; Flores-Montero, J.; Puig, N.; Contreras-Sanfeliciano, T.; Pontes, R.; Corral-Mateos, A.; García-Sánchez, O.; Díez-Campelo, M.; Pessoa de Magalhães, R.J.; García-Martín, L.; et al. Blood monitoring of circulating tumor plasma cells by next generation flow in multiple myeloma after therapy. Blood 2019, 134, 2218–2222, doi:10.1182/blood.2019002610.
- Ravi, P.; Kumar, S.K.; Roeker, L.; Gonsalves, W.; Buadi, F.; Lacy, M.Q.; Go, R.S.; Dispenzieri, A.; Kapoor, P.; Lust, J.A.; et al. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018, 8, 1–6, doi:10.1038/s41408-018-0140-1.
- Touzeau, C.; Pellat-Deceunynck, C.; Gastinne, T.; Accard, F.; Jego, G.; Avet-Loiseau, H.; Robillard, N.; Harousseau, J.L.; Bataille, R.; Moreau, P. Reactive plasmacytoses can mimick plasma cell leukemia: Therapeutical implications. Leuk. Lymphoma 2007, 48, 207–208, doi:10.1080/10428190601016159.
- Gounari, E.; Tsavdaridou, V.; Koletsa, T.; Nikolaidou, A.; Kaiafa, G.; Papaioannou, M.; Kostopoulos, I.; Skoura, L. Utility of hematology analyzer and flow cytometry in timely and correct detection of circulating plasma cells: Report of three cases. Cytometry B. Clin. Cytom. 2016, 90, 531–537, doi:10.1002/cyto.b.21350.
- Huhn, S.; Weinhold, N.; Nickel, J.; Pritsch, M.; Hielscher, T.; Hummel, M.; Bertsch, U.; Huegle-Doerr, B.; Vogel, M.; Angermund, R.; et al. Circulating tumor cells as a biomarker for response to therapy in multiple myeloma patients treated within the GMMG-MM5 trial. Bone Marrow Transplant. 2017, 1–5, doi:10.1038/bmt.2017.91.
- Vij, R.; Mazumder, A.; Klinger, M.; O’Dea, D.; Paasch, J.; Martin, T.; Weng, L.; Park, J.; Fiala, M.; Faham, M.; et al. Deep Sequencing Reveals Myeloma Cells in Peripheral Blood in Majority of Multiple Myeloma Patients. Clin. Lymphoma, Myeloma Leuk. 2014, 14, 131–139, doi:10.1016/j.clml.2013.09.013.
- Gertz, M.A.; Witzig, T.E.; Pineda, A.A.; Greipp, P.R.; Kyle, R.A.; Litzow, M.R. Monoclonal plasma cells in the blood stem cell harvest from patients with multiple myeloma are associated with shortened relapse-free survival after transplantation. Bone Marrow Transplant. 1997, 19, 337–342, doi:10.1038/sj.bmt.1700670.
- Chakraborty, R.; Muchtar, E.; Kumar, S.K.; Jevremovic, D.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Hayman, S.R.; Hogan, W.J.; Kapoor, P.; et al. Risk stratification in myeloma by detection of circulating plasma cells prior to autologous stem cell transplantation in the novel agent era. Blood Cancer J. 2016, 6, 1–6, doi:10.1038/bcj.2016.117.
- Oberle, A.; Brandt, A.; Voigtlaender, M.; Thiele, B.; Radloff, J.; Schulenkorf, A.; Alawi, M.; Akyüz, N.; März, M.; Ford, C.T.; et al. Monitoring multiple myeloma by next-generation sequencing of V(D)J rearrangements from circulating myeloma cells and cell-free myeloma DNA. Haematologica 2017, 102, 1105–1111, doi:10.3324/haematol.2016.161414.
- Korthals, M.; Sehnke, N.; Kronenwett, R.; Schroeder, T.; Strapatsas, T.; Kobbe, G.; Haas, R.; Fenk, R. Molecular Monitoring of Minimal Residual Disease in the Peripheral Blood of Patients with Multiple Myeloma. Biol. Blood Marrow Transplant. 2013, 19, 1109–1115, doi:10.1016/j.bbmt.2013.04.025.
- Garcés, J.-J.; Simicek, M.; Vicari, M.; Brozova, L.; Burgos, L.; Bezdekova, R.; Alignani, D.; Calasanz, M.-J.; Growkova, K.; Goicoechea, I.; et al. Transcriptional profiling of circulating tumor cells in multiple myeloma: a new model to understand disease dissemination. Leukemia 2020, 34, 589–603, doi:10.1038/s41375-019-0588-4.
- Witzig, T.E.; Dhodapkar, M. V; Kyle, R.A.; Greipp, P.R. Quantitation of circulating peripheral blood plasma cells and their relationship to disease activity in patients with multiple myeloma. Cancer 1993, 72, 108–113.
- Foulk, B.; Schaffer, M.; Gross, S.; Rao, C.; Smirnov, D.; Connelly, M.C.; Chaturvedi, S.; Reddy, M.; Brittingham, G.; Mata, M.; et al. Enumeration and characterization of circulating multiple myeloma cells in patients with plasma cell disorders. Br. J. Haematol. 2018, 180, 71–81, doi:10.1111/bjh.15003.
- Witzig, T.E.; Meyers, C.; Therneu, T.; Greipp, P.R. A prospective study of CD38/45 flow cytometry and immunofluorescence microscopy to detect blood plasma cells in patients with plasma cell proliferative disorders. Leuk. Lymphoma 2000, 38, 345–350, doi:10.3109/10428190009087025.
- Periago, A.; Campillo, J.A.; Mrowiec, A.; Gimeno, L.; Montes, N.R.; Martínez-Sánchez, M. V; Cabañas-Perianes, V.; García-Garay, C.; Bolarin, J.M.; Blasco-Mogorrón, A.; et al. Circulating aberrant plasma cells allow risk stratification of patients with myeloma. Am. J. Hematol. 2016, 91, E353–E355, doi:10.1002/ajh.24431.
- Moor, I.; Bacher, V.U.; Jeker, B.; Taleghani, B.M.; Mueller, B.U.; Keller, P.; Betticher, D.; Egger, T.; Novak, U.; Pabst, T. Peripheral flow-MRD status at the time of autologous stem cell collection predicts outcome in multiple myeloma. Bone Marrow Transplant. 2018, 53, 1599–1602, doi:10.1038/s41409-018-0245-y.
- Cowan, A.J.; Stevenson, P.A.; Libby, E.N.; Becker, P.S.; Coffey, D.G.; Green, D.J.; Hyun, T.S.; Fromm, J.R.; Gopal, A.K.; Holmberg, L.A. Circulating Plasma Cells at the Time of Collection of Autologous PBSC for Transplant in Multiple Myeloma Patients is a Negative Prognostic Factor Even in the Age of Post-Transplant Maintenance Therapy. Biol. Blood Marrow Transplant. 2018, 24, 1386–1391, doi:10.1016/j.bbmt.2018.02.017.
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.-J.; Vidriales, M.-B.; García-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103, doi:10.1038/leu.2017.29.
- Paiva, B.; Puig, N.; Cedena, M.-T.; Rosiñol, L.; Cordón, L.; Vidriales, M.-B.; Burgos, L.; Flores-Montero, J.; Sanoja-Flores, L.; Lopez-Anglada, L.; et al. Measurable Residual Disease by Next-Generation Flow Cytometry in Multiple Myeloma. J. Clin. Oncol. 2019, 38, 1–10, doi:10.1200/JCO.19.01231.
- Billadeau, D.; Quam, L.; Thomas, W.; Kay, N.; Greipp, P.; Kyle, R.; Oken, M.M.; Van Ness, B. Detection and quantitation of malignant cells in the peripheral blood of multiple myeloma patients. Blood 1992, 80, 1818–1824.
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346, doi:10.1016/S1470-2045(16)30206-6.
- Chapman, J.R.; Thoren, K.L. Tracking of low disease burden in multiple myeloma: Using mass spectrometry assays in peripheral blood. Best Pract. Res. Clin. Haematol. 2020, 33, 101142, doi:10.1016/j.beha.2020.101142.
- Drayson, M.; Morgan, G.; Jackson, G.; Davies, F.; Owen, R.; Ross, F.; Gregory, W.; Navarro-Coy, N.; Heatley, F.; Bell, S.; et al. Prospective Study of Serum FLC and Other M-Protein Assays: When and How to Measure Response? Clin. Lymphoma Myeloma 2012, 9, S56, doi:10.1016/s1557-9190(11)70552-2.
- Bai, Y.; Orfao, A.; Chim, C.S. Molecular detection of minimal residual disease in multiple myeloma. Br. J. Haematol. 2018, 181, 11–26, doi:10.1111/bjh.15075.
- Puig, N.; Balanzategui, A.; Martínez, J.; Paiva, B.; García, H.; Fumero, S.; Jimé Nez, C.; Alcoceba, M.; Chilló, M.C.; Sebastián, E.; et al. Critical evaluation of ASO RQ-PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia 2014, 28, 391–397, doi:10.1038/leu.2013.217.
- Paiva, B.; Dongen, J.J.M. Van; Orfao, A. New criteria for response assessment : role of minimal residual disease in multiple myeloma. Blood 2015, 125, 3059–3069, doi:10.1182/blood-2014-11-568907.
- Yao, Q.; Bai, Y.; Orfao, A.; Kumar, S.; Chim, C.S. Upgraded Standardized Minimal Residual Disease Detection by Next-Generation Sequencing in Multiple Myeloma. J. Mol. Diagnostics 2020, doi:10.1016/j.jmoldx.2020.02.005.
- Mishima, Y.; Paiva, B.; Shi, J.; Michor, F.; San Miguel, J.F.; Ghobrial Correspondence, I.M.; Mishima, Y.; Park, J.; Manier, S.; Takagi, S.; et al. The Mutational Landscape of Circulating Tumor Cells in Multiple Myeloma. Cell Rep. 2017, 19, 218–224, doi:10.1016/j.celrep.2017.03.025.
- Ledergor, G.; Weiner, A.; Zada, M.; Wang, S.Y.; Cohen, Y.C.; Gatt, M.E.; Snir, N.; Magen, H.; Koren-Michowitz, M.; Herzog-Tzarfati, K.; et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 2018, 24, 1867–1876, doi:10.1038/s41591-018-0269-2.
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.C.; et al. Whole-exome sequencing of cell-free DNA and circulating tumor cells in multiple myeloma. Nat. Commun. 2018, 9, 1–11, doi:10.1038/s41467-018-04001-5.
- Schmidt-Hieber, M.; Pérez-Andrés, M.; Paiva, B.; Flores-Montero, J.; Perez, J.J.; Gutierrez, N.C.; Vidriales, M.B.; Matarraz, S.; San Miguel, J.F.; Orfao, A. CD117 expression in gammopathies is associated with an altered maturation of the myeloid and lymphoid hematopoietic cell compartments and favorable disease features. Haematologica 2011, 96, 328–332, doi:10.3324/haematol.2010.031872.