
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Clémence Rougeaux | + 2745 word(s) | 2745 | 2020-08-07 04:34:31 | | | |
| 2 | Conner Chen | -81 word(s) | 2664 | 2020-10-29 07:25:43 | | |
Video Upload Options
Bacillus anthracis is a Gram-positive spore-forming bacterium, considered one of the most potent and critical bioterrorist agents. It is still as important to quickly diagnose this disease, a rapid diagnosis improving the effective management of the patient. Significant progress has been made in the detection of toxins produced by Bacillus anthracis. These toxins appear as early targets for diagnosis, predictive tools for host survival, and they help to monitor the efficiency of treatment.
1. Introduction
Bacillus anthracis is a Gram-positive spore-forming bacterium, considered one of the most potent and critical bioterrorist agents, and subsequently is listed as Category A select agents by the Centers for Disease Control and Prevention (CDC). It is responsible for anthrax, a zoonotic disease, mainly affecting herbivores, humans being only occasional hosts. There are three main forms of human anthrax and a recently described atypical form, depending on the route of entry of the pathogen: cutaneous, gastrointestinal, inhalational, and injectional anthrax.
B. anthracis toxins largely shape the pathogenesis of the disease in mammals, even though these proteins are produced at very low levels. Indeed, anthrax toxins are highly efficient, as most of their effects are biochemically amplified. Thus, their detection is very challenging, as the toxins are present in the blood at very low levels, below classical detection methods, and are not accessible to the genetic amplification methods used for molecular diagnosis.
However, recent studies have proposed new methods for the detection of anthrax toxin, leading to reassessment of the pathogenesis of anthrax through the lens of the toxins, and leading to exciting perspectives for anthrax diagnosis. B. anthracis needs having sensitive, rapid, and scalable methods of detection of the organisms as of its toxins.
2. Why Detect Anthrax Toxins?
Anthrax toxins act at two critical stages of the infection [1]. Early in the infection, they paralyze the immune response of the host by targeting innate and adaptative immune cells. During the late stage of the infection, the toxins are involved in the failure of vital organs by acting on target cells.
An uncharacteristic clinical picture, with the exception of the cutaneous form, and first-line antibiotic treatment can complicate the initial diagnosis of anthrax. It is currently based on bacterial isolation in cultures and the detection of specific markers of B. anthracis, as antigens or nucleic acid using pagA PCR and, more recently, detection of BA_5345, a chromosomal marker allowing differentiation between B. anthracis, B. cereus biovar anthracis, and B. thuringiensis [2][3][4]. However, these diagnostic approaches have their limits, as bacterial clearance due to early antibiotherapy and the low sensitivity of the test and time required to perform them are not compatible with rapid management of the disease. Sensitive and rapid assays for the detection of B. anthracis are needed to facilitate early and accurate diagnosis and post-exposure treatment. During a bioterrorist attack, for example, the screening must be rapid and should allow the testing of a large number of samples.
Many technical approaches for the detection, identification, and quantification of the toxins of B. anthracis have been developed and sometimes used in the laboratory.
3. Toxins In Vivo
3.1. Cutaneous Anthrax
Dal Molin et al. were the first to quantify the level of PA, LF, and EF during cutaneous anthrax in a rabbit model [5]. Blood samples were collected every 24 h and bacterial factors quantified by western blotting. They were not detected 24 h after infection, but at 48 h, PA63, LF, and EF were detected, whereas PA83 was never observed. The LF/EF ratio of ≈ 5 remained relatively constant.
Detection may depend on the bacterial load. When mice were subcutaneously infected with 103 spores of the Sterne strain, PA was never detected (from 6 h to 237 h) [6]. However, when mice were challenged with 107 spores, PA was first detected at 24 h post-infection at a concentration of approximately 68 ng/mL, when the rodents started to present symptoms of the disease. When the mice were ill at 42 h and 48 h, PA concentrations increased to 408 ng/mL. At 6 h and 8 h, PA was not detected in the still healthy mice.
These techniques are not sufficiently sensitive and did not allow observation of what happened earlier during the infection process. However, they correlated the presence of PA in the blood with an advanced state of the disease. The development of MS enabled a more rigorous vision during the course of infection.
In a mouse model of subcutaneous anthrax, LF was quantified 12 h after challenge, in the ear, the draining cervical lymph nodes (cLN), and serum by MALDI-TOF MS [7]. This early presence reinforces the dogma of the paralyzing effect of LT on PMNs, as demonstrated in vitro on cells and in vivo by injection of LT, thus protecting the bacteria from the immune system [8].
A further study defined three stages of infection, depending on the location of bacteria—early, mid, and late. At each stage, LF was quantified in several organs [8]. When the bacilli were detected in the inoculated ear (early stage), LF was detected in many tissues—the infected ear, serum, cLN, heart, lungs, spleen, and liver, but not the brain or bone marrow. LF concentrations then increased during infection, and LF was detected in all tissues analyzed. The authors noted that LF levels at the infection site were higher than those observed in the serum and bone marrow during the early and mid-stages of infection, suggesting that LF found at the site of infection may play a greater role in initial survival and escape from the innate immune response than that of circulating LF.
More recently, the use of LC-MS/MS and EIA assay has provided a picture of the complex kinetics of LF and EF in a mouse model of cutaneous infection [9] (see Figure 1). Thirty minutes to 3.5 h after infection with spores of B. anthracis, LF and EF were detected in the site of inoculation (ear), in accordance with a rapid germination of spores and a rapid toxin production. More surprisingly, despite the absence of circulating bacteria, LF and EF were also detected in the blood. Although only 29% and 38% of the mice were positive for LF and EF at the site of infection, respectively, the percentage increased to 94% positive mice for EF and/or LF. The percentage in the blood was lower (62% positive mice) when detection of the two was combined, LF being the more effective blood marker of disease. In the ear, the percentage of mice positive for EF and LF increased during infection, with an associated decreased level of LF and an increased concentration of EF. The measured LF/EF ratio varied between 320,000 at the early stage of infection and 890 at the terminal phase. As described in the study of Weiner et al. [8], the level of LF was higher at the site of infection than in the blood until the stage with a bioluminescent spleen. LF and EF concentrations in the blood tended to increase during infection, with a slight decrease of LF at the stage of infection preceding the terminal phase. The LF/EF ratio was 3 just before the terminal stage of infection, corresponding to the previous value of 5 determined by Dal Molin et al. in their rabbit model of infection [5] and approaching the values observed in an inhalation model of anthrax in RMs [10].
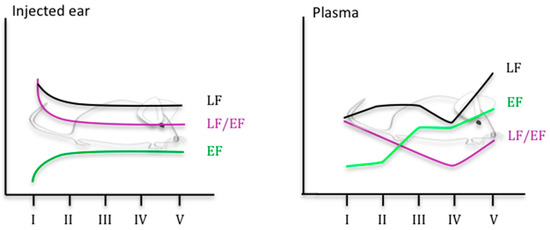
Figure 1. Kinetics of LF and EF level in a mouse model of cutaneous anthrax, adapted from [9] (stage I to V defined through BLI imaging. Stage I: no BLI, stage II: BLI in the injected ear; stage III: BLI in the injected ear and in the draining cLNs; BLI in the injected ear, in the draining cLNs and in the spleen; stage V: mice in septicemia).
Cutaneous anthrax is mainly a local form that leaves a black scar. Patients generally recover without treatment, but in some cases, the infection can spread and kill. The data primarily collected in animal models indicate a more diffuse infection, with virulence factors detected in the blood explaining the rarely fatal outcome of this form.
3.2. Inhalation Anthrax
In the guinea pig model of inhalation anthrax, PA was detected in sera by ELISA just before or just after the death of the animals for four of five infected animals [11]. PA and LF were detected in two infected rabbits after their death. Improvements in techniques have allowed faster detection of PA in these animal models [12]. ECLI allowed the detection of PA in 44.4% of rabbits 18 h after challenge, whereas ELISA allowed the detection of PA in only 11.1% of rabbits 24 h post-infection (both, however, prior to bacterial detection). The discrepancy in the time of detection is explained by the difference in the LOD between ELISA (10 ng/mL) and ECLI (1 ng/mL). The PA concentration increases over time, similar to the increase of bacteremia, with a final concentration that can reach 5 μg/mL. In guinea pigs infected with various doses of Vollum spores, PA was first detected at 24 h in 10% of animals and all bacteremic animals showed detectable PA from 30 h post-challenge, with a maximal concentration of ≈ 40 μg/mL at the final stage of infection.
After focusing on PA detection, techniques were also developed to detect LF and EF.
The MS method was first used in a model of inhalation anthrax in RMs, in which LF was detected in the serum of all three RMs infected at a concentration of 30 ng/mL to 250 ng/mL two days after infection and 30 to ≈ 550 ng/mL the day of the animals’ death (2–4 days) [13]. MS and ELISA were then used in the same animal model for LF and PA detection, respectively [14]. These techniques were compared to classical diagnostic tools for anthrax, which detect the pagA gene by PCR. It allowed the observation of a triphasic kinetic profile (Figure 2B) for LF in the serum of four of the five animals tested: LF was detected in three RMs 24 h after infection (60% of positive RMs), more rapidly than in the first study [13], at levels ranging from 0.006 ng/mL to 0.2 ng/mL. The LF concentrations were higher at 48 h and then decreased by 72 h. By 96 h, the LF levels were increasing for three of the animals, whereas they continued to decrease for the other two. At 120 h, the LF concentration was increasing for all animals. PA was detected only at 96 h and 120 h, the levels of samples for time points earlier than 96 h being lower than the detection limit of 4.8 ng/mL. At the late stages of infection, PA levels were higher than LF levels. The PCR of pagA was positive for four RMs by 48 and 72 h. The PCR for pagA reverted to negative at 72 h for one animal, which showed the lowest LF levels, suggesting microbial clearance. These data suggest that early during infection, either more LF is produced or it is less rapidly sequestered by the host tissues than PA; the circulating level of PA is sufficient to potentiate early infection and anthrax bacteremia. In the same animals, EF was first detected in the serum of two RMs (0.16 pg/mL and 0.42 pg/mL, 40% of positive RM) at 24 h post-challenge and in the serum of the three others at 48 h [10]. The detection of both LF and EF at 24 h post-challenge resulted in 80% positive animals. EF remained detectable throughout infection, with a maximal level of 2220 ng/mL. For the RM that died, the LF/EF ratios ranged from 3.6 to 17.5. The study of Solano et al. completed this kinetic analysis by focusing on the detection of PA83 and PA63 in the same five RMs [15]. PA63 was first detected 48 h after challenge in all RMs, at the intermediate phase of the disease (Figure 2B), at higher levels than LF. Such an excess of circulating active PA could constitute a reservoir for toxin formation throughout the infection. The continuous hydrolysis of PA83 to PA63 may explain the transient presence of PA83 at lower levels and the absence of its detection during cutaneous anthrax, although the technique used was less sensitive [5].
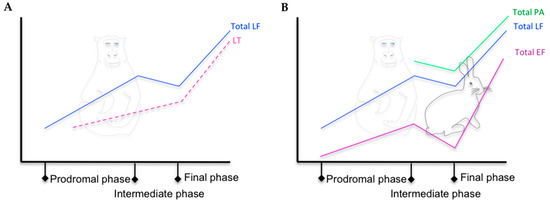
Figure 2. (A) Kinetic trends of total LF and LT level in the serum of RMs with inhalation anthrax, adapted from [16]. (B) Kinetic trends of total LF, EF, and PA level in the serum of RMs and New Zealand white rabbits with inhalation anthrax, adapted from [15][16][17][10][14].
In rabbits, all animals exposed to various doses of Ames spores that developed anthrax had detectable toxins [17]. LF was first detected at 12 h and EF and PA were detected later (Figure 2B). The level of PA was higher than that of LF and EF. As observed in RMs [15][16][10][14], EF concentrations tended to match those of LF and PA at the final phase of infection (Figure 2B). Also as observed in RMs [15], PA63 predominated, PA83 being detected only punctually.
Contrary to the macaque model, intranasally challenged mice showed detectable LF in the plasma of all animals 1 h after challenge, at a mean concentration of 2.63 ng/mL [18]. However, as for RMs, EF was detected in only ≈ 42% of infected animals in the early phase of disease at much lower levels than LF.
Boyer et al. focused on the two forms of LF—free LF and LT, which were quantified in the serum of two RMs during aerosol-inhalation anthrax [16] (Figure 2A). Free LF was first detected at 18 h in the first macaque at a level of 0.026 ng/mL and 24 h post-exposure in the second macaque, before the detection of LT, bacteremia, or pagA by PCR. Both animals were positive for LF, LT, pagA PCR, and bacteremia at 36 post-exposure, the level of LF level being higher than that of LT. The triphasic profile observed in previous and subsequent studies was found in this study for total LF (see Figure 2). This analysis demonstrated a majority of free LF in the earliest stages of infection and a dominant LT form at the late stage, with LT representing 100% and 60% of the total LF for the two animals.
4. Conclusions
Human anthrax is a rare disease, but endemic/enzootic foci persist, and there is an ever-present bioterrorist risk. It its therefore important to have sensitive and ultra-rapid techniques for early diagnosis of the disease. The sooner the patient is diagnosed, the more effective the treatment administered and the better his chances of survival, especially in cases of inhalation anthrax.
The ability to detect the toxins provides several advantages. (1) As their levels increase quickly, they can be detected early, especially when there is a suspicion of anthrax, before any clinical signs, which is very important, as the initiation of adapted anthrax therapy during the prodromal phase significantly improves survival [19]. Moreover, searching for LF and EF increases the chances of detection. (2) Their detection is independent of the presence of the bacteria, which relieves us of the potential problems of antimicrobial or immunological clearance of the organism. (3) Extrapolation of the results obtained in RMs and rabbits for inhalation anthrax [20][17][10][21] to humans makes it possible to predict patient survival based on the level of these toxins, with a threshold beyond which antibiotic treatment is ineffective. The LF/EF ratio can be associated with the stage of the disease and PA is detectable at the intermediate stage of the disease using current techniques. Knowing the stage of the disease also allows the readjustment of treatment. Walsh et al. have shown that LF remains detectable in the blood for 12 days after antimicrobial therapy [22]. Antimicrobial therapy alone may not be sufficient if toxin levels are too high, as shown in the study of Boyer et al. with RMs [20] and as implied in the study of Weiner et al. [54], in which late debridement decreases the chances of survival of the host. 4) It makes it possible to monitor the effectiveness of treatment and seroconversion, either by directly measuring the toxins or by searching for anti-toxin antibodies, as applied in human cases of cutaneous, gastrointestinal, and inhalation anthrax [22][23]. Measuring toxin levels may help to monitor the efficiency of anti-toxin, as it is still the only specific authorized treatment to complement antibiotics [24].
References
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax Pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208, doi:10.1146/annurev-micro-091014–104523.
- Jernigan, J.A.; Stephens, D.S.; Ashford, D.A.; Omenaca, C.; Topiel, M.S.; Galbraith, M.; Tapper, M.; Fisk, T.L.; Zaki, S.; Popovic, T., et al. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg. Infect. Dis. 2001, 7, 933–944, doi:10.3201/eid0706.010604.
- WHO. Anthrax in Humans and Animals. Available online: https://apps.who.int/iris/bitstream/handle/10665/97503/9789241547536_eng.pdf;jsessionid=3BF3DA0CD1CA21CFBCF1160F6B7FB128?sequence=1 (accessed on 1 April 2020)
- Antwerpen, M.H.; Zimmermann, P.; Bewley, K.; Frangoulidis, D.; Meyer, H. Real-time PCR system targeting a chromosomal marker specific for Bacillus anthracis. Mol. Cell. Probes 2008, 22, 313–315, doi:10.1016/j.mcp.2008.06.001.
- Molin, F.D.; Fasanella, A.; Simonato, M.; Garofolo, G.; Montecucco, C.; Tonello, F. Ratio of lethal and edema factors in rabbit systemic anthrax. Toxicon 2008, 52, 824–828, doi:10.1016/j.toxicon.2008.08.011.
- Tang, S.; Moayeri, M.; Chen, Z.; Harma, H.; Zhao, J.; Hu, H.; Purcell, R.H.; Leppla, S.H.; Hewlett, I.K. Detection of anthrax toxin by an ultrasensitive immunoassay using europium nanoparticles. Clin. Vaccine Immunol. 2009, 16, 408–413, doi:10.1128/CVI.00412–08.
- Weiner, Z.P.; Boyer, A.E.; Gallegos-Candela, M.; Cardani, A.N.; Barr, J.R.; Glomski, I.J. Debridement increases survival in a mouse model of subcutaneous anthrax. PLoS ONE. 2012, 7, e30201, doi:10.1371/journal.pone.0030201.
- Weiner, Z.P.; Ernst, S.M.; Boyer, A.E.; Gallegos-Candela, M.; Barr, J.R.; Glomski, I.J. Circulating lethal toxin decreases the ability of neutrophils to respond to Bacillus anthracis. Cell Microbiol 2014, 16, 504–518, doi:10.1111/cmi.12232.
- Rougeaux, C.; Becher, F.; Ezan, E.; Tournier, J.N.; Goossens, P.L. In vivo dynamics of active edema and lethal factors during anthrax. Sci. Rep. 2016, 6, 23346, doi:10.1038/srep23346.
- Lins, R.C.; Boyer, A.E.; Kuklenyik, Z.; Woolfitt, A.R.; Goldstein, J.; Hoffmaster, A.R.; Gallegos-Candela, M.; Leysath, C.E.; Chen, Z.; Brumlow, J.O., et al. Zeptomole per milliliter detection and quantification of edema factor in plasma by LC-MS/MS yields insights into toxemia and the progression of inhalation anthrax. Anal. Bioanal. Chem. 2019, 411, 2493–2509, doi:10.1007/s00216–019–01730–4.
- Mabry, R.; Brasky, K.; Geiger, R.; Carrion, R., Jr.; Hubbard, G.B.; Leppla, S.; Patterson, J.L.; Georgiou, G.; Iverson, B.L. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin. Vaccine Immunol. 2006, 13, 671–677, doi:13/6/671 10.1128/CVI.00023–06.
- Kobiler, D.; Weiss, S.; Levy, H.; Fisher, M.; Mechaly, A.; Pass, A.; Altboum, Z. Protective antigen as a correlative marker for anthrax in animal models. Infect. Immun. 2006, 74, 5871–5876, doi:10.1128/IAI.00792–06.
- Boyer, A.E.; Quinn, C.P.; Woolfitt, A.R.; Pirkle, J.L.; McWilliams, L.G.; Stamey, K.L.; Bagarozzi, D.A.; Hart, J.C., Jr.; Barr, J.R. Detection and quantification of anthrax lethal factor in serum by mass spectrometry. Anal. Chem. 2007, 79, 8463–8470, doi:10.1021/ac701741s.
- Boyer, A.E.; Quinn, C.P.; Hoffmaster, A.R.; Kozel, T.R.; Saile, E.; Marston, C.K.; Percival, A.; Plikaytis, B.D.; Woolfitt, A.R.; Gallegos, M., et al. Kinetics of lethal factor and poly-D-glutamic acid antigenemia during inhalation anthrax in rhesus macaques. Infect. Immun. 2009, 77, 3432–3441, doi:10.1128/IAI.00346–09.
- Solano, M.I.; Woolfitt, A.R.; Boyer, A.E.; Lins, R.C.; Isbell, K.; Gallegos-Candela, M.; Moura, H.; Pierce, C.L.; Barr, J.R. Accurate and selective quantification of anthrax protective antigen in plasma by immunocapture and isotope dilution mass spectrometry. Anal. 2019, 144, 2264–2274, doi:10.1039/c8an02479k.
- Boyer, A.E.; Gallegos-Candela, M.; Quinn, C.P.; Woolfitt, A.R.; Brumlow, J.O.; Isbell, K.; Hoffmaster, A.R.; Lins, R.C.; Barr, J.R. High-sensitivity MALDI-TOF MS quantification of anthrax lethal toxin for diagnostics and evaluation of medical countermeasures. Anal. Bioanal. Chem. 2015, 407, 2847–2858, doi:10.1007/s00216–015–8509–5.
- Woolfitt, A.R.; Juni, B.A.; Gallegos-Candela, M.; Lins, R.; Solano, M.; Lee, J.; Sanford, D.; Stark, G.; Dreier, T.; Barr, J. Development of anthrax toxemia in new zealand white rabbits developing systemic anthrax after exposure to low-dose ames spores. In Proceedings of The International Conference on Bacillus anthracis, B. cereus, B. Thuringiensis, Victoria, Victoria Conference Centre, British Columbia, Canada, 2013.
- Rougeaux, C.; Becher, F.; Goossens, P.L.; Tournier, J.N. Very Early Blood Diffusion of the Active Lethal and Edema Factors of Bacillus anthracis After Intranasal Infection. J. Infect. Dis. 2020, 221, 660–667, doi:10.1093/infdis/jiz497.
- Holty, J.E.; Bravata, D.M.; Liu, H.; Olshen, R.A.; McDonald, K.M.; Owens, D.K. Systematic review: A century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 2006, 144, 270–280, doi:10.7326/0003–4819-144–4-200602210–00009.
- Boyer, A.E.; Woolfitt, A.R.; Candela, M.; Lins, R.C.; Solano, M., Lee, J.; Sanford, D.; Stark, G.; Dreier, T.; Quinn, P.; Barr, J.R. Toxin levels in organ tissues of nonhuman primates with inhalation anthrax. In Proceedings of The International Conference on Bacillus anthracis, B. cereus and B. thuringiensis, Victoria, Victoria Conference Centre, British Columbia, Canada, 2013.
- Gallegos-Candela, M.; Boyer, A.E.; Woolfitt, A.R.; Brumlow, J.; Lins, R.C.; Quinn, C.P.; Hoffmaster, A.R.; Meister, G.; Barr, J.R. Validated MALDI-TOF-MS method for anthrax lethal factor provides early diagnosis and evaluation of therapeutics. Anal. Biochem. 2018, 543, 97–107, doi:10.1016/j.ab.2017.12.007.
- Walsh, J.J.; Pesik, N.; Quinn, C.P.; Urdaneta, V.; Dykewicz, C.A.; Boyer, A.E.; Guarner, J.; Wilkins, P.; Norville, K.J.; Barr, J.R., et al. A case of naturally acquired inhalation anthrax: Clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin Infect. Dis 2007, 44, 968–971, doi:10.1086/512372.
- Sprenkle, M.D.; Griffith, J.; Marinelli, W.; Boyer, A.E.; Quinn, C.P.; Pesik, N.T.; Hoffmaster, A.; Keenan, J.; Juni, B.A.; Blaney, D.D. Lethal factor and anti-protective antigen IgG levels associated with inhalation anthrax, Minnesota, USA. Emerg. Infect. Dis. 2014, 20, 310–314, doi:10.3201/eid2002.130245.
- Tournier, J.N.; Rougeaux, C.; Biot, F.V.; Goossens, P.L. Questionable Efficacy of Therapeutic Antibodies in the Treatment of Anthrax. mSphere. 2019, 4, doi:10.1128/mSphere.00282–19.




