Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalie Mayer | -- | 3440 | 2022-05-19 05:27:37 | | | |
| 2 | Jessie Wu | -3 word(s) | 3437 | 2022-05-19 05:32:23 | | | | |
| 3 | Jessie Wu | Meta information modification | 3437 | 2022-05-19 05:50:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mayer, N.; Widderich, N.; , .; Reckels, B.; Lohkamp, F.; Visscher, C.; Schwaneberg, U.; Kaltschmitt, M.; Bubenheim, P. Improved P Digestibility in Animal Feed and Limitations. Encyclopedia. Available online: https://encyclopedia.pub/entry/23099 (accessed on 07 February 2026).
Mayer N, Widderich N, , Reckels B, Lohkamp F, Visscher C, et al. Improved P Digestibility in Animal Feed and Limitations. Encyclopedia. Available at: https://encyclopedia.pub/entry/23099. Accessed February 07, 2026.
Mayer, Natalie, Niklas Widderich, , Bernd Reckels, Florian Lohkamp, Christian Visscher, Ulrich Schwaneberg, Martin Kaltschmitt, Paul Bubenheim. "Improved P Digestibility in Animal Feed and Limitations" Encyclopedia, https://encyclopedia.pub/entry/23099 (accessed February 07, 2026).
Mayer, N., Widderich, N., , ., Reckels, B., Lohkamp, F., Visscher, C., Schwaneberg, U., Kaltschmitt, M., & Bubenheim, P. (2022, May 19). Improved P Digestibility in Animal Feed and Limitations. In Encyclopedia. https://encyclopedia.pub/entry/23099
Mayer, Natalie, et al. "Improved P Digestibility in Animal Feed and Limitations." Encyclopedia. Web. 19 May, 2022.
Copy Citation
A circular phosphorus (P) bioeconomy is not only worthwhile for conserving limited mineral P reservoirs, but also for minimizing negative environmental impacts caused by human-made alterations. Although P is an essential nutrient, most of the P in concentrates based on cereals, legumes and oilseed byproducts is organically bound to phytate. The latter cannot be efficiently utilized by monogastric animals and is therefore diluted into the environment through the manure pathway.
phosphorus
phytate
grains
1. Mechanical P Separation from Whole Grains
All grains consist of three biological parts: endosperm, germ, and the hull with several different cell layers. From a milling perspective, all components that are not pure endosperm, and thus do not serve as white flour are summarized as bran and account for up to 27 wt.% of the whole grain. The outer layers contain the aleurone, testa and pericarp, as shown in Figure 1 [1][2].
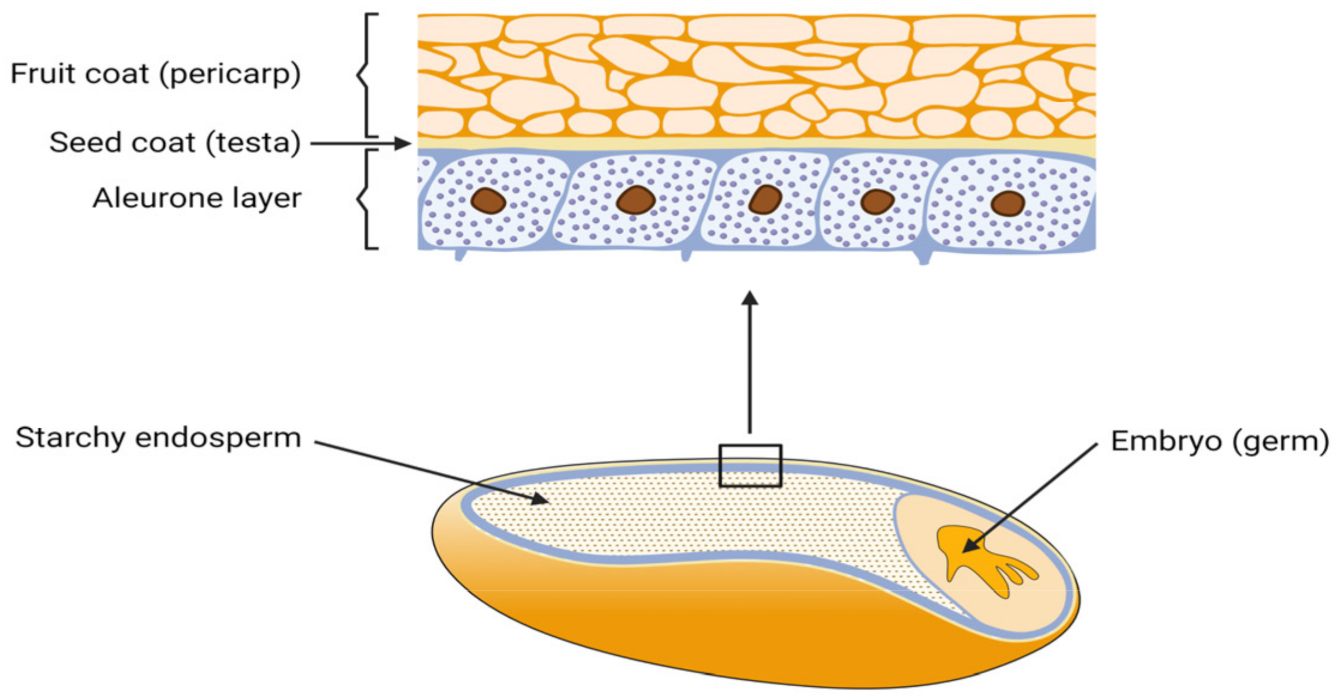
Figure 1. Composition of a grain and the outer bran layers (created with biorender.com, accessed on 20 October 2021).
In Europe, major cereal types produced and used as animal feed are wheat (32 to 45%), corn (32 to 35%) and barley (22 to 35%) [3]. P in common grains, such as wheat and barley is mostly accumulated in the aleurone, which is a layer in between the endosperm and the outer grain hull. Here, P is bound in the form of phytate within protein globoids, accounting for up to 90% of the total P content [4]. Phytate in corn, in contrast, is up to 80 to 90% accumulated within the germ, thus also in the bran fraction [5]. Therefore, two ways to separate a P-rich fraction in a purely mechanical way exist: either grains can be fully debranned which leaves endosperm flour as a P-poor fraction or specific layer exclusion can be performed. The latter aims to separate a very small grain fraction in the region of the P-rich cell layers, which, at the same time, contains maximum P quantity.
1.1. Debranning
Bran, the outer part of the grain, contains many valuable nutrients, such as high amounts of dietary fiber, proteins, and minerals [5]. Among the minerals, especially P is enriched up to a three times higher content in the bran fraction than in whole grains [6]. Wheat in particular typically undergoes a milling process where the starchy endosperm is separated from all other grain components for use in food industry [1], whereas wheat bran is mostly used in animal feeding [7]. Other cereals, such as corn and barley, are specifically grown for animal feeding and used as whole grains in compound feeds [1]. However, debranning processes also exist for barley and corn. Barley is partly fed in a de-hulled form and corn is debranned for use in the starch industry [1][8].
Wheat bran accounts for 20 wt.% of the whole grain on average; for corn and barley, bran makes up approx. 30wt.%. About 72% and 76% of P is accumulated in wheat and corn bran, respectively [4][6][9][10]. Barley bran contains approximately 55 to 70% of all P [1]. Thus, debranning can significantly reduce the total cereal P content by up to 70 to 80%. Especially phytate-P can be separated to an even higher extent of up to 90% [10].
However, with the debranning of grains for animal feed, significant amounts of fiber and proteins can be lost. In the corn hull, especially heteroxylan type dietary fiber is enriched by a factor of 10, whereas proteins are evenly distributed between bran and endosperm [8][11]. Main nutrients in barley bran are the fiber components arabinoxylan and β-glucan. For humans, it was shown that both components show positive health effects, e.g., through prebiotic properties or cholesterol reduction, thus expected to be similarly positive for animal feed as well [9][12]. Likewise, 30% of lipids are accumulated in the barley bran; proteins, in turn, are mostly in the endosperm and therefore remain within a debranned feed fraction [1].
When debranned corn or barley is fed, missing components need to be replaced in the diet [13]. Also, some contaminants, such as pesticides, heavy metals and undesired microorganisms are present in the outer grain layers, so that, for example, removal of only the outer 4 wt.% was shown to reduce bacterial contamination by 87% [12]. Thus, debranning comprises both positive and negative secondary effects on nutritional quality of cereal-based animal feed to be considered in compound feeding.
Particularly for wheat, debranning is already carried out to separate the food from the feed fraction [1]. Thus, the P-poor endosperm is not available as a feed component. Here, a specific layer exclusion is the only mechanical separation technique potentially feasible.
1.2. Specific Layer Exclusion
In contrast to a whole debranning step, a specific separation of the P-rich grain part can prevent the loss of valuable nutrients in the bran and a higher total mass for animal feed is retained. The separated grain mass should be limited to a minimum, while the P content of the separated fraction is maximized [14]. Considering a complete and pure removal of the P-rich grain components, aleurone and germ, a similar amount of P is expected to be removed compared to debranning, but a significantly higher share of the grain remains for feeding [6][15]. The aleurone of wheat and barley accounts for 5 to 6 wt.% and contains 95% of PO [1][15]. For corn, the germ with 7.5 wt.% of the whole grain contains around 90% of all PO [4][16]. Therefore, theoretically, 90 to 95% of PO (equaling 50 to 76% of the total P) can be separated from wheat, barley, and corn, while over 90% of the mass remains available for feeding. In comparison to whole debranning (removal of 20 to 30 wt.% of the grain), 35 to 45 Mt/a additional P-modified feedstuff from wheat, barley and corn can be provided in Europe [17].
Since the germ is located at the outside of the corn grain and differs in certain mechanical properties, its removal is comparably unchallenging. Therefore, various processes have already been established for corn degermination, usually comprising moistening to around 20 wt.% water content, a milling step and subsequent separation by density difference. Degermed corn is used in corn starch production and the germ—being rich in fat—is currently widely fed or extracted to oils [18]. An exact separation of the aleurone layer, in turn, is hardly industrially feasible. The fact that the aleurone is a difficult to access single- to three-cell layer within the grain makes the process less favorable to be implemented than common debranning [15]. A few patents were filed for an industrial separation of aleurone. The processes are all based on various grinding and separation steps, as known from classical milling, although the type of separation significantly differs. Here, the aleurone is separated by electrostatic forces, as aleurone cells contain specific structural sugar compounds and are therefore charged differently than other cell wall components. This is the only yet known technique for a larger scale aleurone removal from a bran mixture which reached purities of 60–90% aleurone cells [15]. Thus, a significant amount of PO can be separated by this process.
2. Enzymatic Feed Treatment
In contrast to mechanical separation techniques, a reduction in inositol-P, while the total amount of P and other valuable nutrients remain in the feed ration, can be achieved by enzymatic hydrolysis. Enzymatic approaches aim for improved bioavailability through hydrolysation of inositol-P present in the feed. This can be either achieved by feed rations with high native phytase activity, therefore simulating the natural hydrolysis during germination, or by supplementation of commercially produced phytases.
2.1. Feeding of Phytase-Rich Feed
When using feed components with elevated plant phytase activity, P digestibility can be increased [19][20]. Under standardized conditions and using a low P ration, the P digestibility in pigs was determined several times using different raw materials (various varying native phytase activities). P digestibility was shown to be highest in feeds with high native phytase activity (wheat and rye), followed by feeds with medium phytase activity (barley) and feed components with low phytase activity (maize or oilseed meals) [21][22][23]. The native phytase activity in feeds is subject to large natural variations (depending on genotype and environmental conditions), resulting in the different digestibility of P [22][24]. In grain-rich rations for monogastrics, it is therefore important whether the native phytase activity has been preserved during the production process (e.g., at storage or drying conditions) [24]. High temperatures (≥70 °C) and humidity often occur during pelleting, significantly reducing the native phytase activity [24][25][26].
With increasing phytase doses, P digestibility can be increased until a certain plateau is reached. Degradation of phytic acid beyond this level is not achieved due to limited solubility and the retention time in the stomach [27].
Schlemmer et al. found in the stomach contents of pigs only 10% of the phytase activity of the feed with high plant phytase activity, and therefore conclude that there is a strong deactivation of phytases during digestive processes within the stomach [28]. According to this, there is only low activity of intrinsic feed phytases in the small intestinal chyme, whereas added microbial phytases show higher activity in the small intestine. This is in line with subsequent research by Schlemmer et al. where microbial phytases are reported to be more stable than intrinsic plant feed phytases to digestive processes in the stomach and small intestine in pigs [29].
2.2. Germination of Phytate-Rich Feed
Germination is the first stage of ontogenesis in seeds as well as sprouting and begins with the uptake of water by dry mature seeds. It is a mechanism in which metabolic processes are activated, resulting in morphological and physiological changes [30]. During germination, phytate is hydrolyzed by endogenous phytase and other phosphatases to release P, inositol and micronutrients for plant development [14]. Inositol-P and its derivatives are implicated in RNA export, DNA repair, ATP synthesis, signaling, endocytosis, cell vesicular trafficking, and cell wall formation [13][14].
Soaking in water reduces phytate content in cereal seeds by the action of endogenous enzymes. It remains unclear whether the increase in phytase activity is the result of the activation of pre-existing enzymes or based on de novo synthesis of the protein [13]. However, phytate hydrolysis during soaking has been shown to be influenced by temperature and pH. Thus, soaking can effectively reduce phytate under maximum conditions for enzymatic activity [31]. In addition, the reduction is also favored by mass transfer, as phytate is water soluble, but loss of minerals, water-extractable proteins, and vitamins also occur [13][14].
The effectiveness of phytate reduction is a species-dependent phenomenon, which is connected to endogenous enzyme activity (Table 1). For lentil, a phytate reduction of up to 60% after 12 h pre-steeping and germination under a wet muslin cloth for 48 h was shown [32]. Greiner et al. showed over 50% reduction in rye grains during two days. After 10 days, phytate content was reduced by over 80% [33]. Pakfetrat et al. investigated the reduction of certain substances in wheat grain during germination, among them phytate. After 14 days, phytate content was reduced by 63% [34]. Reddy et al. summarized phytate degradation rates for various cereal types. Rye showed complete reduction after five days, followed by barley and corn with 66% and wheat with 52% [35].
| Total P (%) |
Phytate-P (%) |
(Phytate-P/ Total P) × 100 |
Phytase Activity (U/kg) |
|
|---|---|---|---|---|
| Cereals | 0.23–0.31 | 0.17–0.23 | 59–78 | 56–5147 |
| Legume seeds | 0.33–0.73 | 0.08–0.33 | 21–56 | 32–258 |
| Oilseeds | 0.6–1.05 | 0.34–0.76 | 57–72 | 73–295 |
| Cereal by-products (bran) | 0.83–1.16 | 0.68–0.88 | 76–82 | 25–7339 |
2.3. Phytase as Feed Additive
Microbial produced phytases are routinely used in monogastric diets, representing the largest market for phytase application. Especially fungal phytases with remarkably high temperature maxima ranging from 45 to 77 °C are well suited to withstand pelleting of animal feed [38]. First commercialized phytases were derived from the fungal Aspergillus niger [39][40], Peniophora lycii and Penicillium funiculosum [41] which were launched in 1991 as feed additive.
Thenceforth, varieties of phytases have been discovered in the last 25 years. Enzymes from the Enterobacteriaceae family, e.g., E. coli or Y. mollaretii [42][43][44] with specific activities above 1000 U/mg [45] lead to commercial bacterial phytase products. Thereby, E. coli phytase AppA is one of the most studied and used enzymes due to its high specific activity. In production, expression hosts are often fungal, even for bacterial phytases, to take advantage of post-translational modifications and high titers [46][47]. Glycosylation is reported to be beneficial for improved thermo-stability [48].
Phytases with outstanding performances have been optimized by rational and semi-rational design or by directed evolution approaches [49][50][51][52][53]. New thermostable phytases were discovered by functional metagenomics [54][55][56]. Furthermore, chimeric enzymes are a future trend and highlight the great potential of DNA recombination strategies to combine improved properties and generate new enzymes. A hybrid 6-phytase based on the genes of the enterobacteria Hafnia sp., Yersinia mollaretii and Buttiauxella gaviniae was released in a commercial product (Natuphos®E) [57]. Recently, a phytase chimera of Citrobacter braakii, Hafnia alvei and Yersinia mollaretii was reported, which exhibit sequence identities of ≤80% to their parental enzymes [58].
Genetic engineering tailors phytases for their application in the feed market. The aim is to increase the enzyme stability in vivo, i.e., in the intestinal transit (specific activity, pH stability and protease resistance) and/or its thermostability to withstand feed pelleting [49][59].
2.4. Enzymatic Hydrolysis
Phytases catalyze the release of P from phytate in a stepwise hydrolysis reaction [60][61]. The sequential hydrolysis of InsP6 proved an accumulation of inositol-triphosphate (InsP3). A full hydrolysis to myo-inositol is not achieved, due to the structural conformation of these phytases. Even if some phytases are able to further hydrolyze to inositol-monophosphate (InsP1), an accumulation of InsP3 was observed [25]. Thereby the hydrolysis pattern is influenced by the enzyme dose and the substrate concentration. In vivo, phytases are able to further hydrolyze inositol-tetraphosphate (InsP4) [62]. Reports show that in the small intestine of piglets [63], InsP3 was detectable; meanwhile, hydrolysis in vitro ended with InsP4 [64]. Such studies can be performed with an in vitro system mimicking the digestive track in monogastric animals and allow for valuable insights into the phytate degradation pattern. Indeed, a remaining challenge is that only up to 50% of phytate is degraded in the animal intestinal tract [31], releasing considerable amounts of lower inositol-P (< InsP4). A challenge in enzyme engineering is the further hydrolysis of InsP4 and InsP3 to InsP1, thereby accessing complete and fast degradation of the available phytate without compromising the thermostability and specific activity. Although phytase from metagenomics analysis like for example rPhyXT52 [54] and phytase blends of a 3- and a 6-phytase (different stereospecificities) [65] were reported in vitro to degrade InsP6 to InsP1, an accumulation of InsP4 in vivo was measured [66][67][68].
Noureddini et al. studied the enzymatic hydrolysis of a side stream in ethanol distillation. Light steep water, a side stream from wet milling of corn prior to fermentation, is a phytate-rich stream with a total P content of approx. 5 mg/g. After 7 h treatment of the substrate with PhyA phytase extracted from Aspergillus niger, it was shown that more than 75% of phytate was degraded, releasing significant amounts of PI [69]. Herrmann et al. established a process using a side product of food oil manufacturing. They reported the hydrolysis of phytate from rapeseed meal and several other deviled seeds by Escherichia coli AppA phytase. An advancement of the process by using a blend of 3-phytase and a 6-phytase (rPhyXT52/Dc phyt) reached 78 to 90% reduction in inositol-P in rape, sunflower, or soya meal. Thereby, the P recovery by enzymatic treatment was increased by 40%, releasing up to 26 g PI/kg of deoiled seed. The hydrolysis took place in a reaction buffer at pH 5 and 37 °C during 6 to 16 h incubation time [65].
The “Value-PP process” (research project) which is still not industrially established, enables the mobilizing of phytate-P from deoiled seeds, nuts and bran. An enzymatic hydrolysis with subsequent biotransformation recovers phytate–P from food manufacturing side products which is accumulated in the yeast S. cerevisiae to polyphosphate (37 mg PO43−/g bran, 38.1 g polyP/L). Biotechnological production of polyphosphate-rich yeast extracts is a valuable food additive (preservative, texture improvement, and flavor) which can be produced from rape meal, rice barn, wheat, soya meal, among others. The emerging value chain leads to the valorization of the biomass as well as to P-reduced feed components. The total P content of the meals after enzymatic treatment is reduced by up to 90% without compromising feed quality or ingredients [70].
3. Chemical Feed Treatment
Similar to the application of enzymes, chemical methods aim for a cleavage of ester bonds between the inositol ring and the P groups. Common carbon esters can be hydrolyzed by catalytic use of acids (e.g., sulphuric acid) or bases (e.g., sodium hydroxide), while carbonic acid (in the case of a basic hydrolysis as the corresponding salt) and alcohol are produced [71]. Also, hydrothermal decomposition is a widely used technique in biomass utilization. Various compounds were shown to be degraded and therefore better available for further processing, among them mainly ether bonds [72][73]. However, also simple esters can partly be degraded via hydrothermal treatment [74]. Phosphomonoesters, in particular, are hydrolyzed by use of acid, water and/or other nucleophiles via either a dissociative or associative mechanism, as shown in Figure 2. Nonetheless, the reaction rate is low, although thermo-dynamically favorable, and therefore needs to be catalyzed [75].
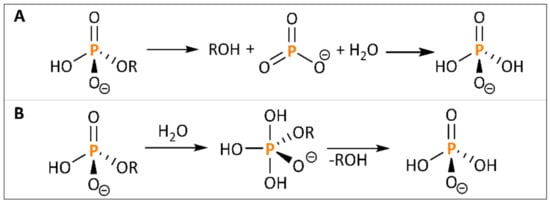
Figure 2. Dissociative (A) and associative (B) mechanism for phosphate ester hydrolysis.
3.1. Hydrothermal Treatment
A treatment of cereal products using water and elevated temperature has several effects:
-
Since phytate is water-soluble, a certain proportion is dissolved into the liquid phase and, therefore, eliminated from the substrate [13].
Up to 100 °C, phytate is reported to be chemically stable [29]. However, soaking in water at lower temperatures can already show significant phytate reduction in cereals. Incubation at about 15 °C for over 30 h is performed during the malting process to promote germination of the grains (Section 3.2.2). Larsson et al. report a maximum phytate reduction of 50% by malting barley. This results from an activation of intrinsic enzymes during the natural process of germination. Other cereal types also show phytate reduction under these conditions, albeit to a lesser extent [79]. With rising temperature above 100 °C and treatment in water only, studies with brown beans showed increasing phytate reduction. A maximum reduction of 65% InsP6 could be obtained by hydrothermal treatment at 140 °C during 1.5 h [29]. Here, a purely thermal effect applies in addition to phytate solubilisation [80].
3.2. Acid Hydrolysis
Since hydrothermal treatment has a limited effect, harsher conditions for phytate degradation might be necessary. Considering the mechanism of phosphate ester hydrolysis (Figure 2), inositol-P esters are particularly stable under basic conditions, and the presence of the anion is favorable for the reaction [75][81]. Therefore, further studies were conducted only on acidic treatment, while there are no approaches for a basic phytate degradation described in literature so far. In fact, a reduction in the pH during extraction was shown to support phytate degradation with an optimum at pH 4–4.5 [81]. Degradation usually comprises two steps: solubilisation of phytate from the substrate into the liquid phase and subsequent cleavage of the ester bonds. Solubilisation of phytate is feasible under mild conditions by incubation of cereal substrate with low concentrated HCl at room temperature [82]. A phytate degradation for a release of free P-groups, in contrast, proves to be more challenging. For a cleavage of the phosphomonoester bonds, elevated temperature is combined with low pH, this being particularly achieved in the literature through utilization of HCl and lactic acid [80].
Lemmens et al. showed a 54% phytate degradation in wheat after 8 h by a single step 60 °C thermal treatment at pH 4. The extraction solution consisted of 100 mM HCl in sodium acetate buffer [80]. Even higher degradation of up to 96% in barley was achieved through a multi-step treatment with 0.8% lactic acid. Bergman et al. examined a two-step acid soaking, with each step followed by a drying step. Best results were reached by soaking barley with 3.2 times the volume of lactic acid at 48 °C for 1 h followed by 5 h drying at 48 °C. The treatment was repeated at 50 °C and followed by additional drying of the substrate at 50 °C for 18 h and at 80 °C for 8 h [83]. Comparably high phytate degradation was also shown in 0.44 M HCl when using microwave radiation. Here, six stages each of 2 min microwave treatment at 650 W were executed, however with a model solution of dissolved pure phytic acid. Likewise, over 99% of initial phytic acid was cleaved for a liberation of free P [84]. Microwave treatment of sorghum seeds alone, i.e., without addition of acid medium, also showed phytate degradation, but with only a few percent and thus nowhere near as effective as presented by March et al. [85]. Therefore, a combined method of acid hydrolysis with energy input by microwave radiation shows the most promising approach for phytate degradation in cereals. Table 2 provides an overview of all the methods discussed.
Table 2. Summary of the most promising methods for a P conditioning of cereal-based animal feed.
| Treatment | Process | Substrate | Phytate Reduction | Process/Conditions |
|---|---|---|---|---|
| Mechanical | debranning | cereal grains | up to 90% | removal of outer hull (debranning) [1] |
| layer exclusion | cereal grains | up to 90% | stepwise debranning/specific electrostatic separation; degermination [15][18] | |
| Enzymatic | co-feeding of phytase | cereal grains | 30–50% | phytase supplementation [31] |
| germination | cereal grains | up to 100% (rye) | 2 days pre-steeping, incubation in H2O, 25 °C, 5 days [35] | |
| biotechnological processing | deoiled seeds, bran | up to 90% | 200 to 400 U phytase, 7 volumes H2O, 37 °C, pH 4.5–6 [82] | |
| Chemical | hydrothermal treatment | brown beans | up to 65% | 140 °C, 90 min, H2O [29] |
| acidic hydrolysis | barley | up to 96% | 0.1 M lactic acid soaking, two-step heating at 48 and 50 °C for 5 h and 1 h, respectively [83] | |
| phytic acid solution | up to 99% | 0.44 M HCl, 6 × 2 min microwave heating at 650 W [84] |
References
- Caballero, B.; Finglas, O.; Toldrá, F. Encyclopedia of food Sciences and Nutrition, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2003; ISBN 9780122270550.
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT 2014, 56, 211–221.
- Knight, S. Grain and Feed Annual_London_EU-28_4-15-2019. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Grain%20and%20Feed%20Annual_London_EU-28_4-15-2019.pdf (accessed on 3 February 2022).
- Loy, D.D.; Lundy, E.L. Nutritional Properties and Feeding Value of Corn and Its Coproducts. In Corn; AACC International Press: Oxford, UK, 2019; pp. 633–659. ISBN 9780128119716.
- Andersson, A.A.M.; Andersson, R.; Jonsäll, A.; Andersson, J.; Fredriksson, H. Effect of Different Extrusion Parameters on Dietary Fiber in Wheat Bran and Rye Bran. J Food Sci. 2017, 82, 1344–1350.
- Steiner, T.; Mosenthin, R.; Zimmermann, B.; Greiner, R.; Roth, S. Distribution of phytase activity, total phosphorus and phytate phosphorus in legume seeds, cereals and cereal by-products as influenced by harvest year and cultivar. Anim. Feed Sci. Technol. 2007, 133, 320–334.
- Alam, S.A.; Järvinen, J.; Kirjoranta, S.; Jouppila, K.; Poutanen, K.; Sozer, N. Influence of Particle Size Reduction on Structural and Mechanical Properties of Extruded Rye Bran. Food Bioprocess Technol. 2014, 7, 2121–2133.
- Ebringerová, A.; Hromádková, Z. Effect of ultrasound on the extractibility of corn bran hemicelluloses. Ultrason. Sonochem. 2002, 9, 225–229.
- Belyea, R.L.; Rausch, K.D.; Tumbleson, M.E. Composition of corn and distillers dried grains with solubles from dry grind ethanol processing. Bioresour. Technol. 2004, 94, 293–298.
- Fretzdorff, B.; Weipert, D. Phytinsäure in Getreide und Getreideerzeugnissen. Mitteilung 1: Phytinsäure und Phytase in Roggen und Roggenprodukten. Bundesforschungsanst. Getreide-Kartoff. 1986, 182, 287–293.
- Luithui, Y.; Baghya Nisha, R.; Meera, M.S. Cereal by-products as an important functional ingredient: Effect of processing. J. Food Sci. Technol. 2019, 56, 1–11.
- Declour, A.J.; Poutanen, K. Fibre-Rich and Wholegrain Foods: Improving Quality; Woodhead Pub Ltd.: Oxford, UK, 2013; ISBN 9780857090386.
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625.
- Bohn, L.; Meyer, A.S.; Rasmussen, S.K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9, 165–191.
- Brouns, F.; Hemery, Y.; Price, R.; Anson, N.M. Wheat aleurone: Separation, composition, health aspects, and potential food use. Crit. Rev. Food Sci. Nutr. 2012, 52, 553–568.
- Rausch, K.D.; Hummel, D.; Johnson, L.A.; May, J.B. Wet Milling: The Basis for Corn Biorefineries. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 501–535. ISBN 9780128119716.
- Comm/Dg/Unit. Cereals Statistics. Available online: https://ec.europa.eu/info/food-farming-fisheries/farming/facts-and-figures/markets/overviews/market-observatories/crops/cereals-statistics_en (accessed on 15 October 2021).
- Anderson, B.; Almeida, H. Corn Dry Milling: Processes, Products, and Applications. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 405–433. ISBN 9780128119716.
- Oloffs, K.; Cossa, J.; Jeroch, H. Die Bedeutung der korneigenen (nativen) Phytaseaktivität im Weizen für die Phosphor-Verwertung bei Broilern und Legehennen. Archiv. Geflugelkd. 2000, 64, 157–161.
- Pointillart, A. Enhancement of phosphorus utilization in growing pigs fed phytate-rich diets by using rye bran. J. Anim. Sci. 1991, 69, 1109–1115.
- Düngelhoef, M.; Rodehutscord, M.; Spiekers, H.; Pfeffer, H.S.E. Effects of supplemental microbial phytase on availability of phosphorus contained in maize, wheat and triticale to pigs. Anim. Feed Sci. Technol. 1994, 49, 1–10.
- Hovenjürgen, M.; Pfeffer, E.; Rodehutscord, M. Effect of fertilization and variety on digestability of phosphorus from plant feedstuffs in pigs. J. Anim. Feed Sci. 2003, 12, 83–93.
- Rodehutscord, M.; Faust, M.; Lorenz, H. Digestibility of phosphorus contained in soybean meal, barley, and different varieties of wheat, without and with supplemental phytase fed to pigs and additivity of digestibility in a wheatsoybean-meal diet. J. Anim. Physiol. Anim. Nutr. 1996, 75, 40–48.
- Rodehutscord, M. Der gegenwärtige Stand der Phosphorbewertung für Nutztiere. Lohmann Inf. 2001, 1, 26–34.
- Walk, C.L. Phytate Destruction-Consequences for Precision Animal Nutrition, 1st ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; ISBN 9789086868360.
- Jongbloed, A.W.; Kemme, P.A. Effect of pelleting mixed feeds on phytase activity and the apparent absorbability of phosphorus and calcium in pigs. Anim. Feed Sci. Technol. 1990, 28, 233–242.
- Kemme, P.A.; Jongbloed, A.W.; Mroz, Z.; Beynen, A.C. Diurnal variation in degradation of phytic acid by plant phytase in the pig stomach. Livest. Prod. Sci. 1998, 54, 33–44.
- Schlemmer, U.; Jany, K.D.; Berk, A.; Schulz, E.; Rechkemmer, G. Degradation of phytate in the gut of pigs-pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch. Tierernahr. 2001, 55, 255–280.
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S330–S375.
- Kumaragamage, D.; Akinremi, O.O. Manure Phosphorus: Mobility in Soils and Management Strategies to Minimize Losses. Curr. Pollut. Rep. 2018, 4, 162–174.
- Greiner, R.; Konietzny, U. Phytase for Food Application. Food Technol. Biotechnol. 2006, 44, 125–140.
- Pal, R.S.; Bhartiya, A.; Yadav, P.; Kant, L.; Mishra, K.K.; Aditya, J.P.; Pattanayak, A. Effect of dehulling, germination and cooking on nutrients, anti-nutrients, fatty acid composition and antioxidant properties in lentil (Lens culinaris). J. Food Sci. Technol. 2017, 54, 909–920.
- Greiner, R.; Alminger, M.L. Purification and characterization of a phytate-degrading enzyme from germinated oat (Avena sativa). J. Sci. Food Agric. 1999, 79, 1453–1460.
- Pakfetrat, S.; Amiri, S.; Radi, M.; Abedi, E.; Torri, L. Reduction of phytic acid, aflatoxins and other mycotoxins in wheat during germination. J. Sci. Food Agric. 2019, 99, 4695–4701.
- Reddy, N.R.; Sathe, S.K.; Salunkhe, D.K. Phytates in Legumes and Cereals. In Phytates in Legumes and Cereals; Advances in Food Research, Ed.; Elsevier: Amsterdam, The Netherlands, 1982; pp. 1–92. ISBN 9780120164288.
- Rodehutscord, M.; Rückert, C.; Maurer, H.P.; Schenkel, H.; Schipprack, W.; Bach Knudsen, K.E.; Schollenberger, M.; Laux, M.; Eklund, M.; Siegert, W.; et al. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 2016, 70, 87–107.
- Viveros, A.; Centeno, C.; Brenes, A.; Canales, R.; Lozano, A. Phytase and acid phosphatase activities in plant feedstuffs. J. Agric. Food Chem. 2000, 48, 4009–4013.
- Singh, B.; Satyanarayana, T. Fungal phytases: Characteristics and amelioration of nutritional quality and growth of non-ruminants. J. Anim. Physiol. Anim. Nutr. 2015, 99, 646–660.
- Ariza, A.; Moroz, O.V.; Blagova, E.V.; Turkenburg, J.P.; Waterman, J.; Roberts, S.M.; Vind, J.; Sjøholm, C.; Lassen, S.F.; de Maria, L.; et al. Degradation of phytate by the 6-phytase from Hafnia alvei: A combined structural and solution study. PLoS ONE 2013, 8, e65062.
- Haefner, S.; Knietsch, A.; Scholten, E.; Braun, J.; Lohscheidt, M.; Zelder, O. Biotechnological production and applications of phytases. Appl. Microbiol. Biotechnol. 2005, 68, 588–597.
- Lassen, S.F.; Breinholt, J.; Østergaard, P.R.; Brugger, R.; Bischoff, A.; Wyss, M.; Fuglsang, C.C. Expression, gene cloning, and characterization of five novel phytases from four basidiomycete fungi: Peniophora lycii, Agrocybe pediades, a Ceriporia sp., and Trametes pubescens. Appl. Environ. Microbiol. 2001, 67, 4701–4707.
- Greiner, R.; Konietzny, U.; Jany, K.D. Purification and characterization of two phytases from Escherichia coli. Arch. Biochem. Biophys. 1993, 303, 107–113.
- Rodriguez, E.; Han, Y.; Lei, X.G. Cloning, sequencing, and expression of an Escherichia coli acid phosphatase/phytase gene (appA2) isolated from pig colon. Biochem. Biophys. Res. Commun. 1999, 257, 117–123.
- Augspurger, N.I.L.; Webel, D.M.; Lei, X.G.; Baker, D.H. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J. Anim. Sci. 2003, 81, 474–483.
- Huang, H.; Luo, H.; Wang, Y.; Fu, D.; Shao, N.; Wang, G.; Yang, P.; Yao, B. A novel phytase from Yersinia rohdei with high phytate hydrolysis activity under low pH and strong pepsin conditions. Appl. Microbiol. Biotechnol. 2008, 80, 417–426.
- Guo, M.; Hang, H.; Zhu, T.; Zhuang, Y.; Chu, J.; Zhang, S. Effect of glycosylation on biochemical characterization of recombinant phytase expressed in Pichia pastoris. Enzyme Microb. Technol. 2008, 42, 340–345.
- Scheers, N.; Sandberg, A.-S. Usefulness of microbial phytases to improve zinc and iron bioavailability. In Proceedings of the International Phytase Summit 2012, Rome, Italy, 11–13 December 2012.
- Wu, T.-H.; Chen, C.-C.; Cheng, Y.-S.; Ko, T.-P.; Lin, C.-Y.; Lai, H.-L.; Huang, T.-Y.; Liu, J.-R.; Guo, R.-T. Improving specific activity and thermostability of Escherichia coli phytase by structure-based rational design. J. Biotechnol. 2014, 175, 1–6.
- Rebello, S.; Jose, L.; Sindhu, R.; Aneesh, E.M. Molecular advancements in the development of thermostable phytases. Appl. Microbiol. Biotechnol. 2017, 101, 2677–2689.
- Wang, X.; Du, J.; Zhang, Z.-Y.; Fu, Y.-J.; Wang, W.-M.; Liang, A.-H. A rational design to enhance the resistance of Escherichia coli phytase appA to trypsin. Appl. Microbiol. Biotechnol. 2018, 102, 9647–9656.
- Niu, C.; Yang, P.; Luo, H.; Huang, H.; Wang, Y.; Yao, B. Engineering of Yersinia Phytases to Improve Pepsin and Trypsin Resistance and Thermostability and Application Potential in the Food and Feed Industry. J. Agric. Food Chem. 2017, 65, 7337–7344.
- Han, N.; Miao, H.; Yu, T.; Xu, B.; Yang, Y.; Wu, Q.; Zhang, R.; Huang, Z. Enhancing thermal tolerance of Aspergillus niger PhyA phytase directed by structural comparison and computational simulation. BMC Biotechnol. 2018, 18, 36.
- Shivange, A.V.; Schwaneberg, U. Recent Advances in Directed Phytase Evolution and Rational Phytase Engineering. In Directed Enzyme Evolution: Advances and Applications; Alcalde, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 145–172. ISBN 978-3-319-50411-7.
- Tan, H.; Wu, X.; Xie, L.; Huang, Z.; Peng, W.; Gan, B. Identification and characterization of a mesophilic phytase highly resilient to high-temperatures from a fungus-garden associated metagenome. Appl. Microbiol. Biotechnol. 2016, 100, 2225–2241.
- Ushasree, M.V.; Shyam, K.; Vidya, J.; Pandey, A. Microbial phytase: Impact of advances in genetic engineering in revolutionizing its properties and applications. Bioresour. Technol. 2017, 245, 1790–1799.
- Farias, N.; Almeida, I.; Meneses, C. New Bacterial Phytase through Metagenomic Prospection. Molecules 2018, 23, 448.
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Flachowsky, G.; Gropp, J.; Kolar, B.; et al. Safety and efficacy of Natuphos® E (6-phytase) as a feed additive for avian and porcine species. EFSA J. 2017, 15, e05024.
- Herrmann, K.; Hofmann, I.; Jungherz, D.; Wittwer, M.; Infanzón, B.; Hamer, S.N.; Davari, M.D.; Ruff, A.J.; Schwaneberg, U. Generation of phytase chimeras with low sequence identities and improved thermal stability. J. Biotechnol. 2021, 339, 14–21.
- Herrmann, K.R.; Ruff, A.J.; Infanzón, B.; Schwaneberg, U. Engineered phytases for emerging biotechnological applications beyond animal feeding. Appl. Microbiol. Biotechnol. 2019, 103, 6435–6448.
- Konietzny, U.; Greiner, R. Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int. J. Food Sci. Technol. 2002, 37, 791–812.
- Wyss, M.; Brugger, R.; Kronenberger, A.; Rémy, R.; Fimbel, R.; Oesterhelt, G.; Lehmann, M.; van Loon, A.P. Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): Catalytic properties. Appl. Environ. Microbiol. 1999, 65, 367–373.
- Zeller, E.; Schollenberger, M.; Kühn, I.; Rodehutscord, M. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 2015, 4, e1.
- Pontoppidan, K.; Glitsoe, V.; Guggenbuhl, P.; Quintana, A.P.; Nunes, C.S.; Pettersson, D.; Sandberg, A.-S. In vitro and in vivo degradation of myo-inositol hexakisphosphate by a phytase from Citrobacter braakii. Arch. Anim. Nutr. 2012, 66, 431–444.
- Dersjant-Li, Y.; Davin, R.; Christensen, T.; Kwakernaak, C. Effect of two phytases at two doses on performance and phytate degradation in broilers during 1–21 days of age. PLoS ONE 2021, 16, e0247420.
- Infanzón, B.; Herrmann, K.R.; Hofmann, I.; Willbold, S.; Ruff, A.J.; Schwaneberg, U. Phytase blends for enhanced phosphorous mobilization of deoiled seeds. Enzym. Microb. Technol. 2021, 153, 109953.
- Espinosa, C.D.; Oliveira, M.S.F.; Velayudhan, D.E.; Dersjant-Li, Y.; Stein, H.H. Influence of a novel consensus bacterial 6-phytase variant on mineral digestibility and bone ash in young growing pigs fed diets with different concentrations of phytate-bound phosphorus. J. Anim. Sci. 2021, 99, skab211.
- Lee, S.A.; Febery, E.; Wilcock, P.; Bedford, M.R. Application of Creep Feed and Phytase Super-Dosing as Tools to Support Digestive Adaption and Feed Efficiency in Piglets at Weaning. Animals 2021, 11, 2080.
- Lu, H.; Cowieson, A.J.; Wilson, J.W.; Ajuwon, K.M.; Adeola, O. Extra-phosphoric effects of super dosing phytase on growth performance of pigs is not solely due to release of myo-inositol. J. Anim. Sci. 2019, 97, 3898–3906.
- Noureddini, H.; Dang, J. Degradation of phytates in distillers’ grains and corn gluten feed by Aspergillus niger phytase. Appl. Biochem. Biotechnol. 2009, 159, 11–23.
- Christ, J.J.; Smith, S.A.; Willbold, S.; Morrissey, J.H.; Blank, L.M. Biotechnological synthesis of water-soluble food-grade polyphosphate with Saccharomyces cerevisiae. Biotechnol. Bioeng. 2020, 117, 2089–2099.
- Breitmaier, E.; Jung, G. Organische Chemie: Grundlagen, Verbindungsklassen, Reaktionen, Konzepte, Molekülstruktur, Naturstof; Georg Thieme Verlag: New York, NY, USA, 2009; ISBN 9783135415062.
- Sun, X.; Liu, Z.; Qu, Y.; Li, X. The Effects of Wheat bran Comosition on the Production of Biomass-Hydrolyzing Enzymes by Penicillium decumbens. In Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2008; pp. 119–128.
- Wu, X.; Fu, J.; Lu, X. Kinetics and Mechanism of Hydrothermal Decomposition of Lignin Model Compounds. Ind. Eng. Chem. Res. 2013, 52, 5016–5022.
- Moriyoshi, T.; Sam, K.; Uosaki, Y. Hydrothermal decomposition of esters under high pressure. High Press. Res. 2001, 20, 491–505.
- Vincent, J.B.; Crowder, M.W.; Averill, B.A. Hydrolysis of phosphate monoesters: A biological problem with multiple chemical solutions. Trends Biochem. Sci. 1992, 17, 105–110.
- Humer, E.; Zebeli, Q. Phytate in feed ingredients and potentials for improving the utilization of phosphorus in ruminant nutrition. Anim. Feed Sci. Technol. 2015, 209, 1–15.
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci. Rep. 2016, 6, 39354.
- Ojo, M.A. Phytic acid in legumes: A review of nutritional importance and hydrothermal processing effect on underutilised species. Food Res. 2021, 5, 22–28.
- Larsson, M.; Sandberg, A.-S. Phytate Reduction in Oats during Malting. J. Food Sci. 1992, 57, 994–997.
- Lemmens, E.; de Brier, N.; Spiers, K.M.; Ryan, C.; Garrevoet, J.; Falkenberg, G.; Goos, P.; Smolders, E.; Delcour, J.A. The impact of steeping, germination and hydrothermal processing of wheat (Triticum aestivum L.) grains on phytate hydrolysis and the distribution, speciation and bio-accessibility of iron and zinc elements. Food Chem. 2018, 264, 367–376.
- Turner, B.L.; Papházy, M.J.; Haygarth, P.M.; McKelvie, I.D. Inositol phosphates in the environment. hilos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 449–469.
- Herrmann, K.R.; Ruff, A.J.; Schwaneberg, U. Phytase-based phosphorus recovery process for 20 distinct press cakes. Sustain. Chem. Eng. 2020, 8, 3913–3921.
- Bergman, E.-L.; Fredlund, K.; Reinikainen, P.; Sandberg, A.-S. Hydrothermal Processing of Barley (cv. Blenheim): Optimisation of Phytate Degradation and Increase of FreeMyo-inositol. J. Cereal Sci. 1999, 29, 261–272.
- March, J.G.; Grases, F.; Salvador, A. Hydrolysis of Phytic Acid by Microwave Treatment: Application to Phytic Acid Analysis in Pharmaceutical Preparations. Microchem. J. 1998, 59, 413–416.
- Hassan, S.; Ahmad, N.; Ahmad, T.; Imran, M.; Xu, C.; Khan, M.K. Microwave processing impact on the phytochemicals of sorghum seeds as food ingredient. J. Food Process. Preserv. 2019, 43, e13924.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
971
Revisions:
3 times
(View History)
Update Date:
19 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




