Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rodrigo Duarte-Casar | -- | 1435 | 2022-05-16 17:18:37 | | | |
| 2 | Rodrigo Duarte-Casar | + 23 word(s) | 1458 | 2022-05-16 17:20:04 | | | | |
| 3 | Conner Chen | -2 word(s) | 1456 | 2022-05-17 02:52:55 | | | | |
| 4 | Conner Chen | Meta information modification | 1456 | 2022-05-18 08:07:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Duarte-Casar, R.; Romero-Benavides, J. Xylosma G. Forst. Genus. Encyclopedia. Available online: https://encyclopedia.pub/entry/22983 (accessed on 07 February 2026).
Duarte-Casar R, Romero-Benavides J. Xylosma G. Forst. Genus. Encyclopedia. Available at: https://encyclopedia.pub/entry/22983. Accessed February 07, 2026.
Duarte-Casar, Rodrigo, Juan Romero-Benavides. "Xylosma G. Forst. Genus" Encyclopedia, https://encyclopedia.pub/entry/22983 (accessed February 07, 2026).
Duarte-Casar, R., & Romero-Benavides, J. (2022, May 16). Xylosma G. Forst. Genus. In Encyclopedia. https://encyclopedia.pub/entry/22983
Duarte-Casar, Rodrigo and Juan Romero-Benavides. "Xylosma G. Forst. Genus." Encyclopedia. Web. 16 May, 2022.
Copy Citation
Xylosma G. Forst. is a genus of plants belonging to the Salicaceae family with intertropical distribution in America, Asia, and Oceania. Of the 100 accepted species, 22 are under some level of conservation risk. Around 13 species of the genus used as medicinal plants were found, mainly in Central and South America, with a variety of uses, among which antimicrobial is the most common.
Xylosma
ethnopharmacology
phytochemicals
Salicaceae
1. Genus
The Salicaceae Mirb. family, to which the Xylosma genus belongs, is famously medicinal because of the Salix genus (willow), the pharmacological properties of which were already used in ancient Mesopotamia, and were extolled in the first century CE, in Dioscorides’ De Materia Medica [1][2].
The Xylosma genus is one of the 55 that conform the Salicaceae family [2], and is composed of 100 accepted species [3], although others list 45 [4]. Until recently, it was included in the now-deprecated Flacourtiaceae family, but has now been assigned to Salicaceae [5]. The name stems from the Greek words for “wood” and “smell” in reference to odoriferous quality of the wood of some Pacific species of the genus [4], presumably X. orbiculata and X. suaveolens used to perfume coconut oil by early South Pacific inhabitants [6]. At first, the genus was named Myroxylon (myrrh-wood) but was changed to Xylosma to avoid confusion with South American balsam trees [7]. Not all species in the genus are sweet-smelling: X. maidenii timber, for example, is foul-smelling. Xylosma species are described in detail by Woodson et al. [8].
In shrubs or small trees, often with axillary spines, the branchlets commonly lenticellate. Leaves alternate, sometimes borne in fascicles, usually short-petiolate, estipulate, the blade is often ±coriaceous, usually glandular-dentate, penninerved, rarely entire-margined, without pellucid-glands. Inflorescences axillary, fasciculate or contracted-racemose, and are rarely racemose. Flowers are small, dioecious, or rarely polygamous; pedicels are articulated above the base, and the bracts are minute; sepals 4-5(-6), imbricate, usually scale-like, slightly connate at the base, often ciliolate along the margins, usually persistent; petals none; stamens ∞ (8–35 in Panamanian spp.), usually surrounded by an annular or glandular, fleshy disc, the filaments free, filiform, short- to usually long-exserted, the anthers minute, basifixed, extrose, longitudinally dehiscent; ovary sessile, inserted on an annular disc, 1-locular, with 2–3, rarely 4–6, parietal placentas, each placenta with 2, sometimes 4–6, ovules, the style entire or ±divided, sometimes very short, the stigmas scarcely dilated to dilated; rudimentary ovary wanting in male flowers. Fruits baccate, rather dry, indehiscent, surmounted by the persistent style, the pericarp rather thin-coriaceous, the seeds 2–8, +angular by mutual pressure, the testa thin; endosperm copious; embryo large, the cotyledons broad.
Species in the Xylosma genus have several uses and properties, from landscaping (Xylosma congesta (Lour.) Merr.), beekeeping (Xylosma venosa N. E. Br. [9]), timber, firewood, to food and medicine; notably Xylosma longifolia Clos. Due to the thorns that some species of the genus have, common names such as “do not touch me” (Xylosma coriacea (Poit.) Eichler) or “deer antlers” (Xylosma spiculifera (Tul.) Triana and Planch.) are used for them [10]. Eleven species of the genus, particularly Xylosma vincentii Guillaumin, are known to be nickel hyperaccumulators [11][12] which presents potential for phytoremediation and phytomining [13].
2. Distribution and Localization
Species belonging to the Xylosma genus are present in subtropical America, Southeast Asia, and Oceania. Of the 100 species listed in the genus [3], 61 are found in America, 8 in Asia, and 31 in Oceania. Figure 1 shows examples of species of the genus. The map in Figure 2 shows the intertropical, and to a lesser extent, temperate, distribution of Xylosma species, by country.

Figure 1. Xylosma flexuosa (Kunth) Hemsl. leaves and berries, left. Xylosma congesta (Lour.) Merr. inflorescence, right. Image sources: left, Public Domain (CC0); right, Miwasatosi, GDFL license.
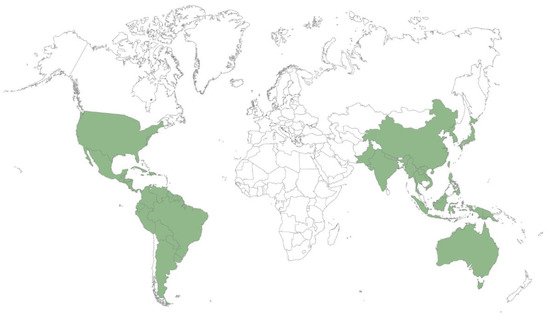
Figure 2. Worldwide Xylosma distribution, by country, after [3].
Of the 100 species of the genus, 7 are listed as vulnerable, 9 as endangered, and 6 as critically endangered. In total, 22% of the species in the genus are considered as species of concern [14]. This should be considered when evaluating potential industrial uses for these species.
3. Ethnopharmacological and Ethnoveterinary Usage
Of the 100 species of the genus, few appear in the scientific literature, and even fewer are mentioned from an ethnopharmacological or ethnoveterinary perspective. Notwithstanding, Xylosma species are a part of the traditional Chinese medicinal system, with documented uses of X. congesta appearing as early as the XVI century CE [15].
Few of the Xylosma species are recognized as medicinal. Table 1 summarizes the species with reported medicinal use along with their stated ethnopharmacological uses, when available. The Anatomical Therapeutic Chemical (ATC) Classification by the World Health Organization (WHO) is used to classify the uses for each species [16]. Not all species are identified in the literature, with general mentions as “Xylosma sp.” in some cases.
Table 1. Medicinal and veterinary use of Xylosma species, listed in alphabetical order.
| No. | Species | Region | Plant Organs Used | Use | Form of Usage |
ATC Category |
Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Xylosma benthamii (Tul.) Triana and Planch. | Brazil | NS | Medicinal (not specified) |
NS | NS | [17] |
| 2 | Xylosma characantha Standl. | Nicaragua | Leaves | Placentary retention in cattle | Decoction | Vet. | [18] |
| 3 | Xylosma chlorantha Donn. Sm. | Costa Rica | Bark | Medicinal (not specified) |
NS | NS | [19] |
| 4 | Xylosma ciliatifolia (Clos) Eichler | Brazil | Root bark | Antibacterial | NS | V | [20] |
| 5 | Xylosma congesta (Lour.) Merr. | China Japan Korea |
Bark Leaves |
NS Anti-inflammatory Disease prevention in suckling piglets Birthing aid |
Bark ashes Poultice |
NS D G Vet. |
[21] [22] [23] [24] |
| 6 | Xylosma controversa Clos. | Guangxi, China | Roots Leaves |
NS | NS | NS | [25] |
| 7 | Xylosma flexuosa (Kunth) Hemsl. | Mexico | NS | Antipyretic Anti-tuberculosis |
NS | N R |
[26][27] |
| 8 | Xylosma horrida Rose. | Mexico Nicaragua Costa Rica |
Bark | Kidneys | Decoction | G | [28] |
| 9 | Xylosma intermedia (Seem.) Triana and Planch. | Bolivia | Bark | Toothache | NS | N | [29] |
| 10 | Xylosma longifolia Clos | India China |
Leaves Stem bark |
Antifungal Antispasmodic Antidiarrheic Anti-tuberculosis Muscle sprains Narcotic |
Paste Decoction Extract |
D A A R M N |
[30] [31] [32] [33] [34] |
| 11 | Xylosma panamensis (Turcz) | Panama Mexico |
Bark Leaves |
Cough Bronchitis |
Dried | R | [35] |
| 12 | Xylosma spiculifera (Tul.) Triana and Planch | Colombia, Venezuela | Leaves | Ulcers, Dermatitis | Decoction | D | [36] |
| 13 | Xylosma tessmanii Sleumer | Ecuador | Leaves | Medicinal (NS) | NS | NS | [37] |
| 14 | Xylosma sp. (not specified) | Panama | Stem Root |
Spider bites | Infusion | V | [38] |
| 15 | Xylosma sp. (not specified) | Perú | Bark | Bronchitis (with other plant species) | Decoction | R | [39] |
NS: Not specified. ATC categories are as follows. A: Alimentary tract and metabolism, B: Blood and blood forming organs, C: Cardiovascular system, D: Dermatological, G: Genito urinary system and sex hormones, H: Systemic hormonal preparations, excluding sex hormones and insulins, J: Anti-infective for systemic use, L: Antineoplastic and immunomodulating agents, M: Musculo-skeletal system, N: nervous system, P: Antiparasitic products, insecticides, and repellents, R: Respiratory system, S: Sensory organs; V: Various [16]; STDs: Sexually transmitted diseases, Vet: veterinary.
Most Xylosma species in use are from Central and South America (38% and 31%), followed by China (23%) and India (8%). This is roughly in accordance with the local abundance of species. There are no reports of ethnomedicinal uses of Xylosma in Oceania. Uses by country are shown in Figure 3.
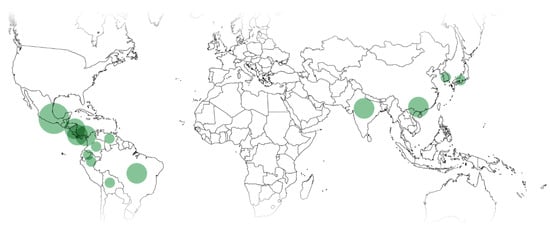
Figure 3. Ethnopharmacological and ethnoveterinary uses of Xylosma spp. Circle diameter proportional to use reports for the country.
According to the ATC classification, the most frequent uses of Xylosma spp. in ethnopharmacology are dermatological, nervous system, and respiratory system, with 17% of the uses each, alimentary tract and metabolism with 11%, and genitourinary system and sex hormones with 6%. Additionally, 11% of the uses are veterinary.
As to the morphological structures used, the most common are leaves and barks with 33% each, and both stems and roots with 11% each.
4. Biological Activity
Biological activity tests of Xylosma have been carried out mostly in vitro, with no reported in vivo research, with plant extracts, be they leaf, root, bark, or the whole plant. Different solvents and solvent mixtures have been used for the extracts, mainly methanol and ethanol.
In Vitro Activity
In vitro research on biological activity of Xylosma species centers around 7 identified species and one unspecified one. The research figures are summarized in Figure 4, and the research is detailed in Table 2.
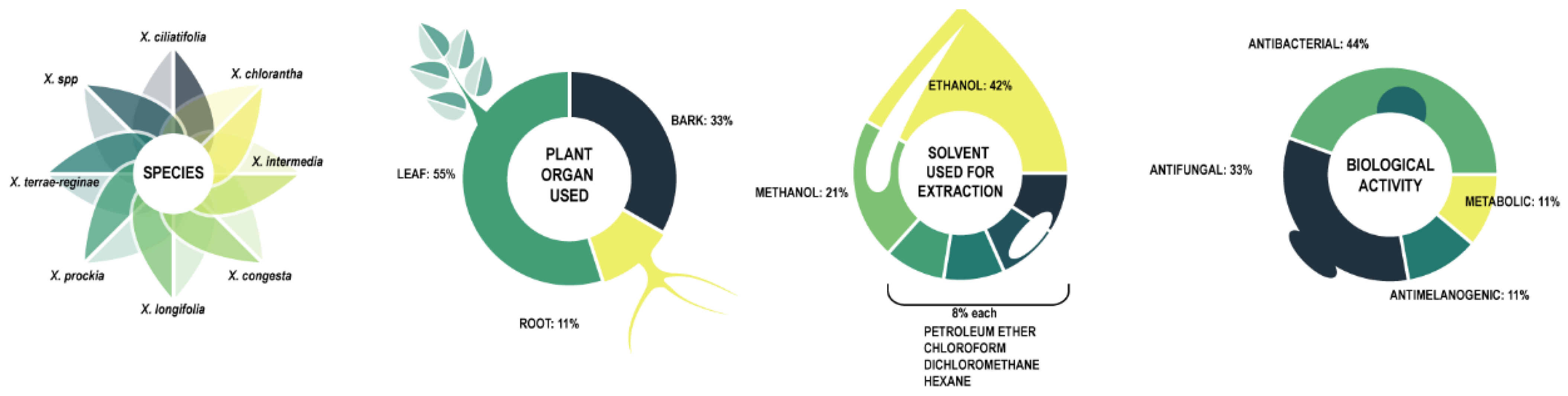
Figure 4. Summary of in vitro activity of Xylosma species.
Table 2. In vitro activity of Xylosma extracts. Species are in alphabetical order.
| Species | Extract | Plant Organs Used | Biological Activity |
Biological Model |
Effect | Methodology | Ref. |
|---|---|---|---|---|---|---|---|
| X. ciliatifolia | Ethanol/Hexane partition | Root bark | Antibacterial | S. aureus S. epidermis S. typihimurium E. coli |
Effective against S. aureus S. epidermis MIC (µg/mL) 250, 500 |
Disk diffusion assay | [20] |
| X. clorantha | Ethanol | Leaves | Metabolic syndrome | HepG2 cells | LXR 2.14 ± 0.11: 100 µg/mL |
LXR transcriptional activity | [40] |
| X. congesta | Ethanol | Leaves | Anti-melanogenic | B16F10 cells | Melanin synthesis inhibition: up to 57.9% | α-MSH | [22] |
| X. intermedia | DCM/Ethanol | Bark | Antibacterial | Bacillus cereus S. aureus |
MIC (ppm) 156 512 |
Microbroth dilution | [41] |
| X. longifolia | Petroleum ether Chloroform Methanol |
Leaves, Stem bark | Antifungal | Microsporum boullardii, M. canis, M. gypseum Trichophyton ajelloi T. rubrum |
MIC (mg/mL) 0.141–9.0 |
Agar diffusion Micro wells diffusion |
[30] |
| X. prockia | Ethanol | Leaves | Antifungal | Cryptococcus spp. | MIC (ppm) 8–64 |
Antifungal microdilution susceptibility standard test | [42] |
| X. terrae reginae | Methanol | Root | Antibacterial Antifungal |
S. aureus C. albicans |
MIC (mg/mL) 2.5 1.2 |
Dilution method | [43] |
| X. sp II | Methanol | Leaves | Antibacterial | Flavobacterium columnae | MIC 375 µg/mL |
Agar diffusion assay | [44] |
DCM: Dichloromethane; MIC: Minimum inhibitory concentration; α-MSH: melanocyte-stimulating hormone. LXR: LXRα Fold Activation.
In vitro biological activity tests devote the most attention to leaves (55%), with bark (33%) and root (11%) used to a lesser extent. Extraction solvents are ethanol (42%), methanol (21%), and to a lesser extent petroleum ether, chloroform, dichloromethane, and hexane, with 8% each. The solvent choices support the assumption that most active compounds are polar, and are thus extracted with polar solvents.
Testing centers on antibacterial (44%) and antifungal (44%) activity reflects the main ethnopharmacological use but appears to leave other traditional uses unexplored.
Cytotoxicity assays involving Xylosma extracts show no significant cytotoxicity for Xylosma prockia nor for Xylosma congesta leaf extracts [42][45]. Moderate cytotoxicity was reported for methanol Xylosma terrae reginae extracts [43]. 2,6-dimethoxybenzoquinone (33) isolated from Xylosma velutina is reported as cytotoxic [46].
Even though there is no in vivo research concerning Xylosma species in the literature, there are several patents that include Xylosma extracts for cosmetic, veterinary, and traditional medicinal uses, such as hangover cures [23].
References
- Calixto, J.B. The Role of Natural Products in Modern Drug Discovery. An. Acad. Bras. Ciênc. 2019, 91, e20190105.
- Salicaceae Mirb.|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30002644-2 (accessed on 25 January 2022).
- Xylosma G. Forst.|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:332071-2 (accessed on 25 January 2022).
- WFO. Xylosma G. Forst. Available online: http://www.worldfloraonline.org/taxon/wfo-4000041044;jsessionid=402EBB0471E61A8E4D367F550003835E (accessed on 25 January 2022).
- The Angiosperm Phylogeny Group. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20.
- Uphof, J. The Dictionary of Economic Plants; Cramer: Lehre, Germany, 1968; ISBN 978-3-7682-0001-1.
- Berry, S.S.; Chapman, J.; Jones, G.; Jones, J.; Pass, J.; Roper, C.F.E.; Wilkes, J. Encyclopaedia Londinensis, or, Universal Dictionary of Arts, Sciences, and Literature: Comprehending, under One General Alphabetical Arrangement, All the Words and Substance of Every Kind of Dictionary Extant in the English Language: In Which the Improved Departments of the Mechanical Compiled, Digested, and Arranged, by John Wilkes, of Milland House…; Assisted by Eminent Scholars of the English, Scotch, and Irish, Universities; Champante and Whitrow: London, UK, 1810.
- Woodson, R.E.; Schery, R.W.; Robyns, A. Flora of Panama. Family 128. Flacourtiaceae. Ann. Mo. Bot. Gard. 1968, 55, 93.
- Vossler, F.G. Flower Visits, Nesting and Nest Defence Behaviour of Stingless Bees (Apidae: Meliponini): Suitability of the Bee Species for Meliponiculture in the Argentinean Chaco Region. Apidologie 2012, 43, 139–161.
- García, R.; Pegüero, B.; Jiménez, F.; Veloz, A.; Clase, T. Lista Roja de La Flora Vascular en República Dominicana; Ministerio de Educación Superior Ciencia y Tecnología (MESCyT): Santo Domingo, Dominican Republic, 2016.
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; Ent, A. A Global Database for Plants That Hyperaccumulate Metal and Metalloid Trace Elements. New Phytol. 2018, 218, 407–411.
- Seregin, I.V.; Kozhevnikova, A.D. Physiological Role of Nickel and Its Toxic Effects on Higher Plants. Russ. J. Plant Physiol. 2006, 53, 257–277.
- Chaney, R.L.; Angle, J.S.; Broadhurst, C.L.; Peters, C.A.; Tappero, R.V.; Sparks, D.L. Improved Understanding of Hyperaccumulation Yields Commercial Phytoextraction and Phytomining Technologies. J. Environ. Qual. 2007, 36, 1429–1443.
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 1 February 2022).
- Ye, H.; Li, C.; Ye, W.; Zeng, F.; Liu, F.; Liu, Y.; Wang, F.; Ye, Y.; Fu, L.; Li, J. Medicinal Angiosperms of Flacourtiaceae, Tamaricaceae, Passifloraceae, and Cucurbitaceae. In Common Chinese Materia Medica; Ye, H., Li, C., Ye, W., Zeng, F., Eds.; Springer: Singapore, 2021; pp. 51–130. ISBN 9789811658792.
- WHO. Anatomical Therapeutic Chemical (ATC) Classification. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 8 June 2021).
- Van den Berg, M.E.; da Silva, M.H.L.; da Silva, M.G. Plantas Aromáticas Da Amazônia. In Proceedings of the 1er Simpósio do Trópico Úmido, 1986; Volume II, p. 95.
- Rodríguez-Flores, O.; Torréz-Centeno, E.; Valenzuela-Betanco, R. Plantas Utilizadas Para el Tratamiento de Enfermedades en los Animales Domésticos, Reserva Natural El Tisey, Estelí. Ph.D. Thesis, Universidad Católica Agropecuaria del Trópico Seco Pbro, Francisco Luis Espinoza Pineda, Estelí, Nicaragua, 2005.
- Juep, A. Rescate del Conocimiento Tradicional y Biológico Para el Manejo de Productos Forestales no Maderables en la Comunidad Indígena Jameykari, Costa Rica. Master’s Thesis, Centro Agronómico Tropical de Educación y Enseñanza, Turrialba, Costa Rica, 2008.
- Philippsen, A.F.; Miguel, O.G.; Miguel, M.D.; de Lima, C.P.; Kalegari, M.; Lordello, A.L.L. Validation of the antibacterial activity of root bark of Xylosma ciliatifolia (Clos) Eichler (Flacourtiaceae/Salicaceae sensu lato). Rev. Cuba. Plantas Med. 2013, 18, 258–267.
- Shizhen, L. Ben Cao Gang Mu, Volume II: Waters, Fires, Soils, Metals, Jades, Stones, Minerals, Salts; University of California Press: Berkeley, CA, USA, 2021; ISBN 978-0-520-97697-9.
- Lee, J.Y.; Ahn, E.-K.; Ko, H.-J.; Cho, Y.-R.; Ko, W.C.; Jung, Y.-H.; Choi, K.-M.; Choi, M.-R.; Oh, J.S. Anti-Melanogenic, Anti-Wrinkle, Anti-Inflammatory and Anti-Oxidant Effects of Xylosma congesta Leaf Ethanol Extract. J. Appl. Biol. Chem. 2014, 57, 365–371.
- Yungui, W. A Disease Control Method for Suckling Piglets. Patent CN108235969A, 3 July 2018.
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Medicinal Plants of the World; Reference Publications: Algonac, MI, USA, 1985; ISBN 978-0-917256-20-2.
- Xu, Z.-R.; Chai, X.-Y.; Bai, C.-C.; Ren, H.-Y.; Lu, Y.-N.; Shi, H.-M.; Tu, P.-F. Xylocosides A-G, Phenolic Glucosides from the Stems of Xylosma controversum. Helv. Chim. Acta 2008, 91, 1346–1354.
- Cornejo-Báez, A. Evaluación de la actividad antibacteriana de los extractos y fracciones de Bidens pilosa L. y Xylosma flexuosum (H. B. & K.) Hemsl y estudio quimiométrico de la actividad antituberculosa de los perfiles cromatográficos de Bidens pilosa L. Master’s Thesis, Universidad Veracruzana, Xalapa, Mexico, 2016.
- Grijalva Pineda, A. Flora útil: Etnobotánica de Nicaragua; Ministerio del Ambiente y Recursos Naturales de Nicaragua, Ed.; MARENA: Managua, Gobierno de Nicaragua, 2006; ISBN 978-99924-903-8-9.
- Pérez-Torres, F. Manual de Plantas Medicinales más Comunes del Occidente de Nicaragua; Universidad Autónoma de Nicaragua: León, Spain, 2007.
- Grandtner, M.M. Elsevier’s Dictionary of Trees: With Names in Latin, English, French, Spanish and Other Languages, 1st ed.; Elsevier: Amsterdam, The Netherlands; San Diego, CA, USA, 2005; ISBN 978-0-444-51784-5.
- Devi, W.R.; Singh, S.B.; Singh, C.B. Antioxidant and Anti-Dermatophytic Properties Leaf and Stem Bark of Xylosma longifolium Clos. BMC Complement. Altern. Med. 2013, 13, 155.
- Truong, B.N.; Pham, V.C.; Mai, H.D.T.; Nguyen, V.H.; Nguyen, M.C.; Nguyen, T.H.; Zhang, H.; Fong, H.H.S.; Franzblau, S.G.; Soejarto, D.D.; et al. Chemical Constituents from Xylosma longifolia and Their Anti-Tubercular Activity. Phytochem. Lett. 2011, 4, 250–253.
- Swapana, N.; Noji, M.; Nishiuma, R.; Izumi, M.; Imagawa, H.; Kasai, Y.; Okamoto, Y.; Iseki, K.; Singh, C.B.; Asakawa, Y.; et al. A New Diphenyl Ether Glycoside from Xylosma longifolium Collected from North-East India. Nat. Prod. Commun. 2017, 12, 1934578X1701200.
- Salam, S. Medicinal Plant Used for the Treatment of Muscular Sprain by the Tangkhul Tribe of Ukhrul District, Manipur, India. Int. J. Adv. Res. 2020, 8, 167–170.
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Reference; Springer: New York, NY, USA, 2007; ISBN 978-0-387-70637-5.
- Joly, L.G.; Guerra, S.; Séptimo, R.; Solís, P.N.; Correa, M.; Gupta, M.; Levy, S.; Sandberg, F. Ethnobotanical Inventory of Medicinal Plants Used by the Guaymi Indians in Western Panama. Part I. J. Ethnopharmacol. 1987, 20, 145–171.
- García-Barriga, H. Flora Medicinal de Colombia: Botánica Médica; Instituto de Ciencias Naturales: Bogotá, Colombia, 1992.
- Cerón, C.; Montalvo, C. Reserva Biológica Limoncocha: Formaciones Vegetales, Diversidad y Etnobotánica. Cinchonia 2000, 1, 20.
- Caballero-George, C.; Gupta, M. A Quarter Century of Pharmacognostic Research on Panamanian Flora: A Review. Planta Med. 2011, 77, 1189–1202.
- Brown, M. Una paz Incierta: Comunidades Aguarunas Frente al Impacto de la Carretera Marginal; Serie Antroplógica; CAAAP: Magdalena, Perú, 1984; ISBN 84-89290-008-0.
- Vasquez, Y. Biological and Chemical Investigation of Panamanian Plants for Potential Utility against Metabolic Syndrome; University of Mississippi: Oxford, MS, USA, 2016.
- Setzer, M.C.; Moriarity, D.M.; Lawton, R.O.; Setzer, W.N.; Gentry, G.A.; Haber, W.A. Phytomedicinal Potential of Tropical Cloudforest Plants from Monteverde, Costa Rica. Rev. Biol. Trop. 2003, 51, 647–673.
- Folly, M.L.C.; Ferreira, G.F.; Salvador, M.R.; Sathler, A.A.; da Silva, G.F.; Santos, J.C.B.; dos Santos, J.R.A.; Nunes Neto, W.R.; Rodrigues, J.F.S.; Fernandes, E.S.; et al. Evaluation of In Vitro Antifungal Activity of Xylosma prockia (Turcz.) Turcz. (Salicaceae) Leaves Against Cryptococcus spp. Front. Microbiol. 2020, 10, 3114.
- Mosaddik, M.A.; Banbury, L.; Forster, P.; Booth, R.; Markham, J.; Leach, D.; Waterman, P.G. Screening of Some Australian Flacourtiaceae Species for in vitro Antioxidant, Cytotoxic and Antimicrobial Activity. Phytomedicine 2004, 11, 461–466.
- Castro, S.B.R.; Leal, C.A.G.; Freire, F.R.; Carvalho, D.A.; Oliveira, D.F.; Figueiredo, H.C.P. Antibacterial Activity of Plant Extracts from Brazil against Fish Pathogenic Bacteria. Braz. J. Microbiol. 2008, 39, 756–760.
- Kuete, V.; Seo, E.-J.; Krusche, B.; Oswald, M.; Wiench, B.; Schröder, S.; Greten, H.J.; Lee, I.-S.; Efferth, T. Cytotoxicity and Pharmacogenomics of Medicinal Plants from Traditional Korean Medicine. Evid. Based Complement. Alternat. Med. 2013, 2013, 341724.
- Jones, E.; Ekundayo, O.; Kingston, D.G.I. Plant Anticancer Agents. XI. 2,6-Dimethoxybenzoquinone as a Cytotoxic Constituent of Tibouchina pulchra. J. Nat. Prod. 1981, 44, 493–494.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
4 times
(View History)
Update Date:
18 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




