
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shahna Fathima | -- | 4099 | 2022-05-18 23:14:15 | | | |
| 2 | Beatrix Zheng | + 1 word(s) | 4100 | 2022-05-19 08:02:59 | | |
Video Upload Options
The gut barrier is comprised of intestinal microbiota and their metabolites, mucins secreted by goblet cells, host-derived antimicrobial peptides such as defensins, and cathelicidins, IgA, intestinal epithelium, microfold cells (M cells), Paneth cells, tuft cells and lymphoid tissues in the sub-epithelium and lamina propria. The gut barrier serves to contain the gut microbiota within the lumen while permitting the absorption of nutrients. Intestinal health, tolerance to food and microbial antigens, and homeostasis are achieved through complex interactions between the multiple components in the gut.
1. Introduction
The gut barrier is comprised of intestinal microbiota and their metabolites, mucins secreted by goblet cells, host-derived antimicrobial peptides such as defensins, and cathelicidins, IgA, intestinal epithelium, microfold cells (M cells), Paneth cells, tuft cells and lymphoid tissues in the sub-epithelium and lamina propria [1][2]. The gut barrier serves to contain the gut microbiota within the lumen while permitting the absorption of nutrients [1][3]. Intestinal health, tolerance to food and microbial antigens, and homeostasis are achieved through complex interactions between the multiple components in the gut [2].
2. Probiotics in Broiler Production
3. Routes of Administration
4. Factors to Be Considered during Probiotics Supplementation
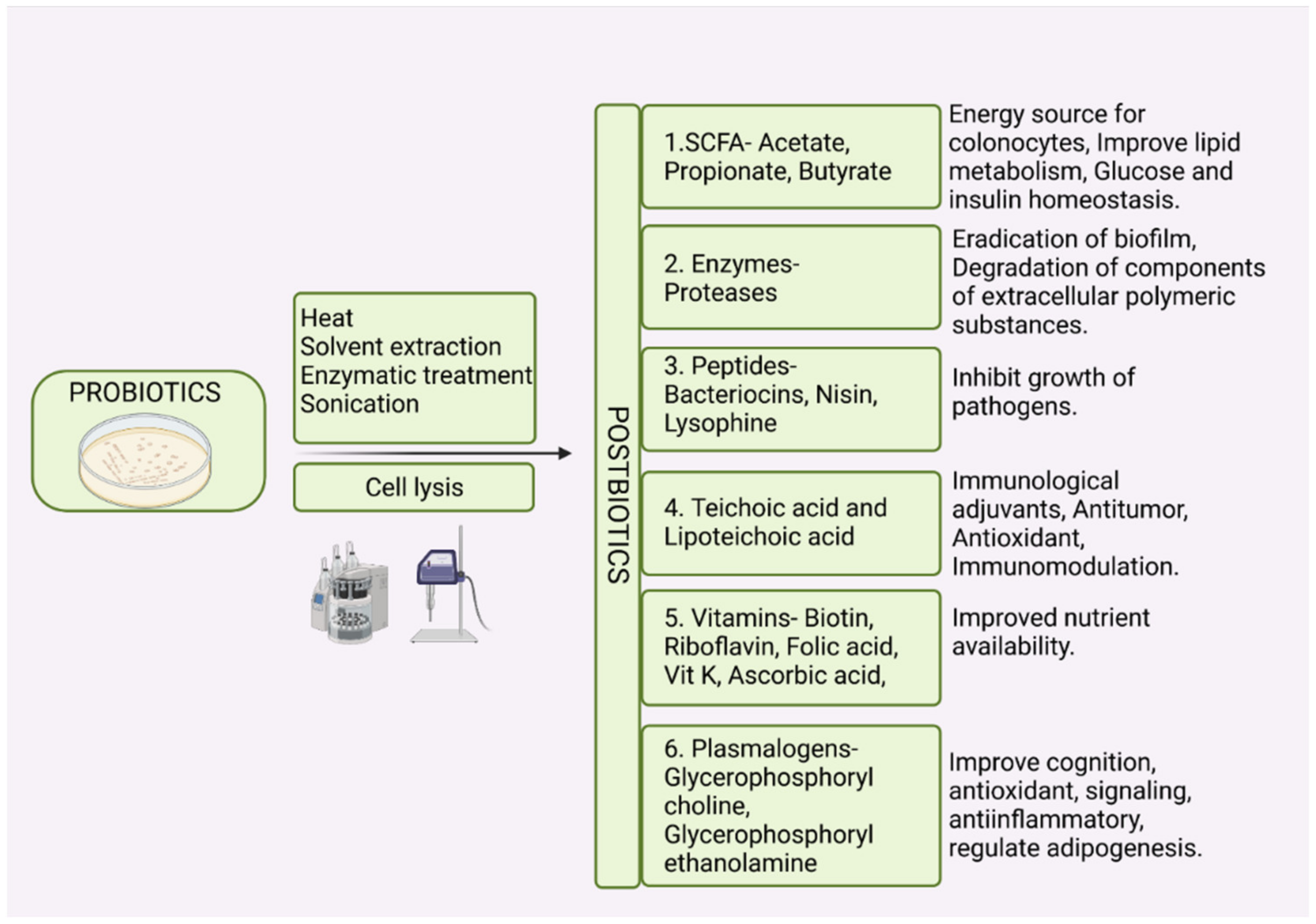
5. Postbiotics and Paraprobiotics
References
- Broom, L.J. Gut barrier function: Effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 2018, 97, 1572–1578.
- Broom, L.J.; Kogut, M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51.
- Befus, A.D.; Johnston, N.; Leslie, G.A.; Bienenstock, J. Gut-associated lymphoid tissue in the chicken. I. Morphology, ontogeny, and some functional characteristics of Peyer’s patches. J. Immunol. 1980, 125, 2626–2632.
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618.
- Vila, B.; Esteve-Garcia, E.; Brufau, J. Probiotic micro-organisms: 100 years of innovation and efficacy; modes of action. Worlds Poult. Sci. J. 2010, 66, 369–380.
- Joint FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food. In Food and Agriculture Organization; World Health Organization: London, UK, 2002.
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–Nomad and Ideal Probiotic. Front. Microbiol. 2021, 12, 2911.
- Ma, T.; Suzuki, Y. Dissect the mode of action of probiotics in affecting host-microbial interactions and immunity in food producing animals. Vet. Immunol. Immunopathol. 2018, 205, 35–48.
- Patterson, J.A.; Burkholder, K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003, 82, 627–631.
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P.; Singh, B.R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 2018, 13, e0192978.
- Ehrmann, M.A.; Kurzak, P.; Bauer, J.; Vogel, R.F. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002, 92, 966–975.
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253.
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSa J. 2018, 16, e05077.
- Hesari, M.R.; Darsanaki, R.K.; Salehzadeh, A. Antagonistic activity of probiotic bacteria isolated from traditional dairy products against E. coli O157: H7. J. Med. Bacteriol. 2017, 6, 23–30.
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343.
- Garriga, M.; Pascual, M.; Monfort, J.M.; Hugas, M. Selection of lactobacilli for chicken probiotic adjuncts. J. Appl. Microbiol. 1998, 84, 125–132.
- Farner, D.S. The hydrogen ion concentration in avian digestive tracts. Poult. Sci. 1942, 21, 445–450.
- Pan, X.; Chen, F.; Wu, T.; Tang, H.; Zhao, Z. The acid, bile tolerance and antimicrobial property of Lactobacillus acidophilus NIT. Food Control 2009, 20, 598–602.
- Nallala, V.; Sadishkumar, V.; Jeevaratnam, K. Molecular characterization of antimicrobial Lactobacillus isolates and evaluation of their probiotic characteristics in vitro for use in poultry. Food Biotechnol. 2017, 31, 20–41.
- Lin, J.; Sahin, O.; Michel, L.O.; Zhang, Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect. Immun. 2003, 71, 4250–4259.
- Taheri, H.R.; Moravej, H.; Tabandeh, F.; Zaghari, M.; Shivazad, M. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poult. Sci. 2009, 88, 1586–1593.
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073.
- Dec, M.; Urban-Chmiel, R.; Stępień-Pyśniak, D.; Wernicki, A. Assessment of antibiotic susceptibility in Lactobacillus isolates from chickens. Gut Pathog. 2017, 9, 1–16.
- Ramlucken, U.; Ramchuran, S.O.; Moonsamy, G.; van Rensburg, C.J.; Thantsha, M.S.; Lalloo, R. Production and stability of a multi-strain Bacillus based probiotic product for commercial use in poultry. Biotechnol. Rep. 2021, 29, e00575.
- Mountzouris, K.C.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010, 89, 58–67.
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241.
- Lee, Y.K.; Salminen, S. Handbook of Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2009.
- Teixeira, P.C.; Castro, M.H.; Malcata, F.X.; Kirby, R.M. Survival of Lactobacillus delbrueckii ssp. bulgaricus following spray-drying. J. Dairy Sci. 1995, 78, 1025–1031.
- Tamime, A.Y.; Saarela, M.; Sondergaard, A.K.; Mistry, V.V.; Shah, N.P. Production and maintenance of viability of probiotic microorganisms in dairy products. Probiotic Dairy Prod. 2005, 3, 39–63.
- Teixeira, P.; Castro, H.; Kirby, R. Inducible thermotolerance in Lactobacillus bulgaricus. Lett. Appl. Microbiol. 1994, 18, 218–221.
- Park, Y.H.; Hamidon, F.; Rajangan, C.; Soh, K.P.; Gan, C.Y.; Lim, T.S.; Abdullah, W.N.W.; Liong, M.T. Application of probiotics for the production of safe and high-quality poultry meat. Korean J. Food Sci. Anim. Resour. 2016, 36, 567.
- Thomke, S.; Elwinger, K. Growth Promotants in Feeding Pigs and Poultry. III. Alternatives to Antibiotic Growth Promotants. Ann. Zootech. INRA/EDP Sci. 1998, 47, 245–271. Available online: https://hal.archives-ouvertes.fr/hal-00889716 (accessed on 1 March 2022).
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45.
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188.
- Gauthier, R. Defining the Alternatives. Canadian Poultry, 10 January 2008.
- Huyghebaert, G. Alternatives for Antibiotic in Poultry; CONFERENCE SPONSORS: Timonium, MD, USA, 2005; p. 38.
- Jeurissen, S.H.; Lewis, F.; van der Klis, J.D.; Mroz, Z.; Rebel, J.M.; Ter Huurne, A.A. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Curr. Issues Intest. Microbiol. 2002, 3, 1–14.
- Figueroa-González, I.; Quijano, G.; Ramirez, G.; Cruz-Guerrero, A. Probiotics and prebiotics—Perspectives and challenges. J. Sci. Food Agric. 2011, 91, 1341–1348.
- Markazi, A.; Luoma, A.; Shanmugasundaram, R.; Mohnl, M.; Murugesan, G.R.; Selvaraj, R. Effects of drinking water synbiotic supplementation in laying hens challenged with Salmonella. Poult. Sci. 2018, 97, 3510–3518.
- Kabir, S.M. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009, 10, 3531–3546.
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683.
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; Federici, S. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423.e16.
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal Microbiota Transplantation: In perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239.
- Seekatz, A.M.; Aas, J.; Gessert, C.E.; Rubin, T.A.; Saman, D.M.; Bakken, J.S.; Young, V.B. Recovery of the gut microbiome following fecal microbiota transplantation. mBio 2014, 5, 893.
- Nurmi, E.; Rantala, M. New aspects of Salmonella infection in broiler production. Nature 1973, 241, 210–211.
- Stern, N.J.; Cox, N.A.; Bailey, J.S.; Berrang, M.E.; Musgrove, M.T. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci. 2001, 80, 156–160.
- Lee, Y.K.; Mazmanian, S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010, 330, 1768–1773.
- Blajman, J.E.; Frizzo, L.S.; Zbrun, M.V.; Astesana, D.M.; Fusari, M.L.; Soto, L.P.; Rosmini, M.R.; Signorini, M.L. Probiotics and broiler growth performance: A meta-analysis of randomised controlled trials. Br. Poult. Sci. 2014, 55, 483–494.
- Edens, F.W. An alternative for antibiotic se in poultry: Probiotics. Braz. J. Poult. Sci. 2003, 5, 75–97.
- Olnood, C.G.; Beski, S.S.; Iji, P.A.; Choct, M. Delivery routes for probiotics: Effects on broiler performance, intestinal morphology and gut microflora. Anim. Nutr. 2015, 1, 192–202.
- Karimi Torshizi, M.A.; Moghaddam, A.R.; Rahimi, S.H.; Mojgani, N. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Br. Poult. Sci. 2010, 51, 178–184.
- Eckert, N.H.; Lee, J.T.; Hyatt, D.; Stevens, S.M.; Anderson, S.; Anderson, P.N.; Beltran, R.; Schatzmayr, G.; Mohnl, M.; Caldwell, D.J. Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets. J. Appl. Poult. Res. 2010, 19, 59–67.
- Hogg, S. Essential Microbiology; John Wiley & Sons: West Sussex, UK; Hoboken, NJ, USA, 2013.
- Higgins, J.P.; Higgins, S.E.; Vicente, J.L.; Wolfenden, A.D.; Tellez, G.; Hargis, B. Temporal effects of lactic acid bacteria probiotic culture on Salmonella in neonatal broilers. Poult. Sci. 2007, 86, 1662–1666.
- De Oliveira, J.E.; Van der Hoeven-Hangoor, E.; Van de Linde, I.B.; Montijn, R.C.; Van der Vossen, J. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014, 93, 818–829.
- Roto, S.M.; Kwon, Y.M.; Ricke, S.C. Applications of in ovo technique for the optimal development of the gastrointestinal tract and the potential influence on the establishment of its microbiome in poultry. Front. Vet. Sci. 2016, 3, 63.
- Ferket, P.R. Embryo Epigenomic Response to Breeder Management and Nutrition; World’s Poultry Congress (Abstr.); Semantic Scholar: Seattle, WA, USA, 2012.
- Gulewicz, K.; Bednarczyk, M. Method for Stimulating Favourable Bacteria Profile in Hatched Chicks. Sposób Stymulacji Korzystnego Profilu Bakteryjnego Wylężonych Piskląt. Poland Application PL20030364037, 12 December 2003.
- Uni, Z.; Ferket, P. Enhancement of Development of Oviparous Species by in OVO Feeding. U.S. Patent 6,592,878 B2, 15 July 2003.
- Sławińska, A.; Siwek, M.; Żylińska, J.; Bardowski, J.; Brzezińska, J.; Gulewicz, K.A.; Nowak, M.; Urbanowski, M.; Płowiec, A.; Bednarczyk, M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biol. 2014, 62, 277–285.
- Bednarczyk, M.; Stadnicka, K.; Kozłowska, I.; Abiuso, C.; Tavaniello, S.; Dankowiakowska, A.; Sławińska, A.; Maiorano, G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal 2016, 10, 1271–1279.
- Li, T.; Castañeda, C.D.; Miotto, J.; McDaniel, C.; Kiess, A.S.; Zhang, L. Effects of in ovo probiotic administration on the incidence of avian pathogenic Escherichia coli in broilers and an evaluation on its virulence and antimicrobial resistance properties. Poult. Sci. 2021, 100, 100903.
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and synbiotics–in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018, 14, 1–17.
- Villaluenga, C.M.; Wardeńska, M.; Pilarski, R.; Bednarczyk, M.; Gulewicz, K. Utilization of the chicken embryo model for assessment of biological activity of different oligosaccharides. Folia Biol. 2004, 52, 135–142.
- Blankenship, L.C.; Bailey, J.S.; Cox, N.A.; Stern, N.J.; Brewer, R.; Williams, O. Two-step mucosal competitive exclusion flora treatment to diminish salmonellae in commercial broiler chickens. Poult. Sci. 1993, 72, 1667–1672.
- Wolfenden, A.D.; Pixley, C.M.; Higgins, J.P.; Higgins, S.E.; Vicente, J.; Torres-Rodriguez, A.; Hargis, B.M.; Tellez, G. Evaluation of spray application of a Lactobacillus-based probiotic on Salmonella enteritidis colonization in broiler chickens. Int. J. Poult. Sci. 2007, 6, 493–496.
- Cox, N.A.; Bailey, J.S.; Blankenship, L.C. Alternative administration of competitive exclusion treatment. In Colonization Control of Human Bacterial Enteropathologens in Poultry; Academic Press: Cambridge, MA, USA, 1991; pp. 105–118.
- Nicetic, M.; Kailasapathy, K.; Tarasoff, L. Mechanical Stability of Food Gum Gels for Immobilization of Probiotic Bacteria. In Proceedings of the 8th International Workshop on Bioencapsulation, Recent Progress in Research and Technology, Trondheim, Norway, 13–15 September 1999; Abstract, P11. pp. 13–15.
- Würth, R.; Hörmannsperger, G.; Wilke, J.; Foerst, P.; Haller, D.; Kulozik, U. Protective effect of milk protein based microencapsulation on bacterial survival in simulated gastric juice versus the murine gastrointestinal system. J. Funct. Foods 2015, 15, 116–125.
- Cottyn, B.; Heylen, K.; Heyrman, J.; Vanhouteghem, K.; Pauwelyn, E.; Bleyaert, P.; Van Vaerenbergh, J.; Höfte, M.; De Vos, P.; Maes, M. Pseudomonas cichorii as the causal agent of midrib rot, an emerging disease of greenhouse-grown butterhead lettuce in Flanders. Syst. Appl. Microbiol. 2009, 32, 211–225.
- Nkukwana, T.T.; Muchenje, V.; Masika, P.J.; Mushonga, B. Intestinal morphology, digestive organ size and digesta pH of broiler chickens fed diets supplemented with or without Moringa oleifera leaf meal. S. Afr. J. Anim. Sci. 2015, 45, 362–370.
- Shanmugasundaram, R.; Mortada, M.; Murugesan, G.R.; Selvaraj, R.K. In vitro characterization and analysis of probiotic species in the chicken intestine by real-time polymerase chain reaction. Poult. Sci. 2019, 98, 5840–5846.
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018, 174, 1388–1405.e21.
- Mullineaux-Sanders, C.; Suez, J.; Elinav, E.; Frankel, G. Sieving through gut models of colonization resistance. Nat. Microbiol. 2018, 3, 132–140.
- Ferreiro, A.; Dantas, G.; Ciorba, M.A. Insights into how probiotics colonize the healthy human gut. Gastroenterology 2019, 156, 820–822.
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227.
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’sullivan, O.; Fouhy, F.; Clarke, S.F.; O’toole, P.W.; Quigley, E.M.; Stanton, C. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642.
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112.
- Bozdogan, B.; Galopin, S.; Leclercq, R. Characterization of a new erm-related macrolide resistance gene present in probiotic strains of Bacillus clausii. Appl. Environ. Microbiol. 2004, 70, 280–284.
- Gueimonde, M.; Sánchez, B.; de Los Reyes-Gavilán Clara, G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202.
- Feld, L.; Schjørring, S.; Hammer, K.; Licht, T.R.; Danielsen, M.; Krogfelt, K.; Wilcks, A. Selective pressure affects transfer and establishment of a Lactobacillus plantarum resistance plasmid in the gastrointestinal environment. J. Antimicrob. Chemother. 2008, 61, 845–852.
- Witte, W. Ecological impact of antibiotic use in animals on different complex microflora: Environment. Int. J. Antimicrob. Agents 2000, 14, 321–325.
- Schjørring, S.; Krogfelt, K.A. Assessment of bacterial antibiotic resistance transfer in the gut. Int. J. Microbiol. 2011, 2011, 312956.
- Hidayat, M.N.; Malaka, R.; Agustina, L.; Pakiding, W. Abdominal fat percentage and carcass quality of broiler given probiotics Bacillus spp. Metab. Clin. Exp. 2016, 22, 3–60.
- Haines, M.D.; Parker, H.M.; McDaniel, C.D.; Kiess, A.S. When rooster semen is exposed to Lactobacillus fertility is reduced. Int. J. Poult. Sci. 2015, 14, 541–547.
- Peralta-Sánchez, J.M.; Martín-Platero, A.M.; Ariza-Romero, J.J.; Rabelo-Ruiz, M.; Zurita-González, M.J.; Baños, A.; Rodríguez-Ruano, S.M.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M. Egg production in poultry farming is improved by probiotic bacteria. Front. Microbiol. 2019, 10, 1042.
- Haines, M.D.; Parker, H.M.; McDaniel, C.D.; Kiess, A.S. Impact of 6 different intestinal bacteria on broiler breeder sperm motility in vitro. Poult. Sci. 2013, 92, 2174–2181.
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114.
- Lee, M.; Zang, Z.; Choi, E.; Shin, H.; Ji, G. Cytoskeleton reorganization and cytokine production of macrophages by bifidobacterial cells and cell-free extracts. J. Microbiol. Biotechnol. 2002, 12, 398–405.
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012, 135, 1914–1919.
- Kim, H.G.; Lee, S.Y.; Kim, N.R.; Lee, H.Y.; Ko, M.Y.; Jung, B.J.; Kim, C.M.; Lee, J.M.; Park, J.H.; Han, S.H. Lactobacillus plantarum lipoteichoic acid down-regulated Shigella flexneri peptidoglycan-induced inflammation. Mol. Immunol. 2011, 48, 382–391.
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 2016, 11, e0147960.
- Matsuguchi, T.; Takagi, A.; Matsuzaki, T.; Nagaoka, M.; Ishikawa, K.; Yokokura, T.; Yoshikai, Y. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Vaccine Immunol. 2003, 10, 259–266.
- Sawada, H.; Furushiro, M.; Hirai, K.; Motoike, M.; Watanabe, T.; Yokokura, T. Purification and Characterization of an Antihypertensive Compound from Lactohacillus casei. Agric. Biol. Chem. 1990, 54, 3211–3219.
- De Almada, C.N.; Almada, C.N.; Martinez, R.C.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114.
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274.
- Ou, C.; Lin, S.; Tsai, J.; Lin, M. Heat-killed lactic acid bacteria enhance immunomodulatory potential by skewing the immune response toward Th1 polarization. J. Food Sci. 2011, 76, M260–M267.
- Shin, H.S.; Park, S.Y.; Lee, D.K.; Kim, S.; An, H.M.; Kim, J.R.; Kim, M.J.; Cha, M.G.; Lee, S.W.; Kim, K.J. Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Arch. Pharm. Res. 2010, 33, 1425–1431.
- Ananta, E.; Knorr, D. Comparison of inactivation pathways of thermal or high pressure inactivated Lactobacillus rhamnosus ATCC 53103 by flow cytometry analysis. Food Microbiol. 2009, 26, 542–546.
- Gandhi, A.; Shah, N.P. Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microbiol. 2015, 49, 197–202.
- Tsilingiri, K.; Rescigno, M. Postbiotics: What else? Benef. Microb. 2013, 4, 101–107.
- Konstantinov, S.R.; Kuipers, E.J.; Peppelenbosch, M.P. Functional genomic analyses of the gut microbiota for CRC screening. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 741–745.
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L.; Chaturvedi, R.; Peek, R.M.; Wilson, K.T.; Polk, D.B. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Investig. 2011, 121, 2242–2253.
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals 2019, 9, 644.
- Kareem, K.Y.; Hooi Ling, F.; Teck Chwen, L.; May Foong, O.; Anjas Asmara, S. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23.
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015.
- Choi, S.S.; Kim, Y.; Han, K.S.; You, S.; Oh, S.; Kim, S.H. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett. Appl. Microbiol. 2006, 42, 452–458.
- Nakamura, F.; Ishida, Y.; Sawada, D.; Ashida, N.; Sugawara, T.; Sakai, M.; Goto, T.; Kawada, T.; Fujiwara, S. Fragmented lactic acid bacterial cells activate peroxisome proliferator-activated receptors and ameliorate dyslipidemia in obese mice. J. Agric. Food Chem. 2016, 64, 2549–2559.
- Thanh, N.T.; Loh, T.C.; Foo, H.L.; Hair-Bejo, M.; Azhar, B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br. Poult. Sci. 2009, 50, 298–306.
- Compare, D.; Rocco, A.; Coccoli, P.; Angrisani, D.; Sgamato, C.; Iovine, B.; Salvatore, U.; Nardone, G. Lactobacillus casei DG and its postbiotic reduce the inflammatory mucosal response: An ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2017, 17, 53.
- Verma, A.; Shukla, G. Synbiotic (Lactobacillus rhamnosus Lactobacillus acidophilus inulin) attenuates oxidative stress and colonic damage in 1, 2 dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague–Dawley rats. Eur. J. Cancer Prev. 2014, 23, 550–559.
- Abd El-Ghany, W.; Hosny, F.; Quesnell, R.; Sakai, L. The Effect of a Postbiotic Produced by Stabilized Non-Viable Lactobacilli on the Health, Growth Performance, Immunity, and Gut Status of Colisepticaemic Broiler Chickens; Research Square: Durham, NC, USA, 2022.
- Abdulamir, A.S.; Hafidh, R.R.; Bakar, F.A. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: Inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol. Cancer 2010, 9, 249.
- Shigwedha, N. Probiotical cell fragments (PCFs) as “novel nutraceutical ingredients”. J. Biosci. Med. 2014, 2, 43–55.
- Lin, C.; Fung, Z.; Wu, C.; Chung, T. Molecular Characterization of a Plasmid-Borne (pTC82) Chloramphenicol Resistance Determinant (cat-TC) fromLactobacillus reuteriG4. Plasmid 1996, 36, 116–124.




