Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shanshan Li | -- | 1947 | 2022-05-13 06:36:36 | | | |
| 2 | Dean Liu | -1 word(s) | 1946 | 2022-05-16 03:40:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, S.; , .; Li, J.; Yang, J.; Li, W. Olfactory Evaluation in Model Mice. Encyclopedia. Available online: https://encyclopedia.pub/entry/22899 (accessed on 07 February 2026).
Li S, , Li J, Yang J, Li W. Olfactory Evaluation in Model Mice. Encyclopedia. Available at: https://encyclopedia.pub/entry/22899. Accessed February 07, 2026.
Li, Shanshan, , Jing Li, Jing Yang, Weiyun Li. "Olfactory Evaluation in Model Mice" Encyclopedia, https://encyclopedia.pub/entry/22899 (accessed February 07, 2026).
Li, S., , ., Li, J., Yang, J., & Li, W. (2022, May 13). Olfactory Evaluation in Model Mice. In Encyclopedia. https://encyclopedia.pub/entry/22899
Li, Shanshan, et al. "Olfactory Evaluation in Model Mice." Encyclopedia. Web. 13 May, 2022.
Copy Citation
Olfactory dysfunction occurs in a variety of diseases, including COVID-19, neurodegenerative diseases, etc. This topic summarizes commonly used olfactory behavioral methods from the AD perspective.
olfactory evaluation
Alzheimer’s disease model mice
olfactory behavior test
1. Introduction
Alzheimer’s disease (AD) is a slowly progressing disease that remains dormant in the preclinical stage for more than ten years. Early research on the pathological mechanisms of AD primarily focused on irreversible brain damage [1]. Although most clinical symptoms are linked to cognitive impairment, other cognition-related factors should also be considered. Several studies have revealed a direct link between smell and learning and memory [2][3]. Numerous studies also have found that AD patients experience olfactory dysfunction in the early stages, such as decreased odor discrimination ability, increased olfactory threshold, and olfactory memory loss [4][5][6][7][8][9]. Olfactory detection has been a means of early diagnosis [10][11].
Olfaction is one of the oldest primary sensory systems (i.e., vision, olfaction, taste, hearing, and balance) [12]. The nature of odor receptor proteins, perireceptor processes, the organization of the olfactory central nervous system, and odor-guided behavior and memory are all highly conserved across species [13]. Thus, AD mouse models corresponding to different etiologies of AD have been bred to conduct many studies on AD anosmia, including APP/PS1, Tg2576, 5 × FAD, 3 × Tg, P301S, andApoE4 mice. The olfactory function test is an indispensable research method for verifying AD-like anosmia.
2. The Structure of the Olfactory System
Primary olfactory areas (the nasal cavity and olfactory epithelium), secondary olfactory areas (the olfactory bulb and lateral olfactory tract), and primary olfactory cortex (the anterior olfactory nucleus, olfactory tubercle, piriform cortex, amygdala, and entorhinal cortex) comprise the olfactory system, which is a component of the sensory nervous system [12][14][15]. Olfactory signals are transmitted step by step in the olfactory system, and volatile odorants reach the olfactory epithelium via the nasal cavity or nasopharynx. Odorant information is converted from chemical signals to neuro-electrical signals after being recognized explicitly by olfactory sensory neurons (OSNs) in the olfactory epithelium and then projected to the main olfactory bulb in a highly precise manner by OSN axons. OSNs expressing the same odorant receptors project to one or more olfactory glomeruli on the surface of the olfactory bulb, forming synaptic connections with mitral cells and tuft cells (M/T) in the olfactory bulb to complete the odor signal handover [16][17]. M/T axons from the lateral olfactory tract further form the anterior olfactory nucleus with medium-sized neurons scattered along the olfactory tract, which eventually branch out at the olfactory tubercle to the fornix (medial striatum) or the piriform cortex, the medial olfactory cortex, and the amygdala (lateral striatum) [15].
3. Olfactory Behavioral Tests in Mice
The design of olfactory behavior tests in mice is based on spontaneous drinking or foraging, innate odor memory, and curiosity for novel odors. Integrating all behavioral tests, researchers classify them into three categories: odor recognition tests, odor discrimination tests, and odor memory tests.
3.1. Odor Recognition Tests
3.1.1. Food-Seeking Test
The food-seeking test is performed to assess general olfactory ability. The mice are trained to look for buried and exposed food with volatile odors (Figure 1A) [18][19][20][21]. To make mice more motivated to seek food, the usual method is to restrict or deprive the mice of food before the experiment and then allow the mice to find the food buried in the bedding and record their latency to find the food. In addition, under the same conditions, an exposed food-seeking test is performed, and the results are compared with those obtained from the buried food-seeking test to confirm olfactory dysfunction without cognitive impairment.
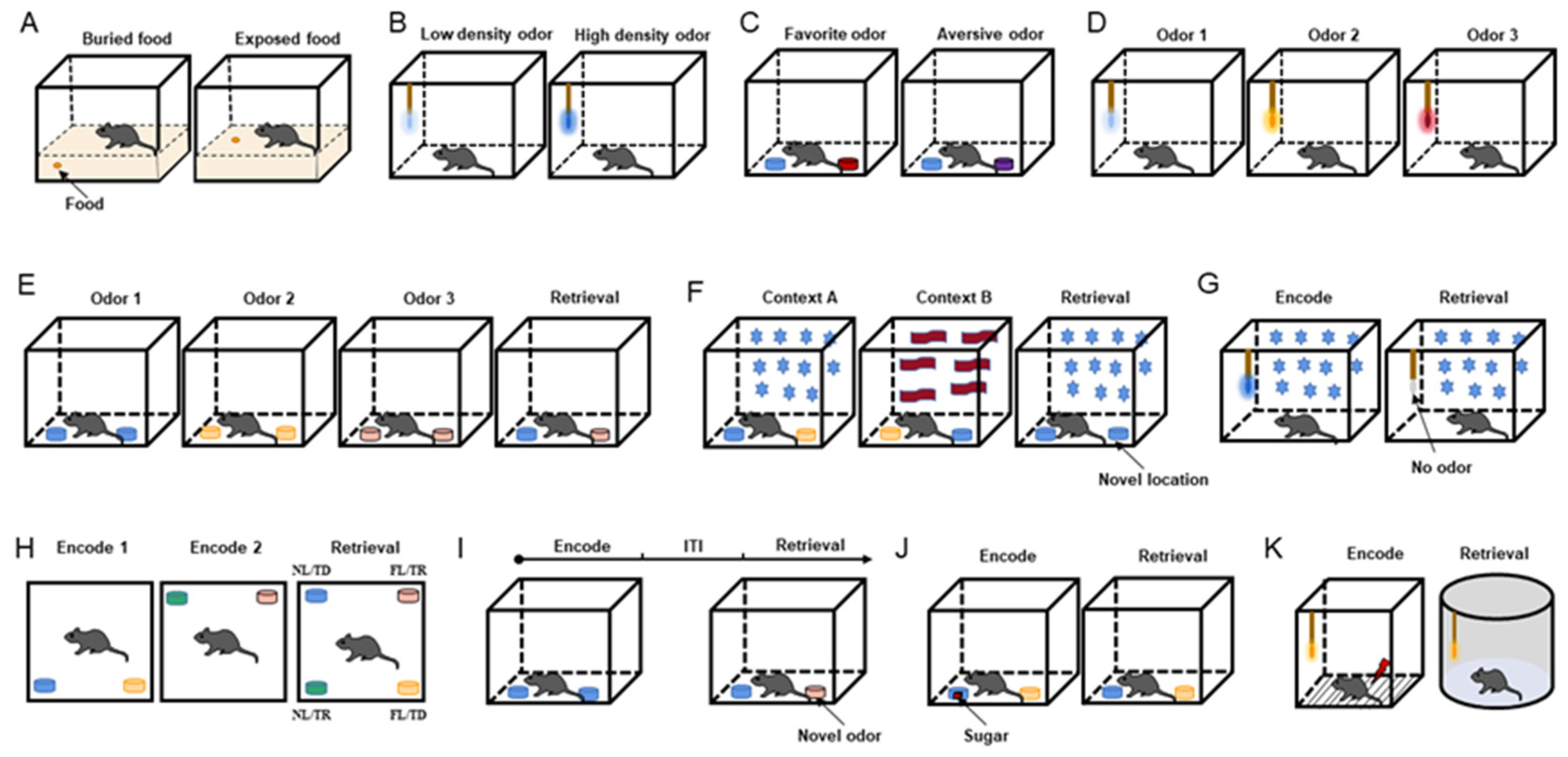
Figure 1. Behavioral paradigms. (A) Diagram of food-seeking test. (B) Diagram of odor sensitivity test. (C) Diagram of odor preference test. Blue is neutral, red is preferred, and purple is aversive. (D) Diagram of odor discrimination test. (E) Diagram of temporal odor memory test. (F) Diagram of spatial odor memory test. Star and wave patterns represent different context decorations. (G) Diagram of context odor memory test. (H) Diagram of spatiotemporal odor memory test. (I) Diagram of context-independent odor memory test. (J) Diagram of odor–reward associative memory test. The red square represents sugar cube. (K) Diagram of odor–aversion (foot shock) associative memory test. Odorant was applied to the cotton tip (B,D,G,K) or odor cup (C,E,F,H–J). Different colors of cotton swabs or smell cups represent the presentation of different odors.
3.1.2. Odor Sensitivity Test
The odor sensitivity test was designed to evaluate the olfactory threshold in mice (Figure 1B) [22][23]. In this experiment, neutral odorants are diluted to various concentrations, such as 1/100, 1/1000, 1/10,000, and 1/100,000, and then presented in increasing order of odor concentration. When the concentration of the presented odor reaches the olfactory threshold, its odor-sniffing behavior increases. In odor concentration presentation, the olfactory threshold experiment outperforms the food-seeking test in terms of controllability and precision.
3.2. Odor Discrimination Tests
Odor discrimination tests are classified into two types on the basis of their degree of difficulty: simple and fine. The former selects odors with a significant difference, whereas the latter chooses odors that are similar or contain a mixture of various odors to increase the difficulty of odor discrimination.
3.2.1. Simple Odor Discrimination Tests
Odor Preference Test
According to the degree of the innate preference of mice, odors can be classified into three types: innate preferred odor, neutral odor, and innate aversive odor. Researchers designed an odor preference test on the basis of how the mice perceived the three types of odors [23]. During the experiment, mice receive two odor combinations—the preferred odor and water and the aversive odor and water—and the sniffing time for each odor is recorded. When the exploring time for the preferred odor is longer than that of water and the exploring time for the aversive odor is shorter than that of water, the mice are considered to have normal discrimination ability (Figure 1C).
Neutral Odor Discrimination Test
The neutral odor discrimination test is based on the nature of mice to explore novel odors. The mice are first tested with a non-odorant liquid (mineral oil or water) as a blank odor and then presented with 2–8 odors in sequence to observe the mice’s sniffing behavior for novel odors (Figure 1D). Sniffing for every odor is extremely high in mice with a good sense of smell [24].
Odor Cross-Habituation Test
The odor cross-habituation test is employed to evaluate odor habituation and the ability to distinguish between old and new odors (odor dishabituation) [20][21][23]. When mice with intact olfactory function are repeatedly exposed to the same odor, their olfactory exploratory behavior is significantly reduced during the second to fourth exposures, and when a new odor is introduced, they exhibit more sniffing behavior. In general, 3–5 neutral odors are chosen for the experiment, and each odor is presented to the mice 3–4 times consecutively, with a time of 20 s for a single presentation and an interval of 30 s. The difference between the sniffing times for the first and last presentations of each odor represented the degree of habituation, and the difference between the last sniffing time of the previous odor and the first sniffing time of the subsequent new odor represented the degree of dishabituation.
3.2.2. Fine Odor Discrimination Test
The fine odor discrimination test is a more challenging task for mice. Its difference from simple odor discrimination tests is mainly reflected in odor selection. To test the mice’s olfactory fine resolution ability, odors with similar aromas or mixtures of different odors are chosen [23][25][26].
3.3. Odor Memory Tests
Context-independent odor memory, context-dependent episodic odor memory, and odor-emotion memory are three types of odor memory tests linked with different olfactory associative cues.
3.3.1. Episodic Odor Memory Tests
Temporal Odor Memory Test
This behavioral paradigm was performed to detect the odor temporal order in specific contexts (Figure 1E) [27]. This test included three acquisition phases and one retrieval phase. Different odors were presented in each acquisition stage, and the first odor and the last odor were selected for testing in the retrieval phase with a constant odor spatial context. Correct memory expression prompts the mice to sniff the first odor.
Spatial Odor Memory Test
Mice were tested for memory of odor location in a specific context in this paradigm (Figure 1F) [27]. The test chamber was decorated to create two different contexts. Two distinct odors were placed at opposite ends of the chamber in context A (odor 1 on the left and odor 2 on the right). Both odors were placed in opposing positions in context B (odor 2 on the left and odor 1 on the right). The chamber was reconfigured as context A for the retrieval phase, but this time, two copies of odor 1 were presented. The time spent investigating the odor cups (the familiar odor in the old and novel positions) was recorded.
Context Odor Memory Test
The context odor memory test was utilized to detect context-driven odor memory (Figure 1G) [27]. In this paradigm, the mice were trained for 30 min on the first nine days to associate a specific environment with an odor presented via a cotton swab, and on the tenth day, the mice were placed in the same context but without the odor. On days 9 and 10, their odor-sniffing behavior was recorded during the first 5 min of their exposure to the context. When the mice failed to detect the expected odor, they used context odor memory by spending more time investigating the swab.
Spatiotemporal Odor Memory Test
This behavioral paradigm integrated temporal and spatial odor memory tests (Figure 1H) [27]. During the first acquisition phase, the mice investigated two distinct odors located in two adjacent chamber corners. Following that, two new odors were introduced and placed in the other two corners. Two groups of odors were presented during the retrieval phase, and each group exchanged the spatial location of one odor. Finally, the odor presented four spatiotemporal combinations: familiar location/temporally distant (FL/TD), familiar location/temporally recent (FL/TR), novel location/temporally distant (NL/TD), and novel location/temporally recent (NL/TR). Successful odor memory in time and space leads to a preference for investigating the odor with NL/TR combinations and the least probability of investigating the odor with FL/TD combinations.
3.3.2. Context-Independent Odor Memory Test
This behavioral paradigm conducted the acquisition phase and the retrieval phase with the same spatial cues in the same context (Figure 1I) [26]. During the acquisition phase, mice were placed in the chamber with two small cups containing the same odor that were placed on opposite sides of the arena. After exploring both copies, the animal was placed in a holding cage for 5, 15, or 30 min. During the retrieval phase, mice investigated both old and new odors. If mice retained memories of old odors, they were more inclined to explore new odors.
3.3.3. Odor–Emotion Memory Tests
Odor–Reward Associative Memory Test
The reward in an odor–reward associative memory test can be food, candy, or water. During the acquisition phase, the mice were exposed to two odors, one with a reward (R+) and one without a reward (R-). Mice were repeatedly trained and successfully associated the R+ odor with the behavior of finding hidden food, candy, or drinking water. During the retrieval phase, the association behavior of the R+ odor matches was recorded (Figure 1J). Mice with a well-established odor–reward combined memory were more likely to dig in the odor cup of the R+ odor or drink water when the R+ odor appeared [23][28][29].
Odor–Aversion Associative Memory Test
Aversion can be induced in the odor–aversion associative memory test by using water-containing LiCl to induce stomach upset, causing avoidance behavior, or a shock to startle mice, resulting in freezing behavior (Figure 1K) [30][31]. Like the odor–reward combined memory test, it was necessary to successfully associate an odor with an aversive behavior and then observe the corresponding behavior during the odor retrieval phase.
References
- Sperling, R.; Mormino, E.; Johnson, K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron 2014, 84, 608–622.
- Igarashi, K.M.; Lu, L.; Colgin, L.L.; Moser, M.-B.; Moser, E.I. Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature 2014, 510, 143–147.
- Li, Y.; Xu, J.; Liu, Y.; Zhu, J.; Liu, N.; Zeng, W.; Huang, N.; Rasch, M.J.; Jiang, H.; Gu, X.; et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat. Neurosci. 2017, 20, 559–570.
- Doty, R.L.; Reyes, P.F.; Gregor, T. Presence of both odor identification and detection deficits in alzheimer’s disease. Brain Res. Bull. 1987, 18, 597–600.
- Djordjevic, J.; Jones-Gotman, M.; De Sousa, K.; Chertkow, H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2008, 29, 693–706.
- Vasavada, M.M.; Martinez, B.; Wang, J.; Eslinger, P.J.; Gill, D.J.; Sun, X.; Karunanayaka, P.; Yang, Q.X. Central Olfactory Dysfunction in Alzheimer’s Disease and Mild Cognitive Impairment: A Functional MRI Study. J. Alzheimer’s Dis. 2017, 59, 359–368.
- Jung, H.J.; Shin, I.-S.; Lee, J.-E. Olfactory function in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Laryngoscope 2018, 129, 362–369.
- Gilbert, P.E.; Murphy, C. The effect of the ApoE epsilon4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. J. Clin. Exp. Neuropsychol. 2004, 26, 779–794.
- Gilbert, P.E.; Barr, P.J.; Murphy, C. Differences in olfactory and visual memory in patients with pathologically confirmed Alzheimer’s disease and the Lewy body variant of Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2004, 10, 835–842.
- Silva, M.D.M.E.; Mercer, P.B.S.; Witt, M.C.Z.; Pessoa, R.R. Olfactory dysfunction in Alzheimer’s disease Systematic review and meta-analysis. Dement. Neuropsychol. 2018, 12, 123–132.
- Roberts, R.O.; Christianson, T.J.H.; Kremers, W.K.; Mielke, M.; Machulda, M.M.; Vassilaki, M.; Alhurani, R.E.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol. 2016, 73, 93–101.
- Smith, T.D.; Bhatnagar, K.P. Anatomy of the olfactory system. Handb. Clin. Neurol. 2019, 164, 17–28.
- Ache, B.W.; Young, J.M. Olfaction: Diverse Species, Conserved Principles. Neuron 2005, 48, 417–430.
- Treloar, H.B.; Miller, A.M.; Ray, A.; Greer, C.A. Development of the Olfactory System. In The Neurobiology of Olfaction; Menini, A., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010.
- Shipley, M.T.; Ennis, M.; Puche, A.C. Olfactory system. In The Rat Nervous System, 3rd ed.; Paxinos, G., Ed.; Academic Press: San Diego, CA, USA, 2004; pp. 921–961.
- Firestein, S. How the olfactory system makes sense of scents. Nature 2001, 413, 211–218.
- Harvey, J.D.; Heinbockel, T. Neuromodulation of Synaptic Transmission in the Main Olfactory Bulb. Int. J. Environ. Res. Public Health 2018, 15, 2194.
- Yoo, S.J.; Lee, J.H.; Kim, S.Y.; Son, G.; Kim, J.Y.; Cho, B.; Yu, S.W.; Chang, K.A.; Suh, Y.H.; Moon, C. Differential spatial expression of peripheral olfactory neuron-derived BACE1 induces olfactory impairment by region-specific accumulation of beta-amyloid oligomer. Cell Death Dis. 2017, 8, e2977.
- Li, S.; Li, W.; Wu, X.; Li, J.; Yang, J.; Tu, C.; Ye, X.; Ling, S. Olfactory deficit is associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice. Brain Res. Bull. 2019, 148, 34–45.
- Yang, M.; Crawley, J.N. Simple Behavioral Assessment of Mouse Olfaction. Curr. Protoc. Neurosci. 2009, 48, 8.24.1–8.24.12.
- Lehmkuhl, A.M.; Dirr, E.R.; Fleming, S.M. Olfactory Assays for Mouse Models of Neurodegenerative Disease. J. Vis. Exp. 2014, 90, e51804.
- Witt, R.M.; Galligan, M.M.; Despinoy, J.R.; Segal, R. Olfactory Behavioral Testing in the Adult Mouse. J. Vis. Exp. 2009, 23, e949.
- Zou, J.; Wang, W.; Pan, Y.; Lu, S.; Xia, Z. Methods to Measure Olfactory Behavior in Mice. Curr. Protoc. Toxicol. 2015, 63, 11.18.1–11.18.21.
- Son, G.; Yoo, S.J.; Kang, S.; Rasheed, A.; Jung, D.H.; Park, H.; Cho, B.; Steinbusch, H.W.; Chang, K.A.; Suh, Y.H.; et al. Region-specific amyloid-beta accumulation in the olfactory system influences olfactory sensory neuronal dysfunction in 5xFAD mice. Alzheimer’s Res. Ther. 2021, 13, 4.
- Li, W.; Li, S.; Shen, L.; Wang, J.; Wu, X.; Li, J.; Tu, C.; Ye, X.; Ling, S. Impairment of Dendrodendritic Inhibition in the Olfactory Bulb of APP/PS1 Mice. Front. Aging Neurosci. 2019, 11, 2.
- Rey, N.L.; Jardanhazi-Kurutz, D.; Terwel, D.; Kummer, M.; Jourdan, F.; Didier, A.; Heneka, M.T. Locus coeruleus degeneration exacerbates olfactory deficits in APP/PS1 transgenic mice. Neurobiol. Aging 2012, 33, 426.e1–426.e11.
- Aqrabawi, A.; Kim, J. Behavioral Evaluation of Odor Memory in Mice. Bio-Protocol. 2018, 8, e3023.
- Yassine, N.; Lazaris, A.; Dorner-Ciossek, C.; Després, O.; Meyer, L.; Maitre, M.; Mensah-Nyagan, A.G.; Cassel, J.-C.; Mathis, C. Detecting spatial memory deficits beyond blindness in tg2576 Alzheimer mice. Neurobiol. Aging 2013, 34, 716–730.
- Girard, S.D.; Jacquet, M.; Baranger, K.; Migliorati, M.; Escoffier, G.; Bernard, A.; Khrestchatisky, M.; Féron, F.; Rivera, S.; Roman, F.S.; et al. Onset of hippocampus-dependent memory impairments in 5XFAD transgenic mouse model of Alzheimer’s disease. Hippocampus 2014, 24, 762–772.
- East, B.S.; Fleming, G.; Vervoordt, S.; Shah, P.; Sullivan, R.M.; Wilson, D.A. Basolateral amygdala to posterior piriform cortex connectivity ensures precision in learned odor threat. Sci. Rep. 2021, 11, 21746.
- Terral, G.; Busquets-Garcia, A.; Varilh, M.; Achicallende, S.; Cannich, A.; Bellocchio, L.; Río, I.B.-D.; Massa, F.; Puente, N.; Soria-Gomez, E.; et al. CB1 Receptors in the Anterior Piriform Cortex Control Odor Preference Memory. Curr. Biol. 2019, 29, 2455–2464.e5.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
16 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




